Synthesis of Sb2S3 NRs@rGO Composite as High-Performance Anode Material for Sodium-Ion Batteries
Abstract
:1. Introduction
2. Materials and Methods
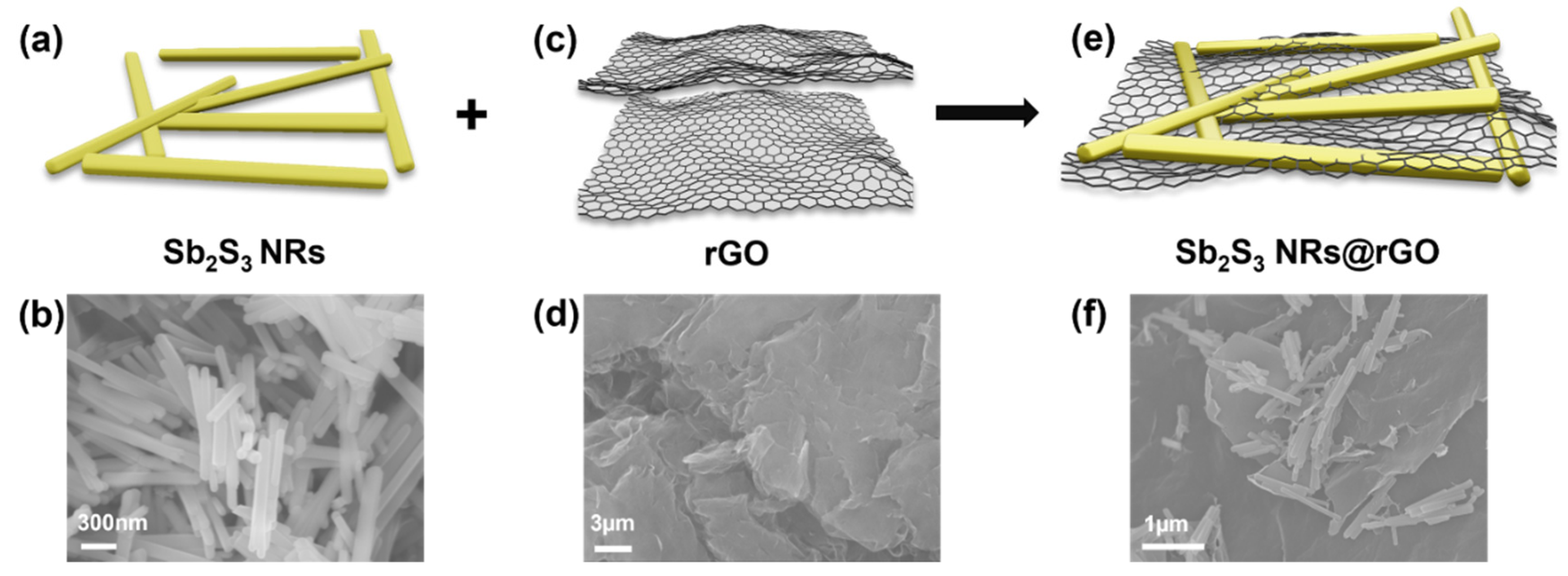
2.1. Synthesis of Sb2S3 NRs
2.2. Synthesis of Graphene Oxide (GO)
2.3. Synthesis of Sb2S3 NRs@rGO
2.4. Structural Refinement
2.5. Electrochemical Investigation
3. Results and Discussion
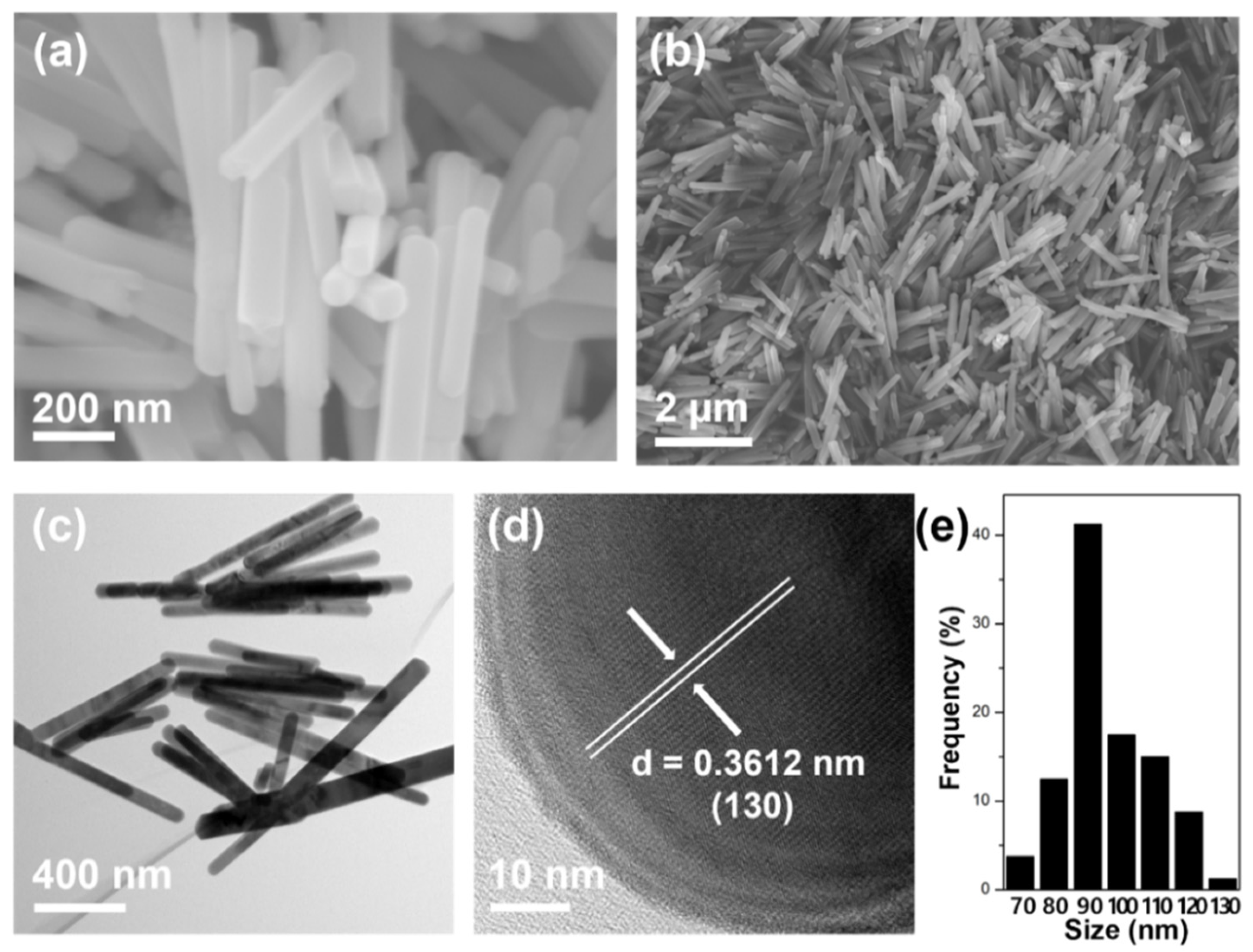
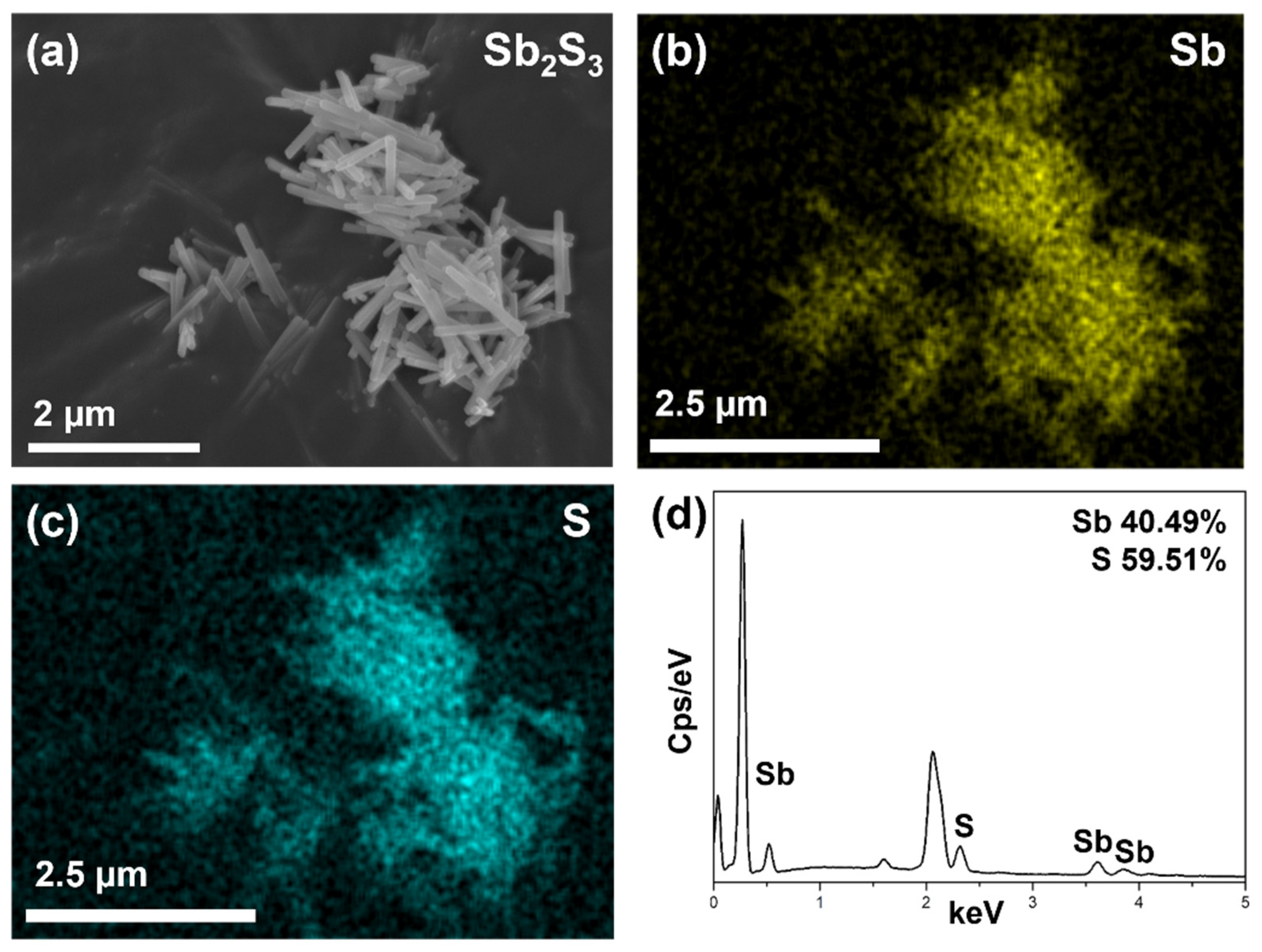
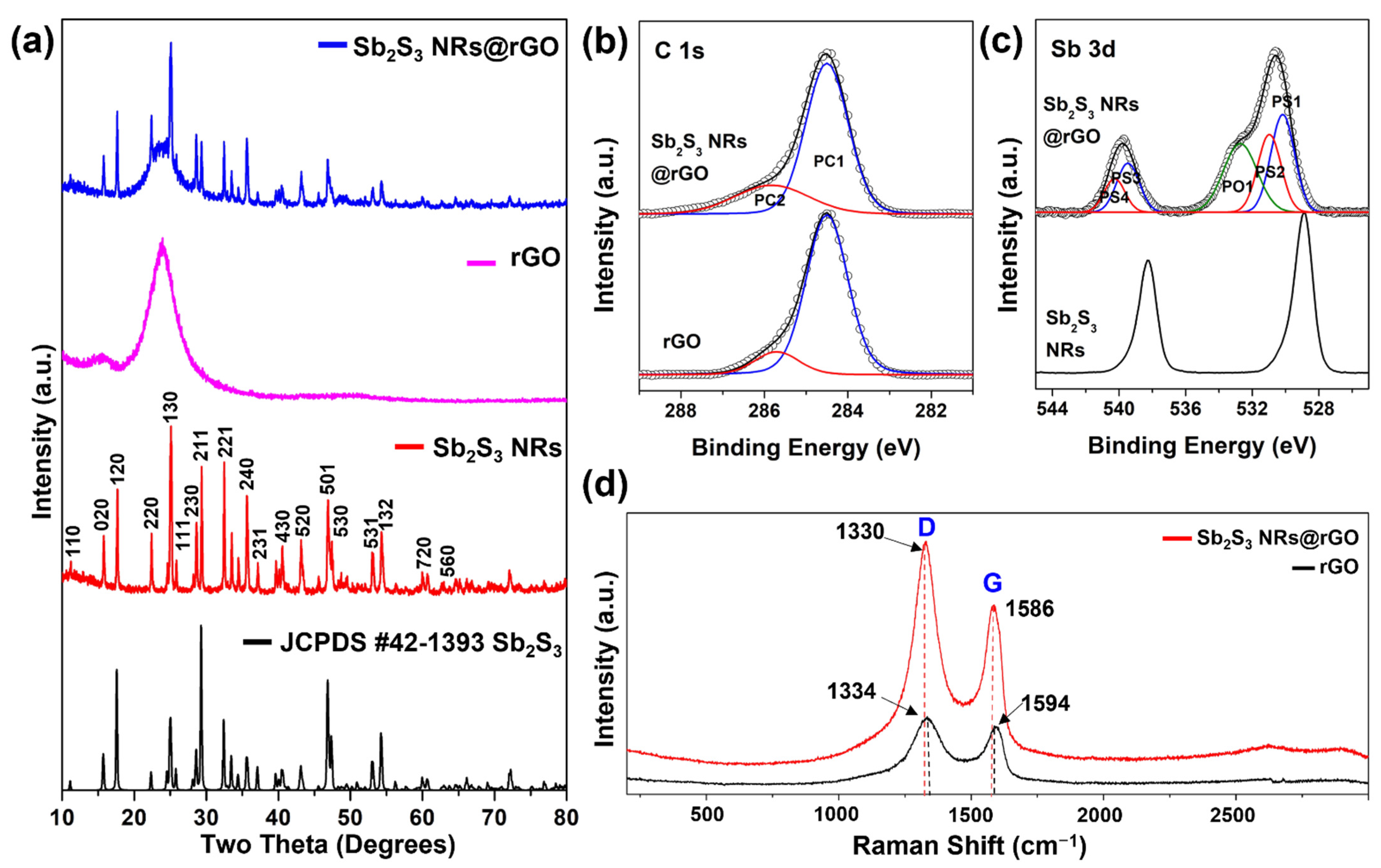
3.1. Morphology, Structure and Composition Analysis
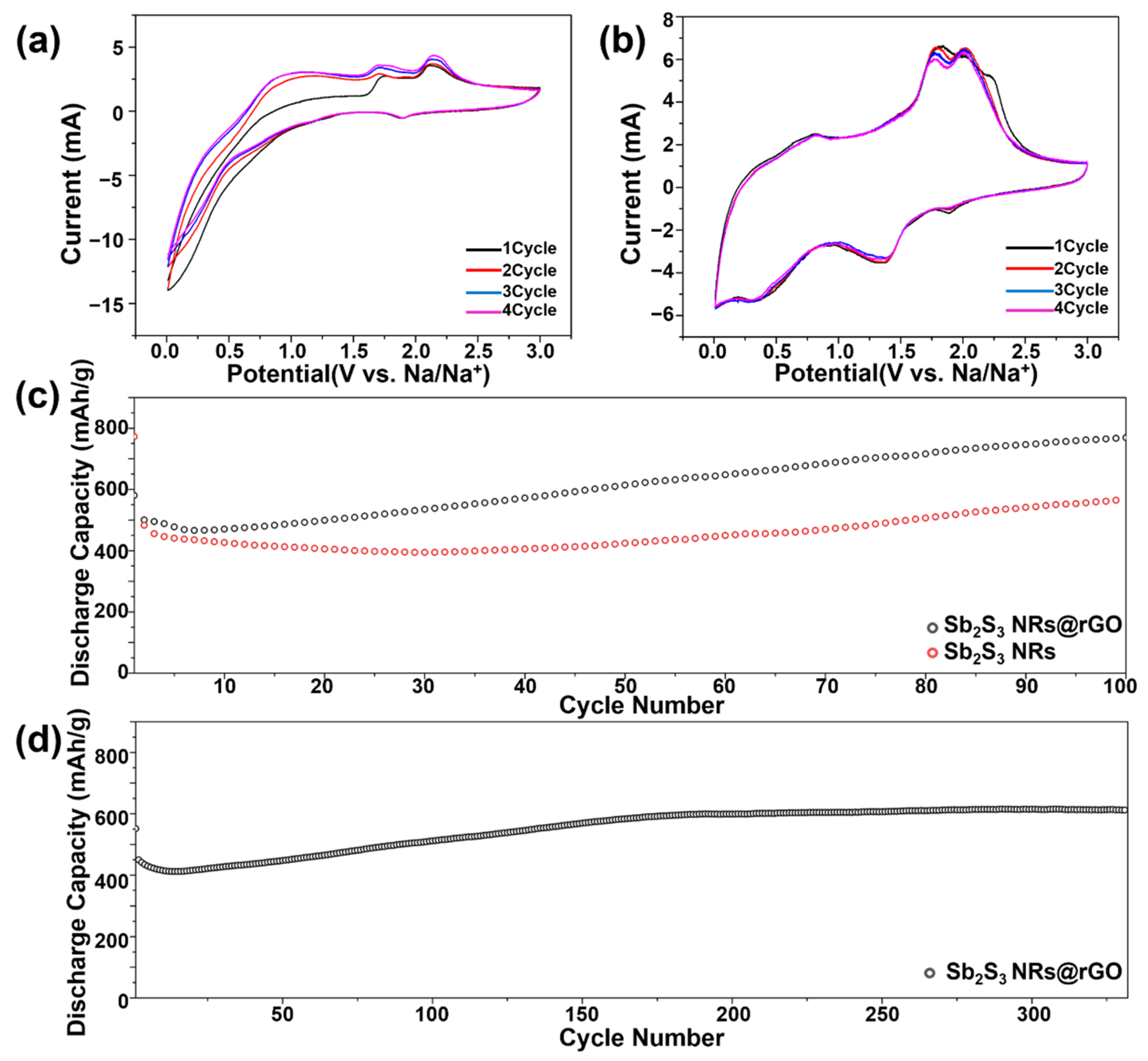
3.2. Sodium Storage Behavior
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goodenough, J.B.; Kim, Y. Challenges for Rechargeable Li Batteries. Chem. Mater. 2019, 22, 587–603. [Google Scholar] [CrossRef]
- Xin, F.; Whittingham, M.S. Challenges and Development of Tin-Based Anode with High Volumetric Capacity for Li-Ion Batteries. Electrochem. Energy Rev. 2020, 3, 643–655. [Google Scholar] [CrossRef]
- Wen, J.; Pei, Y.; Liu, L.; Su, D.; Yang, M.; Wang, Q.; Zhang, W.; Dai, J.; Feng, Y.; Wu, T.; et al. Fully encapsulated Sb2Se3/Sb/C nanofibers: Towards high-rate, ultralong-lifespan lithium-ion batteries. J. Alloys Compd. 2021, 874, 159961. [Google Scholar] [CrossRef]
