Critical Role of Etching Parameters in the Evolution of Nano Micro SLA Surface on the Ti6Al4V Alloy Dental Implants
Abstract
:1. Introduction
2. Materials and Methods
3. Results
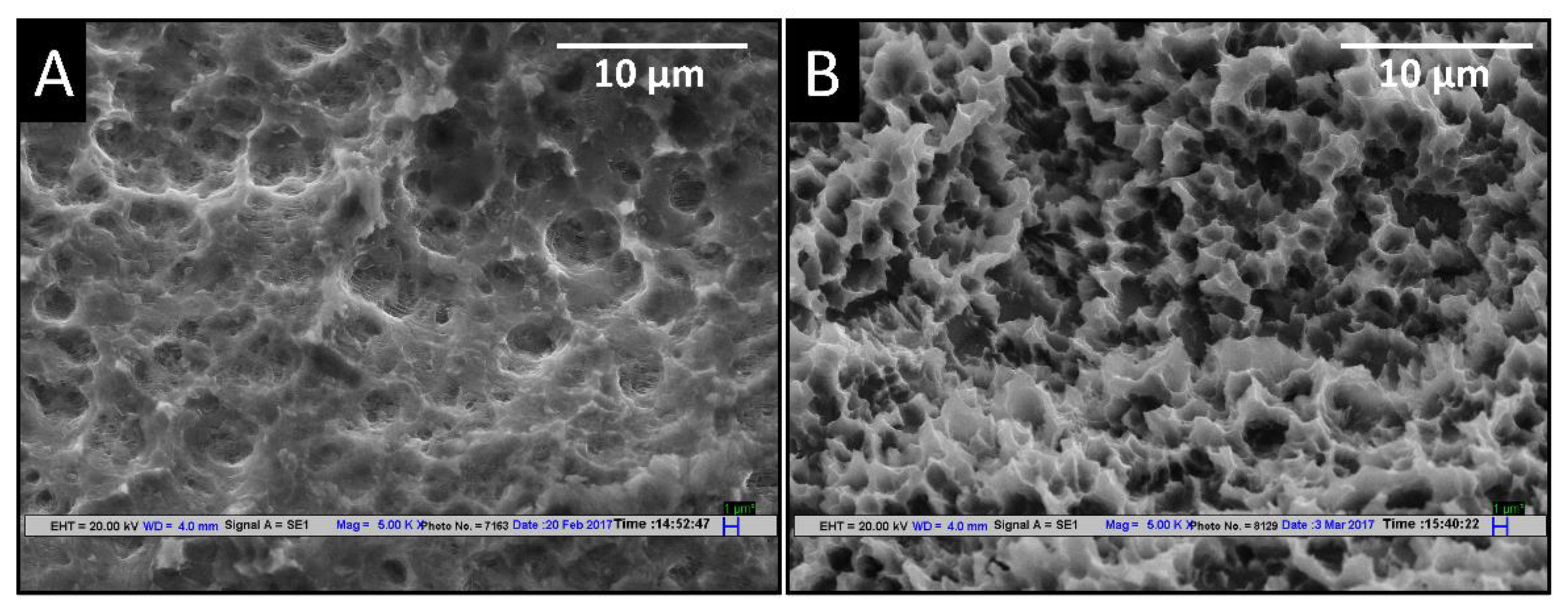
3.1. Effect of Different Etching Parameters on SEM Topography
3.2. Effect of High Temperature (HT) on Thread Geometry of the Dental Implants
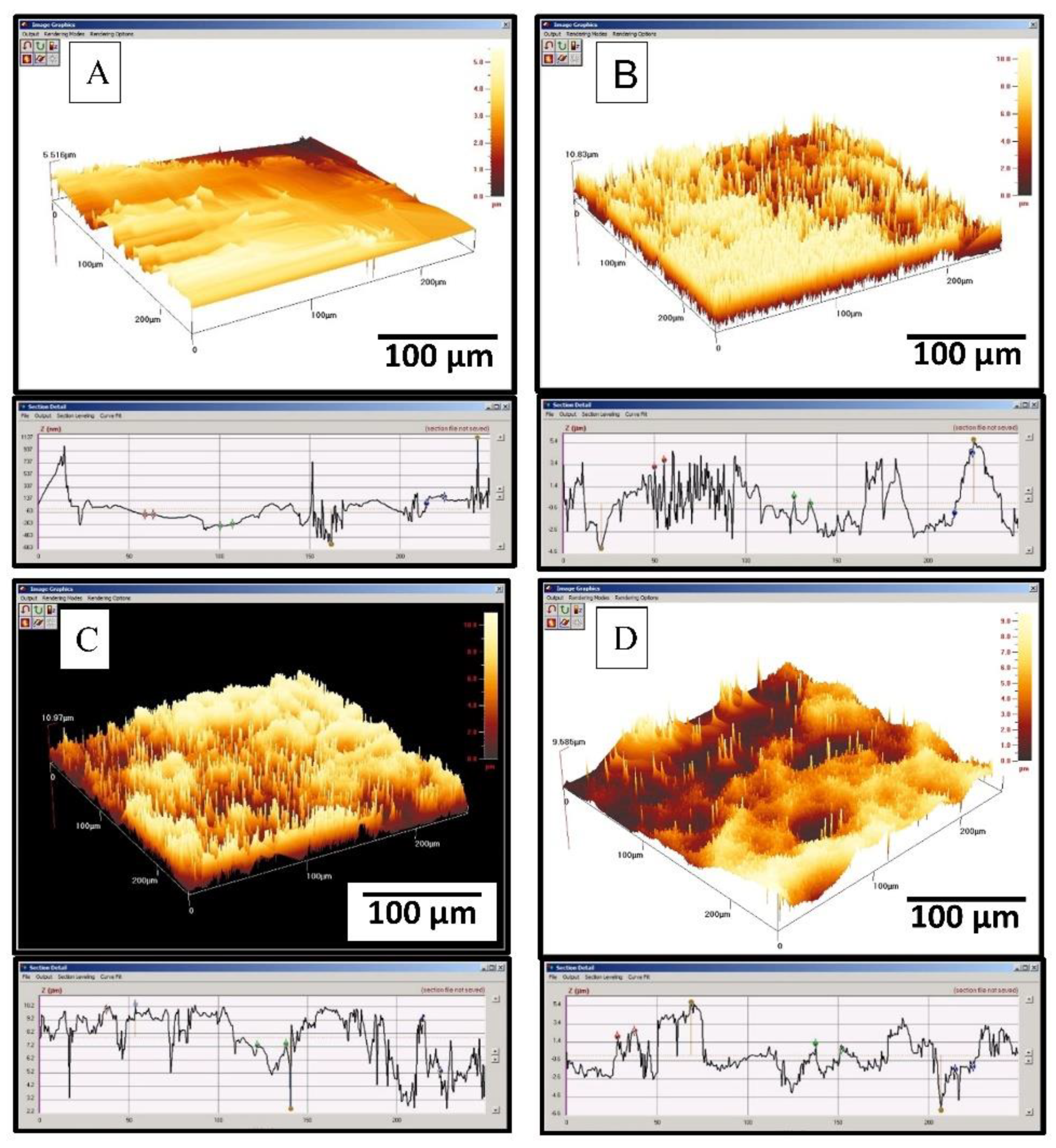
3.3. Surface Roughness and Hydrophilicity
3.4. XPS and XRD Analysis of Surfaces
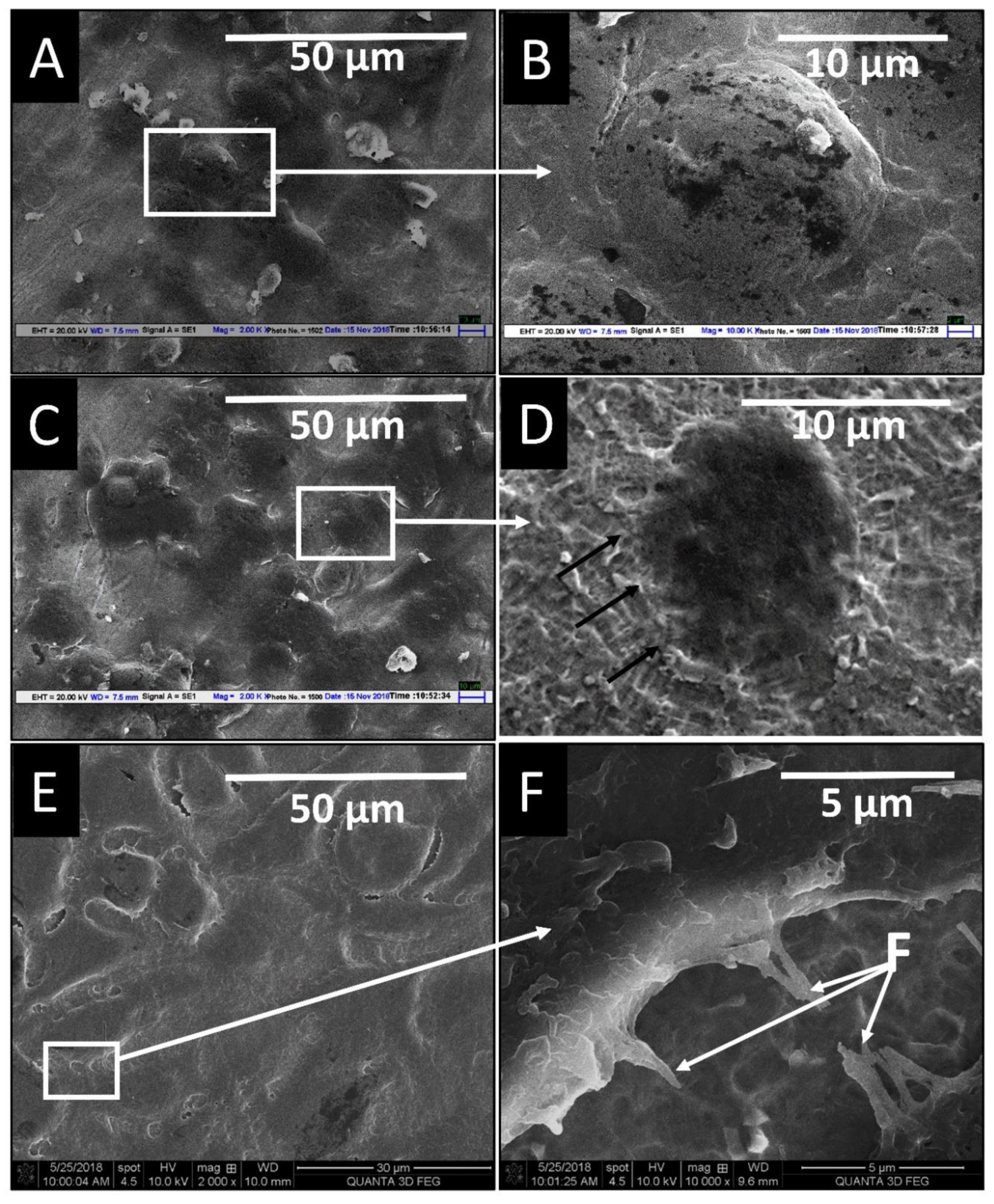
3.5. In Vitro Biocompatibility Study of Two Implant Surfaces
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Brånemark, P.I.; Adell, R.; Breine, U.; Hansson, B.O.; Lindström, J.; Ohlsson, A. Intra-osseous anchorage of dental prostheses. I. Experimental studies. Scand. J. Plast. Reconstr. Surg. 1969, 3, 81–100. [Google Scholar] [CrossRef] [PubMed]
- Siegenthaler, D.W.; Jung, R.E.; Holderegger, C.; Roos, M.; Hämmerle, C.H.F. Replacement of teeth exhibiting periapical pathology by immediate implants. A prospective, controlled clinical trial. Clin. Oral Implant. Res. 2007, 18, 727–737. [Google Scholar] [CrossRef] [PubMed]
- McCracken, M. Dental implant materials: Commercially pure titanium and titanium alloys. J. Prosthodont. 1999, 8, 40–43. [Google Scholar] [CrossRef]
- Liu, X.; Chu, P.K.; Ding, C. Surface modification of titanium, titanium alloys, and related materials for biomedical applications. Mater. Sci. Eng. R Rep. 2004, 47, 49–121. [Google Scholar] [CrossRef] [Green Version]
