Aluminum Doped Titania as a Support of Copper Catalysts for SCR of Nitrogen Oxides
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Composition, Crystallographic Structure, and Morphology
3.2. Textural Properties
3.3. Surface Composition—XPS
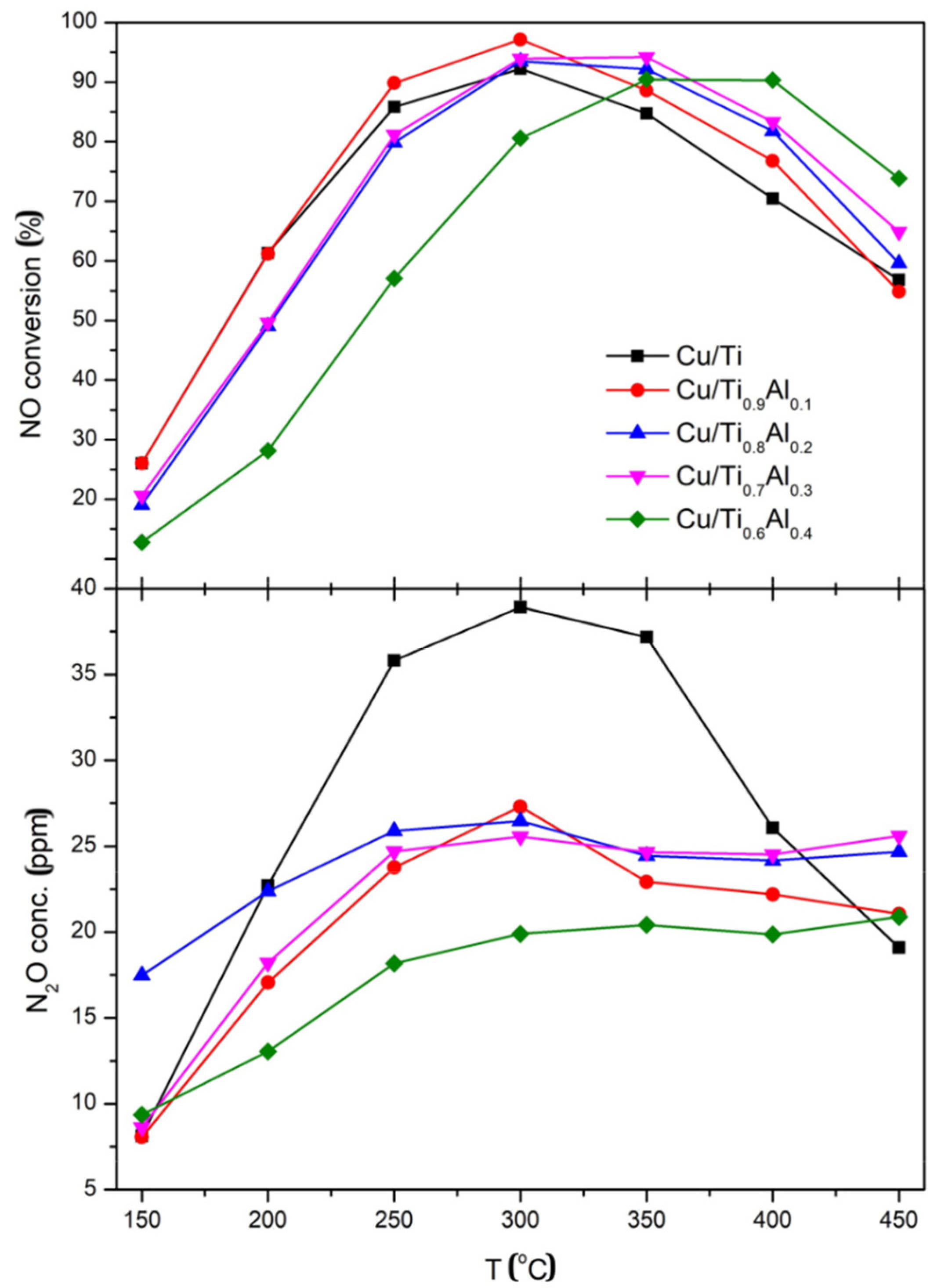
3.4. Catalytic Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Han, L.; Cai, S.; Gao, M.; Hasegawa, J.Y.; Wang, P.; Zhang, J.; Shi, L.; Zhang, D. Selective Catalytic Reduction of NOx with NH3 by Using Novel Catalysts: State of the Art and Future Prospects. Chem. Rev. 2019, 119, 10916–10976. [Google Scholar] [CrossRef]
- Wang, X.; Shi, Y.; Li, S.; Li, W. Promotional synergistic effect of Cu and Nb doping on a novel Cu/Ti-Nb ternary oxide catalyst for the selective catalytic reduction of NOx with NH3. Appl. Catal. B Environ. 2018, 220, 250. [Google Scholar] [CrossRef]
- Blanco, J.; Avila, P.; Suárez, S.; Martín, J.A.; Knapp, C. Alumina- and titania-based monolithic catalysts for low temperature selective catalytic reduction of nitrogen oxides. Appl. Catal. B Environ. 2000, 28, 235–244. [Google Scholar] [CrossRef]
- Suárez, S.; Martín, J.A.; Yates, M.; Avila, P.; Blanco, J. N2O formation in the selective catalytic reduction of NOx with NH3 at low temperature on CuO-supported monolithic catalysts. J. Catal. 2005, 229, 227–236. [Google Scholar] [CrossRef]
- Bennici, S.; Carniti, P.; Gervasini, A. Bulk and surface properties of dispersed CuO phases in relation with activity of NOx reduction. Catal. Lett. 2004, 98, 187–194. [Google Scholar] [CrossRef]
- Tanuma, S.; Powell, C.J.; Penn, D.R. Calculations of electron inelastic mean free paths. V. Data for 14 organic compounds over the 50–2000 eV range. Surf. Interface Anal. 1994, 21, 165–176. [Google Scholar] [CrossRef]
- Yu, Z.; Zhang, Z.; Zhang, Y.; Huang, W. Titania Morphology-Dependent Catalysis of CuOx/TiO2 Catalysts in CO Oxidation and Water Gas Shift Reactions. ChemCatChem 2020, 12, 3679–3686. [Google Scholar] [CrossRef]
- Biesinger, M.C. Advanced analysis of copper X-ray photoelectron spectra. Surf. Interface Anal. 2017, 49, 1325–1334. [Google Scholar] [CrossRef]
- Dhonge, B.P.; Mathews, T.; Sundari, S.T.; Thinaharan, C.; Kamruddin, M.; Dash, S.; Tyagi, A.K. Spray pyrolytic deposition of transparent aluminum oxide (Al2O3) films. Appl. Surf. Sci. 2011, 258, 1091–1096. [Google Scholar] [CrossRef]
