Co-Bonded Hybrid Thermoplastic-Thermoset Composite Interphase: Process-Microstructure-Property Correlation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Interphase between Thermoset and Thermoplastic
2.3. Characterizations
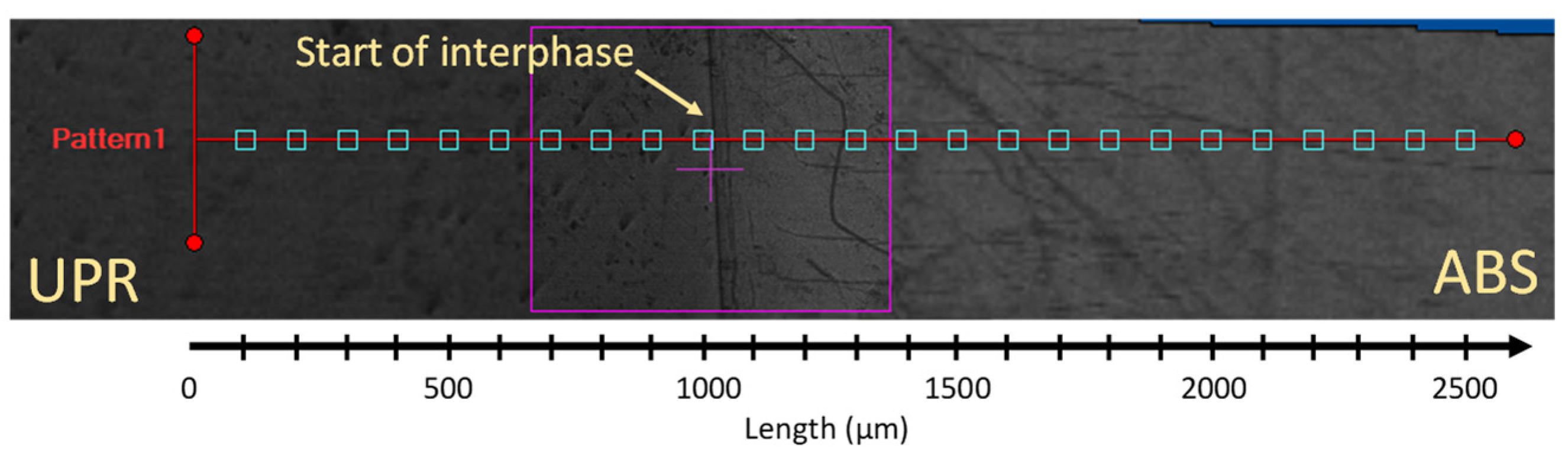
2.3.1. Microscopic Analysis of Unsaturated Polyester Resin-Acrylonitrile Butadiene Styrene (UPR-ABS) Interphase+
2.3.2. Surface Swelling Measurements
2.3.3. Chemo-Rheology of UPR
2.3.4. Diffusivity Coefficient of UPR in ABS
1D Inverse Diffusion Model
- = 1 for and = 0 for where was the gelation time of the polyester resin which was determined experimentally in Section 2.3.3.
- The concentration was assumed to be relatively small by using a value of 10−5 at and at , i.e., (see Figure 3).