- El-Banna, A.; Bissa, M.W.; Khurshid, Z.; Zohaib, S.; Asiri, F.Y.I.; Zafar, M.S. Surface modification techniques of dental implants. Dent. Implant. 2020, 1, 49–68. [Google Scholar] [CrossRef]
- Ionescu, A.C.; Brambilla, E.; Azzola, F.; Ottobelli, M.; Pellegrini, G.; Francetti, L.A. Laser microtextured titanium implant surfaces reduce in vitro and in situ oral biofilm formation. PLoS ONE 2018, 13, e0202262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schupbach, P.; Glauser, R.; Bauer, S. Al2O3 Particles on Titanium Dental Implant Systems following Sandblasting and Acid-Etching Process. Int. J. Biomater. 2019, 2019, 6318429. [Google Scholar] [CrossRef] [Green Version]
- Singh, R. A comparative analysis of sandblasted and acid-etched and polished titanium surface on enhancement of osteogenic potential: An in vitro study. J. Dent. Implant. 2012, 2, 15–18. [Google Scholar] [CrossRef]
- Ban, S.; Iwaya, Y.; Kono, H.; Sato, H. Surface modification of titanium by etching in concentrated sulfuric acid. Dent. Mater. 2006, 22, 1115–1120. [Google Scholar] [CrossRef]
- Juodzbalys, G.; Sapragoniene, M.; Wennerberg, A. New Acid-etched Titanium Dental Implant Surface. Stomatol. Baltic Dent. Maxillofac J. 2003, 5, 101–105. [Google Scholar]
- Bruschi, M.; Steinmüller-nethl, D.; Goriwoda, W.; Rasse, M. Composition and Modifications of Dental Implant Surfaces. J. Oral Implant. 2015, 2015, 14. [Google Scholar] [CrossRef] [Green Version]