- Severino, F.; Brito, J.L.; Laine, J.; Fierro, J.L.G.; López Agudo, A. Nature of copper active sites in the carbon monoxide oxidation on CuAl2O4 and CuCr2O4 spinel type catalysts. J. Catal. 1998, 177, 82–95. [Google Scholar] [CrossRef]
- Ponmudi, S.; Sivakumar, R.; Sanjeeviraja, C.; Gopalakrishnan, C.; Jeyadheepan, K. Facile fabrication of spinel structured n-type CuAl 2 O 4 thin film with nano-grass like morphology by sputtering technique. Appl. Surf. Sci. 2019, 483, 601–615. [Google Scholar] [CrossRef]
- Hu, B.; Yao, M.; Xiao, R.; Chen, J.; Yao, X. Optical properties of amorphous Al2O3 thin films prepared by a sol-gel process. Ceram. Int. 2014, 40, 14133–14139. [Google Scholar] [CrossRef]
- Bertóti, I.; Mohai, M.; Sullivan, J.L.; Saied, S.O. Surface characterisation of plasma-nitrided titanium: An XPS study. Appl. Surf. Sci. 1995, 84, 357–371. [Google Scholar] [CrossRef]
- Greczynski, G.; Hultman, L. Self-consistent modelling of X-ray photoelectron spectra from air-exposed polycrystalline TiN thin films. Appl. Surf. Sci. 2016, 387, 294–300. [Google Scholar] [CrossRef] [Green Version]
- Bukhtiyarov, V.I. XPS and SIMS characterization. Catal. Today 2000, 56, 403–413. [Google Scholar] [CrossRef]
- Jaeger, D.; Patscheider, J. A complete and self-consistent evaluation of XPS spectra of TiN. J. Electron Spectros. Relat. Phenom. 2012, 185, 523–534. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Lau, L.W.; Gerson, A.R.; StC Smart, R. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Sc, Ti, V, Cu and Zn. Appl. Surf. Sci. 2010, 257, 887–898. [Google Scholar] [CrossRef]
- Shen, Y.; Guo, M.; Xia, X.; Shao, G. Role of materials chemistry on the electrical/electronic properties of CuO thin films. Acta Mater. 2015, 85, 122–131. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, B.; Luo, L.; Chen, X.; Wang, Z.; Wu, E.; Su, D.; Huang, W. TiO2/Cu2O Core/Ultrathin Shell Nanorods as Efficient and Stable Photocatalysts for Water Reduction. Angew. Chem. Int. Ed. 2015, 54, 15260–15265. [Google Scholar] [CrossRef] [PubMed]
- Białas, A.; Rugała, K.; Czosnek, C.; Mordarski, G.; Gurgul, J. Copper aluminum spinels doped with cerium as catalysts for NO removal. Catalysts 2020, 10, 1388. [Google Scholar] [CrossRef]
- Ghijsen, J.; Tjeng, L.H.; Van Elp, J.; Eskes, H.; Westerink, J.; Sawatzky, G.A.; Czyzyk, M.T. Electronic structure of Cu2O and CuO. Phys. Rev. B 1988, 38, 11322–11338. [Google Scholar] [CrossRef] [PubMed]
- NIST XPS Database. Available online: https://srdata.nist.gov/xps (accessed on 10 September 2021).