- Hwang, J.Y.; Myung, S.T.; Sun, Y.K. Sodium-ion batteries: Present and future. Chem. Soc. Rev. 2017, 46, 3529–3614. [Google Scholar] [CrossRef] [Green Version]
- Dashairya, L.; Das, D.; Saha, P. Elucidating the role of graphene and porous carbon coating on nanostructured Sb2S3 for superior lithium and sodium storage. J. Alloys Compd. 2021, 883, 160906. [Google Scholar] [CrossRef]
- Zhao, Y.; Manthiram, A. Amorphous Sb2S3 embedded in graphite: A high-rate, long-life anode material for sodium-ion batteries. Chem. Commun. 2015, 51, 13205–13208. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Cui, J.; Lu, Z.; Xu, Z.-L.; Qin, L.; Huang, J.; Sadighi, Z.; Ciucci, F.; Kim, J.-K. Unveiling the Unique Phase Transformation Behavior and Sodiation Kinetics of 1D van der Waals Sb2S3Anodes for Sodium Ion Batteries. Adv. Energy Mater. 2017, 7, 1602149. [Google Scholar] [CrossRef]
- Li, J.; Yan, D.; Zhang, X.; Hou, S.; Li, D.; Lu, T.; Yao, Y.; Pan, L. In situ growth of Sb2S3 on multiwalled carbon nanotubes as high-performance anode materials for sodium-ion batteries. Electrochim. Acta 2017, 228, 436–446. [Google Scholar] [CrossRef]
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Nie, P.; Shen, L.; Dong, S.; Sheng, Q.; Li, H.; Luo, H.; Zhang, X. High rate capability and superior cycle stability of a flower-like Sb2S3 anode for high-capacity sodium ion batteries. Nanoscale 2015, 7, 3309–3315. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.S.; Jing, M.J.; Huang, Z.D.; Yang, Y.C.; Zhang, Y.; Chen, J.; Wu, Z.B.; Ji, X.B. One-Dimensional Rod-Like Sb2S3-Based Anode for High-Performance Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2015, 7, 19362–19369. [Google Scholar] [CrossRef]
- Hameed, A.S.; Reddy, M.V.; Chen, J.L.T.; Chowdari, B.V.R.; Vittal, J.J. RGO/Stibnite Nanocomposite as a Dual Anode for Lithium and Sodium Ion Batteries. ACS Sustain. Chem. Eng. 2016, 4, 2479–2486. [Google Scholar] [CrossRef]
- Chen, B.; Lu, H.; Zhou, J.; Ye, C.; Shi, C.; Zhao, N.; Qiao, S.-Z. Porous MoS2/Carbon Spheres Anchored on 3D Interconnected Multiwall Carbon Nanotube Networks for Ultrafast Na Storage. Adv. Energy Mater. 2018, 8, 1702909. [Google Scholar] [CrossRef]
- Hong, Y.R.; Mhin, S.; Kwon, J.; Han, W.S.; Song, T.; Han, H. Synthesis of transition metal sulfide and reduced graphene oxide hybrids as efficient electrocatalysts for oxygen evolution reactions. R. Soc. Open Sci. 2018, 5, 180927. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Luo, D.; Wang, X.; Seo, M.H.; Hemmati, S.; Yu, A.; Chen, Z. Enhanced Reversible Sodium-Ion Intercalation by Synergistic Coupling of Few-Layered MoS2and S-Doped Graphene. Adv. Funct. Mater. 2017, 27, 1702562. [Google Scholar] [CrossRef]