- Saksø, H.; Jakobsen, T.; Saksø, M.; Baas, J.; Jakobsen, S.; Soballe, K. No positive effect of Acid etching or plasma cleaning on osseointegration of titanium implants in a canine femoral condyle press-fit model. Open Orthop. J. 2013, 7, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Wennerberg, A.; Albrektsson, T. Effects of titanium surface topography on bone integration: A systematic review. Clin. Oral Implant. Res. 2009, 20, 172–184. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Huang, M.; Fisher, S.; Yue, C.Y.; Yang, J. Effects of primer and annealing treatments on the shear strength between anodized Ti6Al4V and epoxy. Int. J. Adhes. Adhes. 2015, 57, 49–56. [Google Scholar] [CrossRef]
- Lord, M.S.; Foss, M.; Besenbacher, F. Influence of nanoscale surface topography on protein adsorption and cellular response. Nano Today 2010, 5, 66–78. [Google Scholar] [CrossRef]
- Karazisis, D.; Ballo, A.M.; Petronis, S.; Agheli, H.; Emanuelsson, L.; Thomsen, P.; Omar, O. The role of well-defined nanotopography of titanium implants on osseointegration: Cellular and molecular events in vivo. Int. J. Nanomed. 2016, 11, 1367–1382. [Google Scholar] [CrossRef] [Green Version]
- Mendonça, G.; Mendonça, D.B.S.S.; Aragão, F.J.L.L.; Cooper, L.F. Advancing dental implant surface technology—From micron- to nanotopography. Biomaterials 2008, 29, 3822–3835. [Google Scholar] [CrossRef] [PubMed]