Temperature Dependent Diffusivity Model
2.3.5. Resin Uptake
2.3.6. Resin Volume Fraction at Interphase
2.3.7. Hardness Measurement at Interphase
3. Results and Discussion
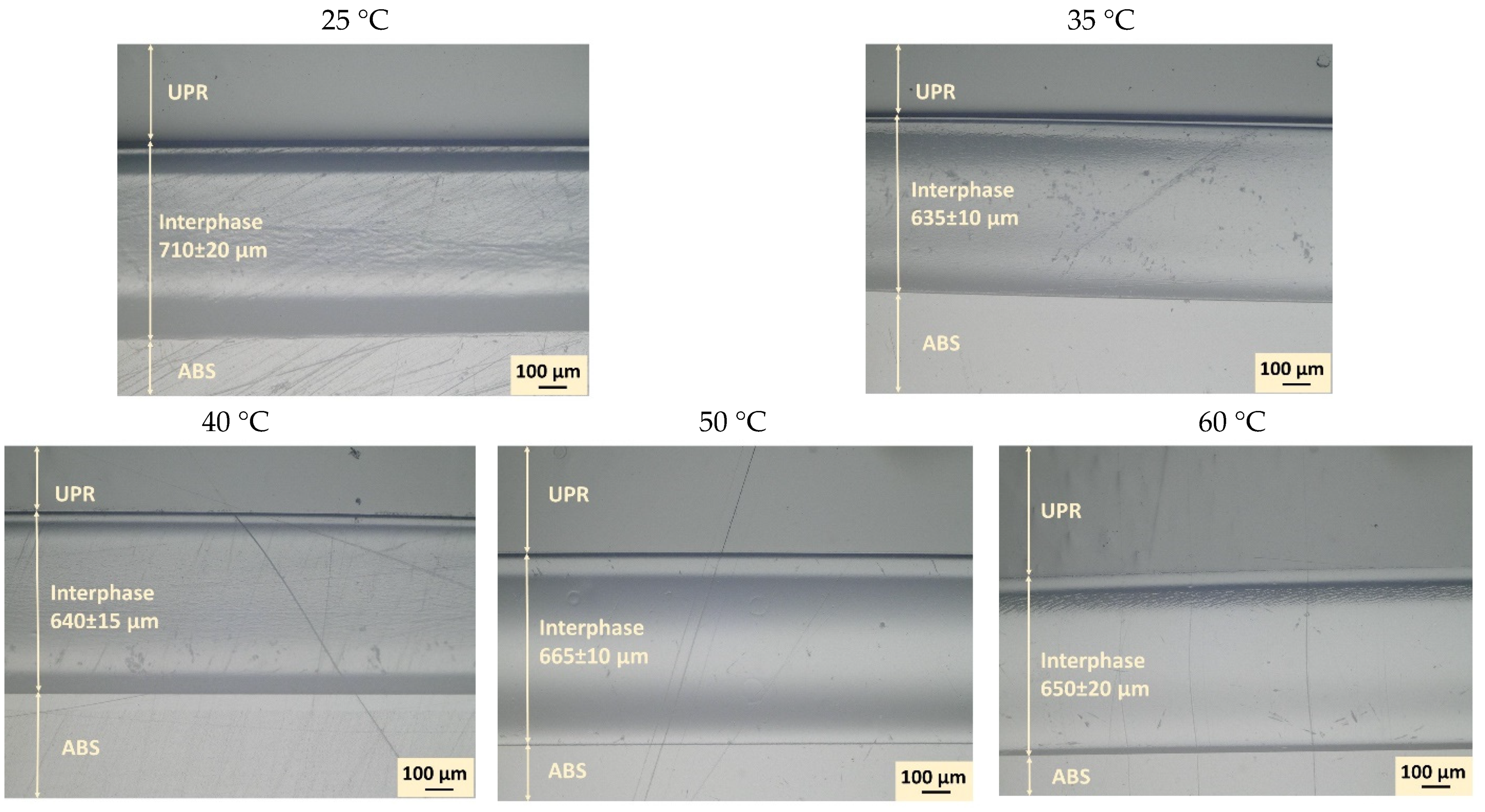
3.1. Interphase Formation and Processing Temperature Effect
3.2. Resin Curing and Diffusion Kinetics
3.3. Diffusivity Coefficient and Model
3.4. Kinetics of Interdiffusion and Its Correlation to Interphase Microstructure
3.5. Interphase’s Mechanical Response by Microhardness
4. Conclusions and Future Work
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Monfared Zanjani, J.S.; Okan, B.S.; Menceloglu, Y.Z.; Yildiz, M. Nano-engineered design and manufacturing of high-performance epoxy matrix composites with carbon fiber/selectively integrated graphene as multi-scale reinforcements. Rsc Adv. 2016, 6, 9495–9506. [Google Scholar] [CrossRef]
- Nash, N.H.; Young, T.M.; McGrail, P.T.; Stanley, W.F. Inclusion of a thermoplastic phase to improve impact and post-impact performances of carbon fibre reinforced thermosetting composites—A review. Mater. Des. 2015, 85, 582–597. [Google Scholar] [CrossRef]
- Seyyed Monfared Zanjani, J.; Baran, I.; Akkerman, R. Combatting rain erosion of offshore wind turbine blades by co-bonded thermoplastic-thermoset hybrid composites. IOP Conf. Ser. Mater. Sci. Eng. 2020, 942, 012024. [Google Scholar] [CrossRef]
- Selver, E.; Potluri, P.; Hogg, P.; Soutis, C. Impact damage tolerance of thermoset composites reinforced with hybrid commingled yarns. Compos. Part B Eng. 2016, 91, 522–538. [Google Scholar] [CrossRef]
- Dogan, A.; Arikan, V. Low-velocity impact response of E-glass reinforced thermoset and thermoplastic based sandwich composites. Compos. Part B Eng. 2017, 127, 63–69. [Google Scholar] [CrossRef]
- Ma, Y.; Yang, Y.; Sugahara, T.; Hamada, H. A study on the failure behavior and mechanical properties of unidirectional fiber reinforced thermosetting and thermoplastic composites. Compos. Part B Eng. 2016, 99, 162–172. [Google Scholar] [CrossRef]
- Thoppul, S.D.; Finegan, J.; Gibson, R.F. Mechanics of mechanically fastened joints in polymer–matrix composite structures—A review. Compos. Sci. Technol. 2009, 69, 301–329. [Google Scholar] [CrossRef]
- Hassan, J.; Feser, T.; O’Higgins, R.M.; Waimer, M.; McCarthy, C.T.; Toso, N.; Byrne, M.E.; McCarthy, M.A. Energy absorption capability of composite bolted joints undergoing extended bearing failure. Compos. Struct. 2020, 237, 111868. [Google Scholar] [CrossRef]
- Cao, Y.; Cao, Z.; Zhao, Y.; Zuo, D.; Tay, T.E. Damage progression and failure of single-lap thin-ply laminated composite bolted joints under quasi-static loading. Int. J. Mech. Sci. 2020, 170, 105360. [Google Scholar] [CrossRef]
- Stavrov, D.; Bersee, H.E.N. Resistance welding of thermoplastic composites-an overview. Compos. Part A Appl. Sci. Manuf. 2005, 36, 39–54. [Google Scholar] [CrossRef]