- Wang, B.; Ruan, T.; Chen, Y.; Jin, F.; Peng, L.; Zhou, Y.; Wang, D.; Dou, S. Graphene-based composites for electrochemical energy storage. Energy Storage Mater. 2020, 24, 22–51. [Google Scholar] [CrossRef]
- Deng, P.; Yang, J.; He, W.; Li, S.; Zhou, W.; Tang, D.; Qu, B. Tin-Assisted Sb2S3Nanoparticles Uniformly Grafted on Graphene Effectively Improves Sodium-Ion Storage Performance. ChemElectroChem 2018, 5, 811–816. [Google Scholar] [CrossRef]
- Jiang, Y.; Wei, M.; Feng, J.; Ma, Y.; Xiong, S. Enhancing the cycling stability of Na-ion batteries by bonding SnS2ultrafine nanocrystals on amino-functionalized graphene hybrid nanosheets. Energy Environ. Sci. 2016, 9, 1430–1438. [Google Scholar] [CrossRef]
- Youn, D.H.; Stauffer, S.K.; Xiao, P.; Park, H.; Nam, Y.; Dolocan, A.; Henkelman, G.; Heller, A.; Mullins, C.B. Simple Synthesis of Nanocrystalline Tin Sulfide/N-Doped Reduced Graphene Oxide Composites as Lithium Ion Battery Anodes. ACS Nano 2016, 10, 10778–10788. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.G.; Park, H.; Park, T.J.; Yang, M.H.; Kim, J.S.; Jang, S.Y.; Heo, N.S.; Lee, S.Y.; Kong, J.; Hong, W.H. Solution chemistry of self-assembled graphene nanohybrids for high-performance flexible biosensors. ACS Nano 2010, 4, 2910–2918. [Google Scholar] [CrossRef]