- Gittens, R.A.; Olivares-Navarrete, R.; Cheng, A.; Anderson, D.M.; McLachlan, T.; Stephan, I.; Geis-Gerstorfer, J.; Sandhage, K.H.; Fedorov, A.G.; Rupp, F.; et al. The roles of titanium surface micro/nanotopography and wettability on the differential response of human osteoblast lineage cells. Acta Biomater. 2013, 9, 6268–6277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abuhussein, H.; Pagni, G.; Rebaudi, A.; Wang, H.L. The effect of thread pattern upon implant osseointegration: Review. Clin. Oral Implant. Res. 2010, 21, 129–136. [Google Scholar] [CrossRef] [Green Version]
- Niu, W.; Wang, P.; Zhu, S.; Liu, Z.; Ji, P. Marginal bone loss around dental implants with and without microthreads in the neck: A systematic review and meta-analysis. J. Prosthet. Dent. 2017, 117, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.-I.; Lee, D.-W.; Moon, I.-S. Effect of thread size on the implant neck area: Preliminary results at 1 year of function. Clin. Oral Implant. Res. 2011, 23, 1147–1151. [Google Scholar] [CrossRef] [PubMed]
- Zahran, R.; Rosales Leal, J.I.; Rodríguez Valverde, M.A.; Cabrerizo Vílchez, M.A. Effect of hydrofluoric acid etching time on titanium topography, chemistry, wettability, and cell adhesion. PLoS ONE 2016, 11, 1–12. [Google Scholar] [CrossRef]
- Chrcanovic, B.R.; Lorrayne, N.; Leão, C.; Martins, M.D. Influence of Different Acid Etchings on the Superficial Characteristics of Ti Sandblasted with Al2O3. Mater. Res. 2013, 16, 1006–1014. [Google Scholar] [CrossRef] [Green Version]