- Yousefpour, A.; Hojjati, M.; Immarigeon, J.-P. Fusion Bonding/Welding of Thermoplastic Composites. J. Thermoplast. Compos. Mater. 2004, 17, 303–341. [Google Scholar] [CrossRef]
- Villegas, I.F.; van Moorleghem, R. Ultrasonic welding of carbon/epoxy and carbon/PEEK composites through a PEI thermoplastic coupling layer. Compos. Part A Appl. Sci. Manuf. 2018, 109, 75–83. [Google Scholar] [CrossRef]
- Sahu, S.K.; Mishra, D.; Mahto, R.P.; Sharma, V.M.; Pal, S.K.; Pal, K.; Banerjee, S.; Dash, P. Friction stir welding of polypropylene sheet. Eng. Sci. Technol. Int. J. 2018, 21, 245–254. [Google Scholar] [CrossRef]
- Takeda, T.; Yasuoka, T.; Hoshi, H.; Sugimoto, S.; Iwahori, Y. Effectiveness of flame-based surface treatment for adhesive bonding of carbon fiber reinforced epoxy matrix composites. Compos. Part A Appl. Sci. Manuf. 2019, 119, 30–37. [Google Scholar] [CrossRef]
- Martínez-Landeros, V.H.; Vargas-Islas, S.Y.; Cruz-González, C.E.; Barrera, S.; Mourtazov, K.; Ramírez-Bon, R. Studies on the influence of surface treatment type, in the effectiveness of structural adhesive bonding, for carbon fiber reinforced composites. J. Manuf. Process. 2019, 39, 160–166. [Google Scholar] [CrossRef]
- Çoban, O.; Akman, E.; Bora, M.Ö.; Genc Oztoprak, B.; Demir, A. Laser surface treatment of CFRP composites for a better adhesive bonding owing to the mechanical interlocking mechanism. Polym. Compos. 2019, 40, 3611–3622. [Google Scholar] [CrossRef]
- Dahmen, V.; Redmann, A.J.; Austermann, J.; Quintanilla, A.L.; Mecham, S.J.; Osswald, T.A. Fabrication of hybrid composite T-joints by co-curing with 3D printed dual cure epoxy. Compos. Part B Eng. 2020, 183, 107728. [Google Scholar] [CrossRef]
- Zheng, C.; Wang, S.; Liang, S. Interface bonding mechanisms of co-cured damping carbon fiber reinforced epoxy matrix composites. J. Alloys Compd. 2020, 822, 153739. [Google Scholar] [CrossRef]
- Huang, Y.; Meng, X.; Xie, Y.; Li, J.; Si, X.; Fan, Q. Improving mechanical properties of composite/metal friction stir lap welding joints via a taper-screwed pin with triple facets. J. Mater. Process. Technol. 2019, 268, 80–86. [Google Scholar] [CrossRef]
- Kweon, J.-H.; Jung, J.-W.; Kim, T.-H.; Choi, J.-H.; Kim, D.-H. Failure of carbon composite-to-aluminum joints with combined mechanical fastening and adhesive bonding. Compos. Struct. 2006, 75, 192–198. [Google Scholar] [CrossRef]
- Zhao, T.; Palardy, G.; Villegas, I.F.; Rans, C.; Martinez, M.; Benedictus, R. Mechanical behaviour of thermoplastic composites spot-welded and mechanically fastened joints: A preliminary comparison. Compos. Part B Eng. 2017, 112, 224–234. [Google Scholar] [CrossRef] [Green Version]