- Park, K.H.; Choi, J.; Kim, H.J.; Lee, J.B.; Son, S.U. Synthesis of antimony sulfide nanotubes with ultrathin walls via gradual aspect ratio control of nanoribbons. Chem. Mater. 2007, 19, 3861–3863. [Google Scholar] [CrossRef]
- Xiong, X.; Wang, G.; Lin, Y.; Wang, Y.; Ou, X.; Zheng, F.; Yang, C.; Wang, J.H.; Liu, M. Enhancing Sodium Ion Battery Performance by Strongly Binding Nanostructured Sb2S3 on Sulfur-Doped Graphene Sheets. ACS Nano 2016, 10, 10953–10959. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, N.; Jiang, S.; Zhang, Y.; Zhou, M.; Tao, Z.; Archer, L.A.; Chen, J. High-Capacity and Ultrafast Na-Ion Storage of a Self-Supported 3D Porous Antimony Persulfide-Graphene Foam Architecture. Nano Lett. 2017, 17, 3668–3674. [Google Scholar] [CrossRef] [PubMed]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Stankovich, S.; Piner, R.D.; Chen, X.; Wu, N.; Nguyen, S.T.; Ruoff, R.S. Stable aqueous dispersions of graphitic nanoplatelets via the reduction of exfoliated graphite oxide in the presence of poly(sodium 4-styrenesulfonate). J. Mater. Chem. 2006, 16, 155–158. [Google Scholar] [CrossRef]
- Choi, J.; Myung, Y.; Kim, S.-K. Flexible sodium-ion battery anodes using indium sulfide-based nanohybrid paper electrodes. Appl. Surf. Sci. 2019, 467–468, 1040–1045. [Google Scholar] [CrossRef]