- Chrcanovic, B.R.; Martins, M.D. Study of the influence of acid etching treatments on the superficial characteristics of Ti. Mater. Res. 2014, 17, 373–380. [Google Scholar] [CrossRef] [Green Version]
- Budei, D.V.; Vaireanu, D.I.; Prepelita, P.; Popescu-Pelin, G.; Mincu, M.; Ciobotaru, I.A. A comparative morphological study of titanium dioxide surface layer dental implants. Open Chem. 2021. [Google Scholar] [CrossRef]
- Pimenta, J.; Szmukler-Moncler, S.; Raigrodski, A. Physical characterization of 3 implant systems made of distinct materials with distinct surfaces. J. Prosthet. Dent. 2021. [Google Scholar] [CrossRef]
- Szmukler-Moncler, S.; Blus, C.; Schwarz, D.M.; Orrù, G. Characterization of a macro-and micro-textured titanium grade 5 alloy surface obtained by etching only without sandblasting. Materials 2020, 13, 5074. [Google Scholar] [CrossRef]
- Reshadi, F.; Khorasani, S.; Faraji, G. Surface characterization of nanostructured commercially pure titanium modified by sandblasting and acid-etching for implant applications. Proc. Inst. Mech. Eng. Part J J. Eng. Tribol. 2020. [Google Scholar] [CrossRef]
- Verma, M.; Bhatnagar, N.; Sood, A.; Faraz, F.; Sharma, K.; Rao, G.V.; Kumar, P.S.; Iyer, S. Dental Implant System. Available online: https://implantlogistics.com/pages/dental-implant-systems (accessed on 30 August 2021).