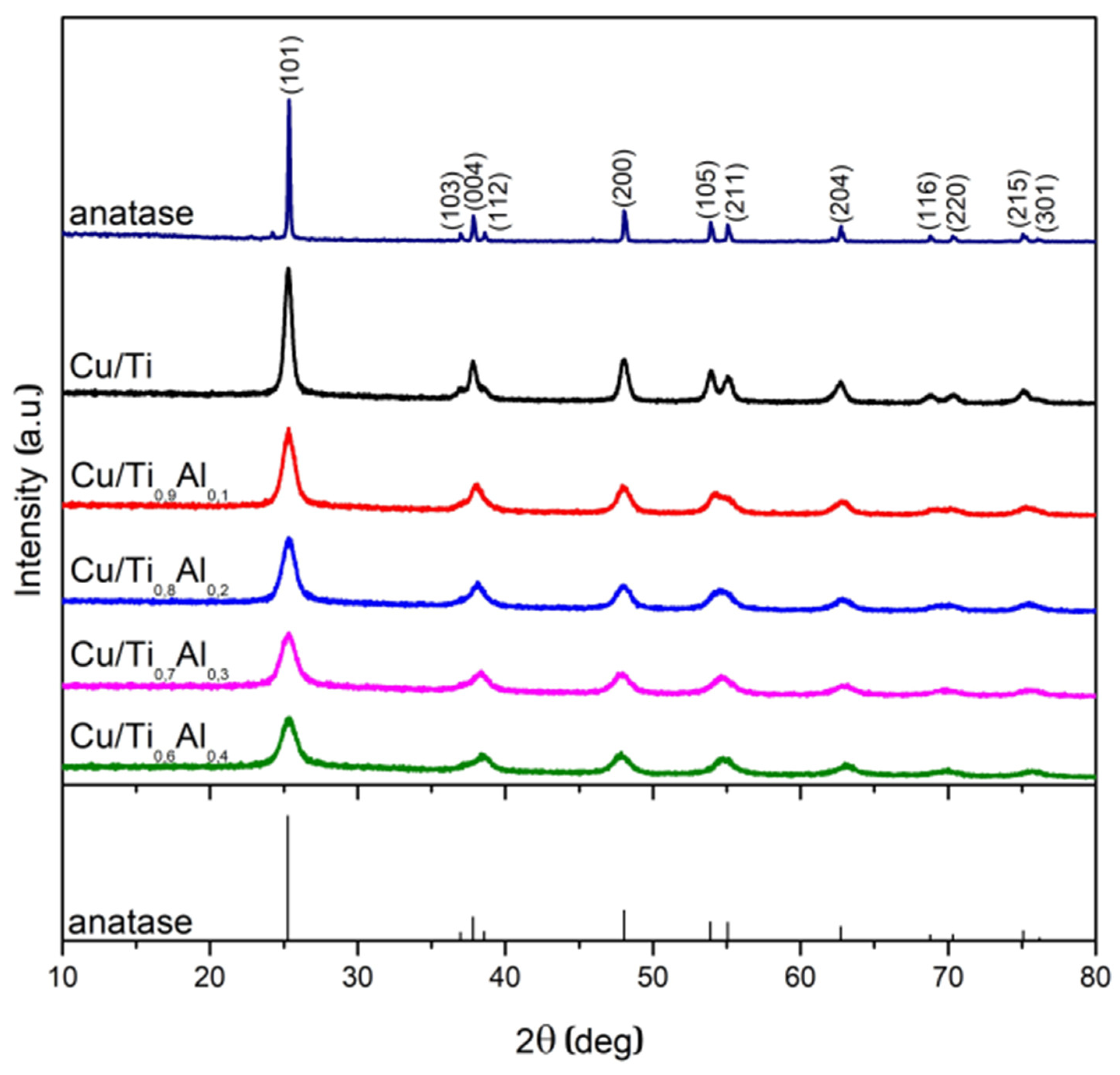
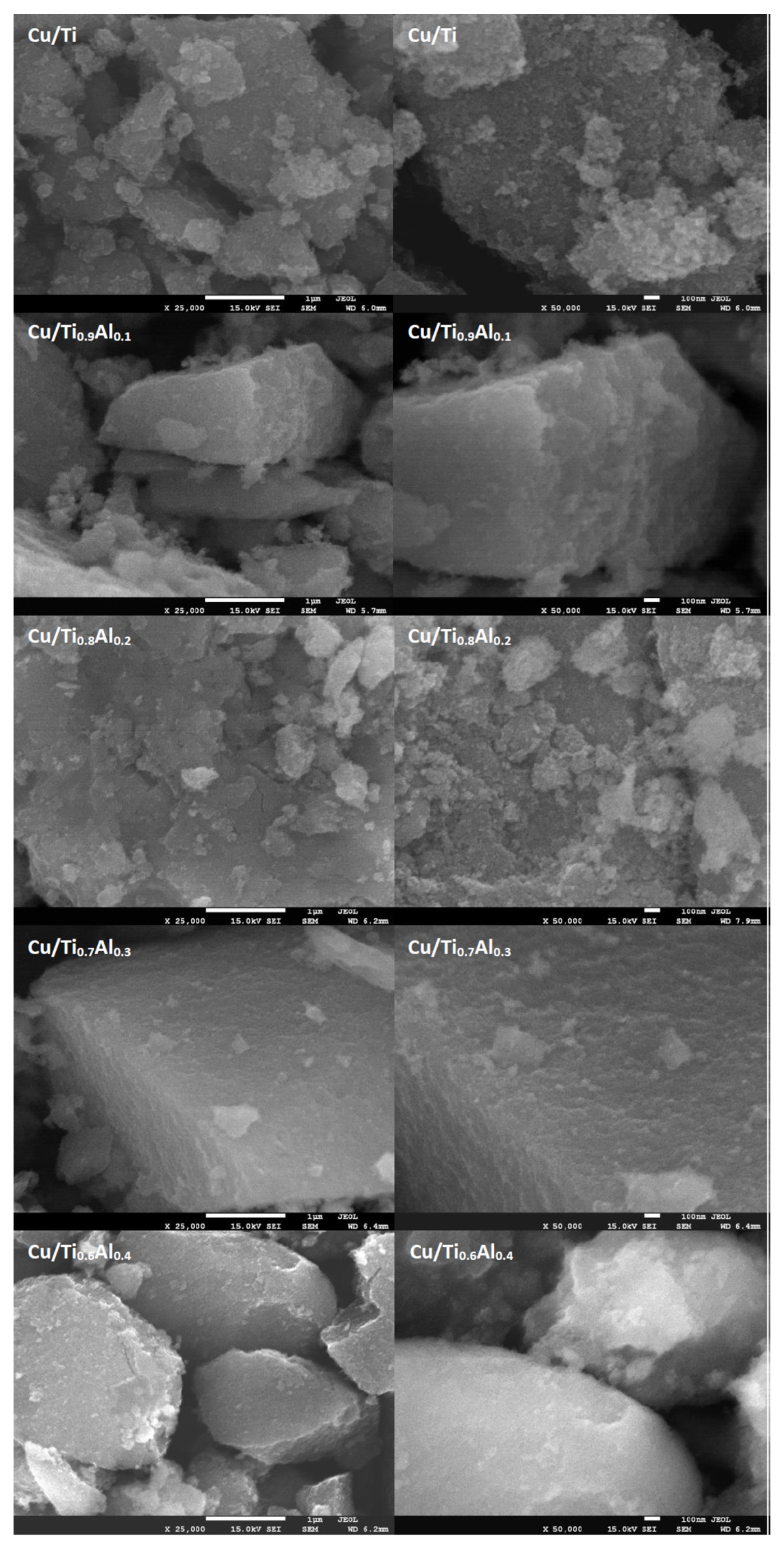
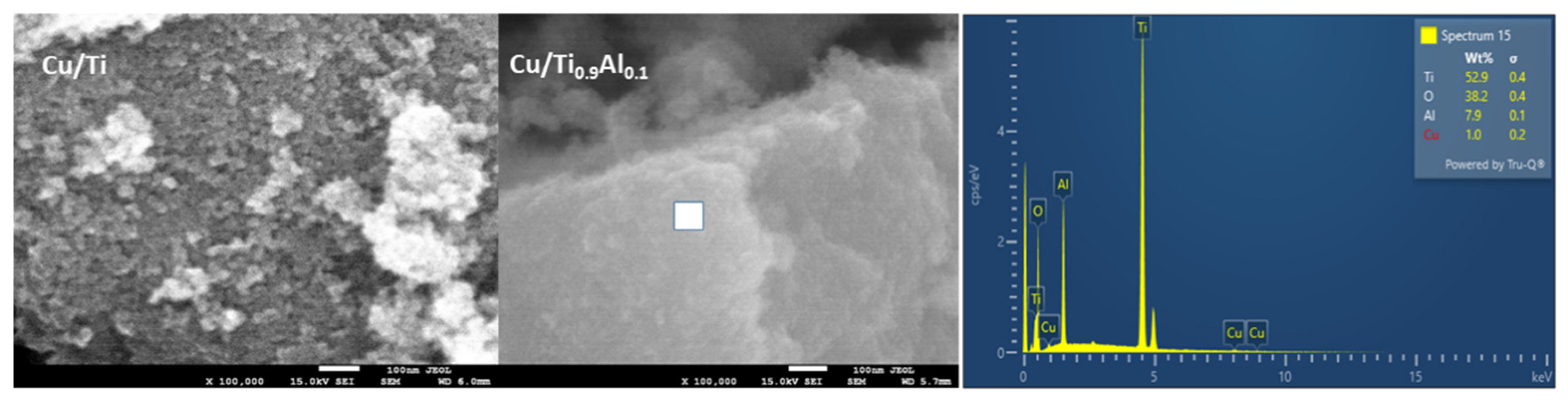
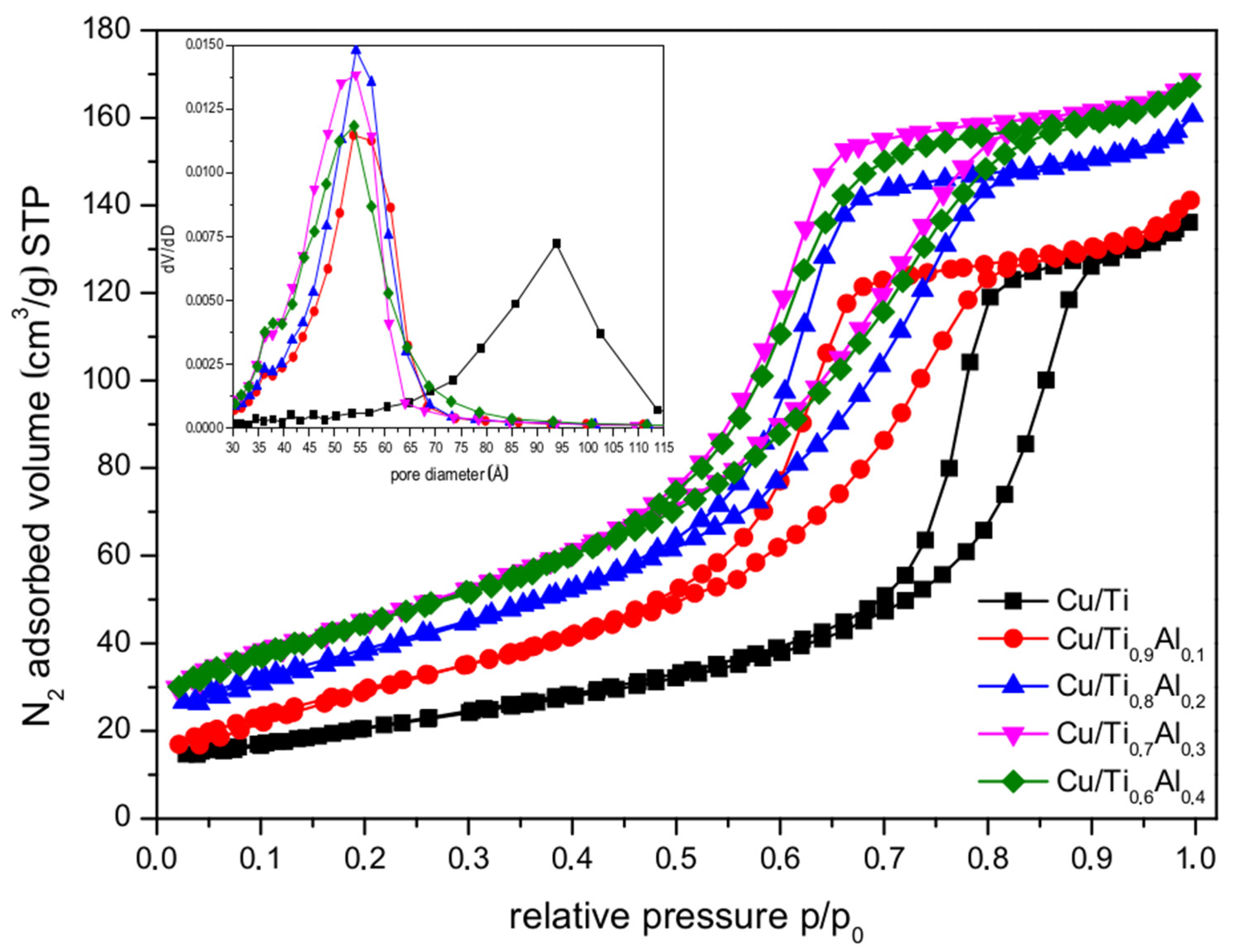
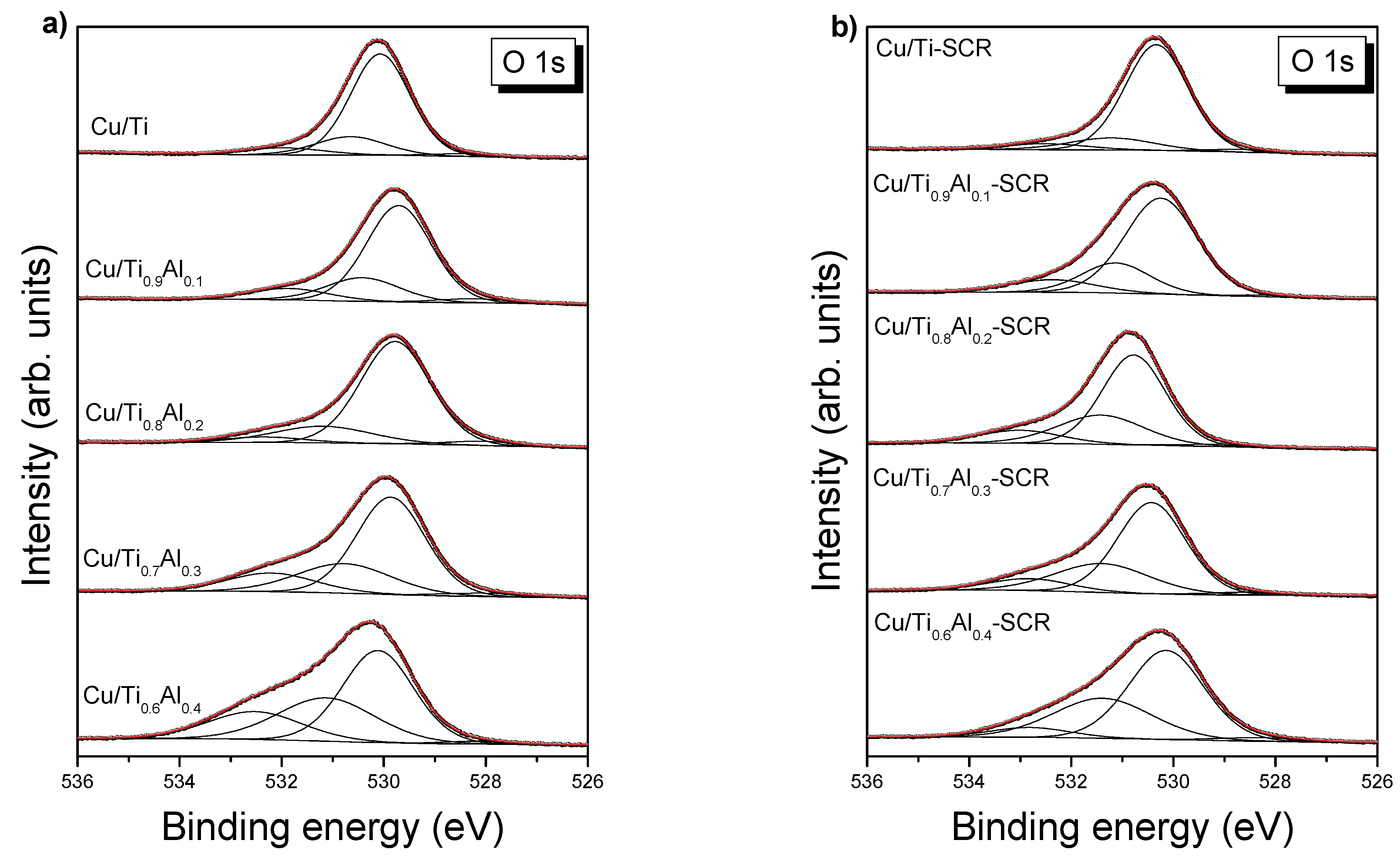

| Sample | Average Crystallite Size [nm] | Surface Area * SSABET [m2 g−1] | Pore Volume * [cm3 g−1] | |
|---|---|---|---|---|
| Nominal Formula | Measured Formula | |||
| Cu/Ti | 1.1Cu/Ti | 14.6 | 75 (97) | 0.203 (0.225) |
| Cu/Ti0.9Al0.1 | 1.3Cu/Ti0.90Al0.10 | 9.7 | 110 (129) | 0.204 (0.277) |
| Cu/Ti0.8Al0.2 | 1.3Cu/Ti0.85Al0.15 | 8.8 | 131 (178) | 0.233 (0.256) |
| Cu/Ti0.7Al0.3 | 1.5Cu/Ti0.74Al0.26 | 7.9 | 148 (190) | 0.247 (0.274) |
| Cu/Ti0.6Al0.4 | 1.6Cu/Ti0.63Al0.37 | 7.5 | 145 (190) | 0.246 (0.269) |
| Sample | C | O | Al | Ti | Cu | Cl * |