- Peng, S.; Han, X.; Li, L.; Zhu, Z.; Cheng, F.; Srinivansan, M.; Adams, S.; Ramakrishna, S. Unique Cobalt Sulfide/Reduced Graphene Oxide Composite as an Anode for Sodium-Ion Batteries with Superior Rate Capability and Long Cycling Stability. Small 2016, 12, 1359–1368. [Google Scholar] [CrossRef] [PubMed]
- Baggetto, L.; Ganesh, P.; Sun, C.-N.; Meisner, R.A.; Zawodzinski, T.A.; Veith, G.M. Intrinsic thermodynamic and kinetic properties of Sb electrodes for Li-ion and Na-ion batteries: Experiment and theory. J. Mater. Chem. A 2013, 1, 7985–7994. [Google Scholar] [CrossRef]
- Wang, S.; Yuan, S.; Yin, Y.-B.; Zhu, Y.-H.; Zhang, X.-B.; Yan, J.-M. Green and Facile Fabrication of MWNTs@Sb2S3@PPy Coaxial Nanocables for High-Performance Na-Ion Batteries. Part. Part. Syst. Charact. 2016, 33, 493–499. [Google Scholar] [CrossRef]
- Pan, Z.-Z.; Yan, Y.; Cui, N.; Xie, J.-C.; Zhang, Y.-B.; Mu, W.-S.; Hao, C. Ionic Liquid-Assisted Preparation of Sb2S3/Reduced Graphene Oxide Nanocomposite for Sodium-Ion Batteries. Adv. Mater. Interfaces 2018, 5, 1701481. [Google Scholar] [CrossRef]
- Fan, A.; Hou, T.; Sun, X.; Xie, D.; Li, X.; Zhang, N.; Guo, J.; Jin, S.; Zhou, Y.; Cai, S.; et al. One-Pot Hydrothermal Synthesis of ZnS Nanospheres Anchored on 3D Conductive MWCNTs Networks as High-Rate and Cold-Resistant Anode Materials for Sodium-Ion Batteries. ChemElectroChem 2020, 7, 1904–1913. [Google Scholar] [CrossRef]