- Zhou, S.; Cui, Z.; Urban, J. Dead cell counts during serum cultivation are underestimated by the fluorescent live/dead assay. Biotechnol. J. 2011, 6, 513–518. [Google Scholar] [CrossRef]
- Balla, V.K.; Soderlind, J.; Bose, S.; Bandyopadhyay, A. Microstructure, mechanical and wear properties of laser surface melted Ti6Al4V alloy. J. Mech. Behav. Biomed. Mater. 2014, 32, 335–344. [Google Scholar] [CrossRef]
- Zeng, X.; Yamaguchi, T.; Nishio, K. Characteristics of Ti (C, N)/TiB composite layer on Ti-6Al-4V alloy produced by laser surface melting. Opt. Laser Technol. 2016, 80, 84–91. [Google Scholar] [CrossRef]
- Vermesse, E.; Mabru, C.; Arurault, L. Surface integrity after pickling and anodization of Ti-6Al-4V titanium alloy. Appl. Surf. Sci. 2013, 285, 629–637. [Google Scholar] [CrossRef] [Green Version]
- Chauhan, P.; Koul, V.; Bhatnagar, N. Effect of acid etching temperature on surface physiochemical properties and cytocompatibility of Ti6Al4V ELI alloy. Mater. Res. Express 2019, 6, 10. [Google Scholar] [CrossRef]
- Thrivikraman, G.; Madras, G.; Basu, B. In vitro/in vivo assessment and mechanisms of toxicity of bioceramic materials and its wear particulates. RSC Adv. 2014, 4, 12763–12781. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Wan, Y.; Wang, T.; Liu, Z. Corrosion Behavior of Titanium Implant with different Surface Morphologies. Procedia Manuf. 2017, 10, 363–370. [Google Scholar] [CrossRef]
- Qu, Q.; He, Y.; Li, L.; Yang, M.; Lai, B.; Chen, Y. Effect of Fluorine Ion on the Corrosion of Ti-6Al-4V Alloy in Artificial Saliva. Int. J. Electrochem. Sci. 2015, 10, 7453–7464. [Google Scholar]
- Das, K.; Bose, S.; Bandyopadhyay, A. Surface modifications and cell-materials interactions with anodized Ti. Acta Biomater. 2007, 3, 573–585. [Google Scholar] [CrossRef] [PubMed]
- Safioti, L.M.; Kotsakis, G.A.; Pozhitkov, A.E.; Chung, W.O.; Daubert, D.M. Increased Levels of Dissolved Titanium Are Associated with Peri-Implantitis—A Cross-Sectional Study. J. Periodontol. 2017, 88, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Iwaya, Y.; Machigashira, M.; Kanbara, K.; Miyamoto, M.; Noguchi, K.; Izumi, Y.; Ban, S. Surface properties and biocompatibility of acid-etched titanium. Dent. Mater. J. 2008, 27, 415–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taborelli, M.; Jobin, M.; François, P.; Vaudaux, P.; Tonetti, M.; Szmukler-Moncler, S.; Simpson, J.P.; Descouts, P. Influence of surface treatments developed for oral implants on the physical and biological properties of titanium. (I) Surface characterization. Clin. Oral Implant. Res. 1997, 8, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Buser, D.; Schenk, R.K.; Steinemann, S.; Fiorellini, J.P.; Fox, C.H.; Stich, H. Influence of surface characteristics on bone integration of titanium implants. A histomorphometric study in miniature pigs. J. Biomed. Mater. Res. 1991, 25, 889–902. [Google Scholar] [CrossRef]
- Vanzillotta, P.S.; Sader, M.S.; Bastos, I.N.; De Almeida Soares, G. Improvement of in vitro titanium bioactivity by three different surface treatments. Dent. Mater. 2006, 22, 275–282. [Google Scholar] [CrossRef]
- Cho, S.A.; Park, K.T. The removal torque of titanium screw inserted in rabbit tibia treated by dual acid etching. Biomaterials 2003, 24, 3611–3617. [Google Scholar] [CrossRef]
- Variola, F.; Yi, J.H.; Richert, L.; Wuest, J.D.; Rosei, F.; Nanci, A. Tailoring the surface properties of Ti6Al4V by controlled chemical oxidation. Biomaterials 2008, 29, 1285–1298. [Google Scholar] [CrossRef]