|---|---|---|---|---|---|---|
| Cu/Ti | 5.84 | 65.93 | --- | 26.84 | 0.99 | 0.41 |
| Cu/Ti-SCR | 8.65 | 65.57 | --- | 24.9 | 0.8 | --- |
| Cu/Ti0.9Al0.1 | 4.59 | 64.89 | 6.44 | 23.08 | 0.59 | 0.4 |
| Cu/Ti0.9Al0.1-SCR | 5.09 | 65.47 | 5.83 | 22.86 | 0.75 | --- |
| Cu/Ti0.8Al0.2 | 4.62 | 65.63 | 8.22 | 20.6 | 0.52 | 0.4 |
| Cu/Ti0.8Al0.2-SCR | 5.55 | 65.28 | 8.55 | 20.04 | 0.58 | --- |
| Cu/Ti0.7Al0.3 | 5.69 | 63.52 | 12.49 | 17.22 | 0.46 | 0.61 |
| Cu/Ti0.7Al0.3-SCR | 5.9 | 64.08 | 12.3 | 17.19 | 0.54 | --- |
| Cu/Ti0.6Al0.4 | 5.33 | 63.82 | 16.83 | 13.03 | 0.41 | 0.57 |
| Cu/Ti0.6Al0.4-SCR | 3.18 | 65.45 | 16.6 | 14.27 | 0.51 | --- |
| Core Excitation | Cu/Ti | Cu/Ti0.9Al0.1 | Cu/Ti0.8Al0.2 | Cu/Ti0.7Al0.3 | Cu/Ti0.6Al0.4 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| BE(eV) Area (%) | BE(eV) Area (%) | BE(eV) Area (%) | BE(eV) Area (%) | BE(eV) Area (%) | ||||||