- Karakaya, N.; Papila, M.; Özkoç, G. Overmolded hybrid composites of polyamide-6 on continuous carbon and glass fiber/epoxy composites: ‘An assessment of the interface’. Compos. Part A Appl. Sci. Manuf. 2020, 131, 105771. [Google Scholar] [CrossRef]
- Baran, I. Warpage prediction in over-infusion process of glass/polyester composite laminates. In Proceedings of the 21st International Conference on Composite Materials 2017, Xi’an, China, 20–25 August 2017. [Google Scholar]
- Leone, C.; Genna, S. Effects of surface laser treatment on direct co-bonding strength of CFRP laminates. Compos. Struct. 2018, 194, 240–251. [Google Scholar] [CrossRef]
- Immordino, K.M.; McKnight, S.H.; Gillespie, J.W. In-Situ Evaluation of the Diffusion of Epoxy and Amine in Thermoplastic Polymers. J. Adhes. 1998, 65, 115–129. [Google Scholar] [CrossRef]
- Rajagopalan, G.; Immordino, K.M.; Gillespie, J.W.; McKnight, S.H. Diffusion and reaction of epoxy and amine in polysulfone studied using Fourier transform infrared spectroscopy: Experimental results. Polymer 2000, 41, 2591–2602. [Google Scholar] [CrossRef]
- Sonnenfeld, C.; Mendil-Jakani, H.; Agogué, R.; Nunez, P.; Beauchêne, P. Thermoplastic/thermoset multilayer composites: A way to improve the impact damage tolerance of thermosetting resin matrix composites. Compos. Struct. 2017, 171, 298–305. [Google Scholar] [CrossRef]
- Van Velthem, P.; Ballout, W.; Daoust, D.; Sclavons, M.; Cordenier, F.; Henry, E.; Dumont, D.; Destoop, V.; Pardoen, T.; Bailly, C. Influence of thermoplastic diffusion on morphology gradient and on delamination toughness of RTM-manufactured composites. Compos. Part A Appl. Sci. Manuf. 2015, 72, 175–183. [Google Scholar] [CrossRef]
- Lu, M.G.; Shim, M.J.; Kim, S.W. Curing behavior of an unsaturated polyester system analyzed by Avrami equation. Thermochim. Acta 1998, 323, 37–42. [Google Scholar] [CrossRef]
- Baran, I.; Akkerman, R.; Hattel, J.H. Material characterization of a polyester resin system for the pultrusion process. Compos. Part B Eng. 2014, 64, 194–201. [Google Scholar] [CrossRef]
- Monfared Zanjani, J.S.; Baran, I.; Akkerman, R. Characterization of interdiffusion mechanisms during co-bonding of unsaturated polyester resin to thermoplastics with different thermodynamic affinities. Polymer 2020, 209, 122991. [Google Scholar] [CrossRef]
- Rajagopalan, G.; Gillespie, J.W., Jr.; McKnight, S.H. Diffusion of reacting epoxy and amine monomers in polysulfone: A diffusivity model. Polymer 2000, 41, 7723–7733. [Google Scholar] [CrossRef]
- Voleppe, Q.; Pardoen, T.; Bailly, C. Interdiffusion and phase separation upon curing in thermoset-thermoplastic interphases unravelled by the characterization of partially cured systems. Polymer 2016, 106, 120–127. [Google Scholar] [CrossRef]
- Lestriez, B.; Chapel, J.-P.; Gérard, J.-F. Gradient Interphase between Reactive Epoxy and Glassy Thermoplastic from Dissolution Process, Reaction Kinetics, and Phase Separation Thermodynamics. Macromolecules 2001, 34, 1204–1213. [Google Scholar] [CrossRef]