- Dong, Y.; Hu, M.; Zhang, Z.; Zapien, J.A.; Wang, X.; Lee, J.M. Hierarchical self-assembled Bi2S3 hollow nanotubes coated with sulfur-doped amorphous carbon as advanced anode materials for lithium ion batteries. Nanoscale 2018, 10, 13343–13350. [Google Scholar] [CrossRef]
- Guo, B.; Fang, X.; Li, B.; Shi, Y.; Ouyang, C.; Hu, Y.-S.; Wang, Z.; Stucky, G.D.; Chen, L. Synthesis and Lithium Storage Mechanism of Ultrafine MoO2 Nanorods. Chem. Mater. 2012, 24, 457–463. [Google Scholar] [CrossRef]
- Liang, H.; Ni, J.; Li, L. Bio-inspired engineering of Bi2S3-PPy yolk-shell composite for highly durable lithium and sodium storage. Nano Energy 2017, 33, 213–220. [Google Scholar] [CrossRef]
- Ni, J.; Zhao, Y.; Li, L.; Mai, L. Ultrathin MoO2 nanosheets for superior lithium storage. Nano Energy 2015, 11, 129–135. [Google Scholar] [CrossRef]
- Shi, Y.; Guo, B.; Corr, S.A.; Shi, Q.; Hu, Y.S.; Heier, K.R.; Chen, L.; Seshadri, R.; Stucky, G.D. Ordered mesoporous metallic MoO2 materials with highly reversible lithium storage capacity. Nano Lett. 2009, 9, 4215–4220. [Google Scholar] [CrossRef]
- Hu, Y.Y.; Liu, Z.; Nam, K.W.; Borkiewicz, O.J.; Cheng, J.; Hua, X.; Dunstan, M.T.; Yu, X.; Wiaderek, K.M.; Du, L.S.; et al. Origin of additional capacities in metal oxide lithium-ion battery electrodes. Nat. Mater. 2013, 12, 1130–1136. [Google Scholar] [CrossRef]