| Cu 2p3/2 | 930.3 932.7 934.1 | 2.0 91.2 6.8 | 930.7 932.6 935.0 | 2.0 90.5 7.5 | 930.3 932.5 935.0 | 4.5 93.3 2.2 | 930.1 932.6 935.6 | 4.1 92.9 3.0 | 930.8 932.8 935.8 | 5.4 91.1 2.5 |
| Ti 2p3/2 | 458.9 | 100 | 458.5 | 100 | 458.6 | 100 | 458.6 | 100 | 458.7 | 100 |
| Al 2p3/2 | --- | 74.0 | 100 | 74.0 | 100 | 74.0 | 100 | 74.2 | 100 | |
| Core Excitation | Cu/Ti-SCR | Cu/Ti0.9Al0.1-SCR | Cu/Ti0.8Al0.2-SCR | Cu/Ti0.7Al0.3-SCR | Cu/Ti0.6Al0.4-SCR | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| BE(eV) Area (%) | BE(eV) Area (%) | BE(eV) Area (%) | BE(eV) Area (%) | BE(eV) Area (%) | ||||||
| Cu 2p3/2 | 930.2 932.4 935.3 | 3.4 95.3 1.3 | 930.6 933.0 935.9 | 4.4 94.1 1.5 | 931.0 933.0 935.2 | 2.8 92.3 4.9 | 931.1 932.9 935.3 | 6.1 92.8 1.1 | 931.4 932.9 935.6 | 8.3 87.0 4.7 |
| Ti 2p3/2 | 459.1 | 100 | 458.5 | 100 | 459.4 | 100 | 458.9 | 100 | 458.8 | 100 |
| Al 2p3/2 | --- | 74.2 | 100 | 74.4 | 100 | 74.2 | 100 | 74.3 | 100 | |
| Sample | Cu0 | Cu+ | Cu2+ | KE of Cu L3M45M45 | Auger Parameter |
|---|---|---|---|---|---|
| Cu/Ti | 930.3 | 932.7 | 934.1 | 916.3 | 1849 |
| (2) | (91.2) | (6.8) | |||
| Cu/Ti0.9Al0.1 | 930.8 | 932.6 | 935 | 916.5 | 1849.1 |
| (7.5) | (90.5) | (2) | |||
| Cu/Ti0.8Al0.2 | 930.3 | 932.5 | 935 | 916.6 | 1849.1 |
| (4.5) | (93.3) | (2.2) | |||
| Cu/Ti0.7Al0.3 | 930.1 | 932.6 | 935.6 | 916.3 | 1848.9 |
| (4.1) | (92.9) | (3) | |||
| Cu/Ti0.6Al0.4 | 931.1 | 933 | 936 | no data * | no data |