- Oyama, H.T.; Solberg, T.N.; Wightman, J.P. Electron microprobe analysis as a novel technique to study the interface between thermoset and thermoplastic polymers. Polymer 1999, 40, 3001–3011. [Google Scholar] [CrossRef]
- Ramos, J.A.; Blanco, M.; Zalakain, I.; Mondragon, I. Nanoindentation study of interphases in epoxy/amine thermosetting systems modified with thermoplastics. J. Colloid Interface Sci. 2009, 336, 431–437. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Petkovich, N.D.; Liu, K.; Qian, Y.; Macosko, C.W.; Stein, A. Unsaturated polyester resin toughening with very low loadings of GO derivatives. Polymer 2017, 110, 149–157. [Google Scholar] [CrossRef]
- Adhikari, J.; Biswas, B.; Chabri, S.; Bandyapadhyay, N.R.; Sawai, P.; Mitra, B.C.; Sinha, A. Effect of functionalized metal oxides addition on the mechanical, thermal and swelling behaviour of polyester/jute composites. Eng. Sci. Technol. Int. J. 2017, 20, 760–774. [Google Scholar] [CrossRef] [Green Version]
- Bee, S.-T.; Sin, L.T.; Ratnam, C.T.; Chew, W.S.; Rahmat, A.R. Enhancement effect of trimethylopropane trimethacrylate on electron beam irradiated acrylonitrile butadiene styrene (ABS). Polym. Bull. 2018, 75, 5015–5037. [Google Scholar] [CrossRef]
- Dunnigan, R.; Clemens, J.; Cavalli, M.N.; Kaabouch, N.; Gupta, S. Beneficial usage of recycled polymer particulates for designing novel 3D printed composites. Prog. Addit. Manuf. 2018, 3, 33–38. [Google Scholar] [CrossRef]
- Hsu, C.; Lee, L.J. Free-radical crosslinking copolymerization of styrene/unsaturated polyester resins: 3. Kinetics-gelation mechanism. Polymer 1993, 34, 4516–4523. [Google Scholar] [CrossRef]
- Amir, Z.; Said, I.M.; Jan, B.M. In situ organically cross-linked polymer gel for high-temperature reservoir conformance control: A review. Polym. Adv. Technol. 2019, 30, 13–39. [Google Scholar] [CrossRef] [Green Version]
- Crank, J. The Mathematics of Diffusion; Oxford University Press: Oxford, UK, 1979. [Google Scholar]
- Tsige, M.; Grest, G.S. Molecular dynamics simulation of solvent–polymer interdiffusion: Fickian diffusion. J. Chem. Phys. 2004, 120, 2989–2995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaw, P.E.; Burn, P.L. Real-time fluorescence quenching-based detection of nitro-containing explosive vapours: What are the key processes? Phys. Chem. Chem. Phys. 2017, 19, 29714–29730. [Google Scholar] [CrossRef]
- Jagannatha, T.; Harish, G. Mechanical properties of carbon/glass fiber reinforced epoxy hybrid polymer composites. Int. J. Mech. Eng. Robot. Res. 2015, 4, 131–137. [Google Scholar]
- Gong, J.; Wu, J.; Guan, Z. Examination of the indentation size effect in low-load vickers hardness testing of ceramics. J. Eur. Ceram. Soc. 1999, 19, 2625–2631. [Google Scholar] [CrossRef]