- Wen, S.; Zhao, J.; Zhao, Y.; Xu, T.; Xu, J. Reduced graphene oxide (RGO) decorated Sb2S3 nanorods as anode material for sodium-ion batteries. Chem. Phys. Lett. 2019, 716, 171–176. [Google Scholar] [CrossRef]
- Bag, S.; Roy, A.; Mitra, S. Sulfur, nitrogen dual doped reduced graphene oxide supported two-dimensional Sb2S3 nanostructures for the anode material of sodium-ion battery. Chem. Sel. 2019, 4, 6679–6686. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, H.; Seong, H.; Lee, S.Y.; Moon, J.H.; Kim, S.K.; Lee, J.B.; Myung, Y.; Na, C.W.; Choi, J. Synthesis of Sb2S3 NRs@rGO Composite as High-Performance Anode Material for Sodium-Ion Batteries. Materials 2021, 14, 7521. https://doi.org/10.3390/ma14247521
Hwang H, Seong H, Lee SY, Moon JH, Kim SK, Lee JB, Myung Y, Na CW, Choi J. Synthesis of Sb2S3 NRs@rGO Composite as High-Performance Anode Material for Sodium-Ion Batteries. Materials. 2021; 14(24):7521. https://doi.org/10.3390/ma14247521
Chicago/Turabian StyleHwang, Hosung, Honggyu Seong, So Yi Lee, Joon Ha Moon, Sung Kuk Kim, Jin Bae Lee, Yoon Myung, Chan Woong Na, and Jaewon Choi. 2021. "Synthesis of Sb2S3 NRs@rGO Composite as High-Performance Anode Material for Sodium-Ion Batteries" Materials 14, no. 24: 7521. https://doi.org/10.3390/ma14247521