| (5.4) | (92.1) | (2.5) |
| Sample | Cu0 | Cu+ | Cu2+ | KE of CuLMM | Auger Parameter |
|---|---|---|---|---|---|
| Cu/Ti-SCR | 930.2 | 932.4 | 935.3 | 915.9 | 1848.7 |
| (3.4) | (95.3) | (1.3) | |||
| Cu/Ti0.9Al0.1-SCR | 930.6 | 933 | 935.9 | 916.4 | 1849.4 |
| (4.4) | (94.1) | (1.5) | |||
| Cu/Ti0.8Al0.2-SCR | 931 | 933 | 935.2 | 916.3 | 1849.3 |
| (4.9) | (93.3) | (2.8) | |||
| Cu/Ti0.7Al0.3-SCR | 931.4 | 932.9 | 935.3 | 916.1 | 1849 |
| (7.8) | (90.8) | (1.4) | |||
| Cu/Ti0.6Al0.4-SCR | 931.4 | 932.9 | 935.6 | no data * | no data |
| (8.3) | (87) | (4.7) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guziewicz, W.; Białas, A.; Napruszewska, B.D.; Zimowska, M.; Gurgul, J. Aluminum Doped Titania as a Support of Copper Catalysts for SCR of Nitrogen Oxides. Materials 2021, 14, 6021. https://doi.org/10.3390/ma14206021
Guziewicz W, Białas A, Napruszewska BD, Zimowska M, Gurgul J. Aluminum Doped Titania as a Support of Copper Catalysts for SCR of Nitrogen Oxides. Materials. 2021; 14(20):6021. https://doi.org/10.3390/ma14206021
Chicago/Turabian StyleGuziewicz, Wojciech, Anna Białas, Bogna D. Napruszewska, Małgorzata Zimowska, and Jacek Gurgul. 2021. "Aluminum Doped Titania as a Support of Copper Catalysts for SCR of Nitrogen Oxides" Materials 14, no. 20: 6021. https://doi.org/10.3390/ma14206021
APA StyleGuziewicz, W., Białas, A., Napruszewska, B. D., Zimowska, M., & Gurgul, J. (2021). Aluminum Doped Titania as a Support of Copper Catalysts for SCR of Nitrogen Oxides. Materials, 14(20), 6021. https://doi.org/10.3390/ma14206021