- Hung, K.Y.; Lin, Y.C.; Feng, H.P. The effects of acid etching on the nanomorphological surface characteristics and activation energy of titanium medical materials. Materials 2017, 10, 1164. [Google Scholar] [CrossRef] [PubMed]
- Szmukler-Moncler, S.; Bischof, M.; Nedir, R.; Ermrich, M. Titanium hydride and hydrogen concentration in acid-etched commercially pure titanium and titanium alloy implants: A comparative analysis of five implant systems. Clin. Oral Implant. Res. 2010, 21, 944–950. [Google Scholar] [CrossRef]
- Nagaoka, A.; Yokoyama, K.K.; Sakai, J.J. Evaluation of hydrogen absorption behaviour during acid etching for surface modification of commercial pure Ti, Ti-6Al-4V and Ni-Ti superelastic alloys. Corros. Sci. 2010, 52, 1130–1138. [Google Scholar] [CrossRef]
- Rodrigues, D.C.; Urban, R.M.; Jacobs, J.J.; Gilbert, J.L. In vivo severe corrosion and hydrogen embrittlement of retrieved modular body titanium alloy hip-implants. J. Biomed. Mater. Res.—Part B Appl. Biomater. 2009, 88, 206–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, X.; Zhou, L.; Li, S.; Lu, H.; Ding, X. Behavior of acid etching on titanium: Topography, hydrophility and hydrogen concentration. Biomed. Mater. 2014, 9, 015002–0150010. [Google Scholar] [CrossRef]
- Naauman, Z.; Rajion, Z.A.B.; Maliha, S.; Hariy, P.; Muhammad, Q.S.; Noor, H.A.R. Ultraviolet A and Ultraviolet C Light-Induced Reduction of Surface Hydrocarbons on Titanium Implants. Eur. J. Dent. 2019, 13, 114–118. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Komasa, S.; Mashimo, C.; Sekino, T.; Okazaki, J. Effect of ultraviolet treatment on bacterial attachment and osteogenic activity to alkali-treated titanium with nanonetwork structures. Int. J. Nanomed. 2017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferraris, S.; Cochis, A.; Cazzola, M.; Tortello, M.; Scalia, A.; Spriano, S.; Rimondini, L. Cytocompatible and anti-bacterial adhesion nanotextured titanium oxide layer on titanium surfaces for dental and orthopedic implants. Front. Bioeng. Biotechnol. 2019, 7, 103. [Google Scholar] [CrossRef] [PubMed]
- Rupp, F.; Scheideier, L.; Olshanska, N.; De Wild, M.; Wieland, M.; Geis-Gerstorfer, J. Enhancing surface free energy and hydrophilicity through chemical modification of microstructured titanium implant surfaces. J. Biomed. Mater. Res.—Part A 2006, 76, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Buser, D. Titanium for Dental Applications (II): Implants with Roughened Surfaces. In Titanium in Medicine. Engineering Materials; Springer: Berlin/Heidelberg, Germany, 2001; pp. 875–888. [Google Scholar]
- Schwartz, Z.; Lohmann, C.H.; Oefinger, J.; Bonewald, L.F.; Dean, D.D.; Boyan, B.D. Implant surface characteristics modulate differentiation behavior of cells in the osteoblastic lineage. Adv. Dent. Res. 1999, 13, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.S.; Richards, R.G. Surfaces to control tissue adhesion for osteosynthesis with metal implants: In vitro and in vivo studies to bring solutions to the patient. Expert Rev. Med. Devices 2010, 7, 131–142. [Google Scholar] [CrossRef] [PubMed]











| Acid Etching Group | Etching Solution | Temperature | Time | |
|---|---|---|---|---|
| Single Step | HF Only | R.T. | 30 s | |
| Double step (Room temperature-RT) | A | HF (1st etching) H2SO4 +HCl (2nd etching) | RT RT | 30 s 1 min |