- Zanjani, J.S.M.; Saner Okan, B.; Menceloglu, Y.Z.; Yildiz, M. Design and fabrication of multi-walled hollow nanofibers by triaxial electrospinning as reinforcing agents in nanocomposites. J. Reinf. Plast. Compos. 2015, 34, 1273–1286. [Google Scholar] [CrossRef]
- Sperling, L.H. Introduction to Physical Polymer Science; John Wiley & Sons: Hoboken, NJ, USA, 2005. [Google Scholar]
- Christensen, H.; Sehested, K.; Corfitzen, H. Reactions of hydroxyl radicals with hydrogen peroxide at ambient and elevated temperatures. J. Phys. Chem. 1982, 86, 1588–1590. [Google Scholar] [CrossRef]
- Dumont, D.; Seveno, D.; De Coninck, J.; Bailly, C.; Devaux, J.; Daoust, D. Interdiffusion of thermoplastics and epoxy resin precursors: Investigations using experimental and molecular dynamics methods. Polym. Int. 2012, 61, 1263–1271. [Google Scholar] [CrossRef]
- Rajagopalan, G.; Narayanan, C.; Gillespie, J.W.; McKnight, S.H. Diffusion and reaction of epoxy and amine in polysulfone-transport modeling and experimental validation. Polymer 2000, 41, 8543–8556. [Google Scholar] [CrossRef]
- Wolf, C.J.; Grayson, M.A. Solubility, diffusion and swelling of fluids in thermoplastic resin systems. Polymer 1993, 34, 746–751. [Google Scholar] [CrossRef]
- Reis, R.; Vladimir Oliveira, J.; Nobrega, R. Diffusion coefficients in polymer-solvent systems for highly concentrated polymer solutions. Braz. J. Chem. Eng. 2001, 18, 367–384. [Google Scholar] [CrossRef]
- Chenyakin, Y.; Ullmann, D.A.; Evoy, E.; Renbaum-Wolff, L.; Kamal, S.; Bertram, A.K. Diffusion coefficients of organic molecules in sucrose–water solutions and comparison with Stokes–Einstein predictions. Atmos. Chem. Phys. 2017, 17, 2423–2435. [Google Scholar] [CrossRef] [Green Version]











| Temperature (°C) | 25 | 35 | 40 | 50 | 60 |
|---|---|---|---|---|---|
| n | 0.7002 | 0.5717 | 0.5538 | 0.5254 | 0.5251 |
| k (gr/mm2·sn) | 3.86 × 10−7 | 1.88 × 10−6 | 2.62 × 10−6 | 4.12 × 10−6 | 5.25 × 10−6 |
| Temperature (°C) | 25 | 35 | 40 | 50 | 60 |
|---|---|---|---|---|---|
| 0.40 | 0.34 | 0.26 | 0.21 | 0.20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zanjani, J.S.M.; Baran, I. Co-Bonded Hybrid Thermoplastic-Thermoset Composite Interphase: Process-Microstructure-Property Correlation. Materials 2021, 14, 291. https://doi.org/10.3390/ma14020291
Zanjani JSM, Baran I. Co-Bonded Hybrid Thermoplastic-Thermoset Composite Interphase: Process-Microstructure-Property Correlation. Materials. 2021; 14(2):291. https://doi.org/10.3390/ma14020291
Chicago/Turabian StyleZanjani, Jamal Seyyed Monfared, and Ismet Baran. 2021. "Co-Bonded Hybrid Thermoplastic-Thermoset Composite Interphase: Process-Microstructure-Property Correlation" Materials 14, no. 2: 291. https://doi.org/10.3390/ma14020291