| B | HF (1st etching) H2SO4 + HCl (2nd etching) | RT RT | 30 s 5 min | |
| C | HF (1st etching) H2SO4 + HCl (2nd etching) | RT RT | 30 s 10 min | |
| Double step (Different temperatures) | A | HF (1st etching) H2SO4 + HCl (2nd etching) | RT 40 °C | 30 s 5 min |
| B | HF (1st etching) H2SO4 + HCl (2nd etching) | RT 80 °C | 30 s 5 min | |
| C | HF (1st etching) H2SO4 + HCl (2nd etching) | RT 120 °C | 30 s 5 min | |
| Double step (High temperature-HT) | A | HF (1st etching) H2SO4 + HCl (2nd etching) | RT 60–80 °C | 30 s 1 min |
| B | HF (1st etching) H2SO4 + HCl (2nd etching) | RT 60–80 °C | 30 s 5 min | |
| C | HF (1st etching) H2SO4 + HCl (2nd etching) | RT 60–80 °C | 30 s 10 min | |
| Surface Type | Ra in µm | Contact Angle in Degree |
|---|---|---|
| Machined | 0.357 ± 0.132 | 110 ± 5 |
| Sandblasted | 2.1 ± 1.1 | 100 ± 2 |
| Sandblasted and acid-etched at RT (10 min) | 1.7 ± 1.2 | 70 ± 7 |
| Sandblasted and acid-etched at HT (5 min) | 1.6 ± 1.2 | 40 ± 3 |
| Elements | C | O | TI | AL | V | F |
|---|---|---|---|---|---|---|
| RT Ac-id-Etched Implant | 46.3 | 39.8 | 9.1 | 1.3 | 0.1 | 3.4 |
| HT Ac-id-Etched Implant | 30.9 | 28.6 | 39 | 0.8 | 0.1 | 0.6 |
| Room Temperature (RT) Acid-Etched Titanium Alloy Dental Implant Surface | High Temperature (HT) Acid-Etched Titanium Alloy Dental Implant Surface | |||||
|---|---|---|---|---|---|---|
| Peak Position | % Area | Oxidation State | Peaks Position | % Area | Oxidation State | |
| TI | 453.48 | 0.00 | Ti(0) 2p3/2 (Ti metal) | 453.43 | 4.18 | Ti(0) 2p3/2 (Ti metal) |
| 455.22 | 0.00 | Ti(II) 2p3/2 Ti-O | 454.99 | 1.28 | Ti(II) 2p3/2 Ti-O | |
| 457.30 | 2.05 | Ti(III) 2p3/2 Ti2O3 | 456.98 | 5.61 | Ti(III) 2p3/2 Ti2O3 | |
| 458.84 | 64.62 | Ti(IV) 2p3/2 TiO2 | 458.69 | 55.60 | Ti(IV) 2p3/2 TiO2 | |
| 459.68 | 0.00 | Ti(0) 2p1/2 (Ti metal) | 459.63 | 2.09 | Ti(0) 2p1/2 (Ti metal) | |
| 460.82 | 0.00 | Ti(II) 2p1/2 Ti-O | 460.59 | 0.64 | Ti(II) 2p1/2 Ti-O | |
| 462.90 | 1.03 | Ti(III) 2p1/2 Ti2O3 | 462.58 | 2.81 | Ti(III) 2p1/2 Ti2O3 | |
| 464.51 | 32.31 | Ti(IV) 2p1/2 TiO2 | 464.36 | 27.80 | Ti(IV) 2p1/2 TiO2 | |
| AL | 74.76 | 81.61 | Al2O3 | 71.32 | 11.89 | Metal |
| 76.34 | 18.39 | AlF3 | 74.22 | 88.11 | Al2O3 | |
| V | 515.85 | 100.00 | Oxide | 511.74 | 5.95 | Metal |
| 515.41 | 94.05 | Oxide | ||||
| C | 284.79 | 84.11 | C–C, C–H | 284.79 | 88.22 | C–C, C-H |
| 286.27 | 9.61 | C–O | 285.92 | 8.77 | C–O | |
| 288.78 | 6.28 | O–C=O | 288.75 | 3.01 | O–C=O | |
| O | 530.19 | 45.27 | Metal oxide | 530.17 | 55.68 | Metal oxide |
| 531.83 | 54.73 | Organic, sulfates | 531.69 | 44.32 | Organic, sulfates | |
| F | 684.89 | 73.77 | Fluoride | 685.01 | 100.00 | Fluoride |
| 686.38 | 26.23 | AlF3 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chauhan, P.; Koul, V.; Bhatnagar, N. Critical Role of Etching Parameters in the Evolution of Nano Micro SLA Surface on the Ti6Al4V Alloy Dental Implants. Materials 2021, 14, 6344. https://doi.org/10.3390/ma14216344
Chauhan P, Koul V, Bhatnagar N. Critical Role of Etching Parameters in the Evolution of Nano Micro SLA Surface on the Ti6Al4V Alloy Dental Implants. Materials. 2021; 14(21):6344. https://doi.org/10.3390/ma14216344
Chicago/Turabian StyleChauhan, Pankaj, Veena Koul, and Naresh Bhatnagar. 2021. "Critical Role of Etching Parameters in the Evolution of Nano Micro SLA Surface on the Ti6Al4V Alloy Dental Implants" Materials 14, no. 21: 6344. https://doi.org/10.3390/ma14216344





