Intrinsic Point Defects in Silica for Fiber Optics Applications
Abstract
:1. Introduction
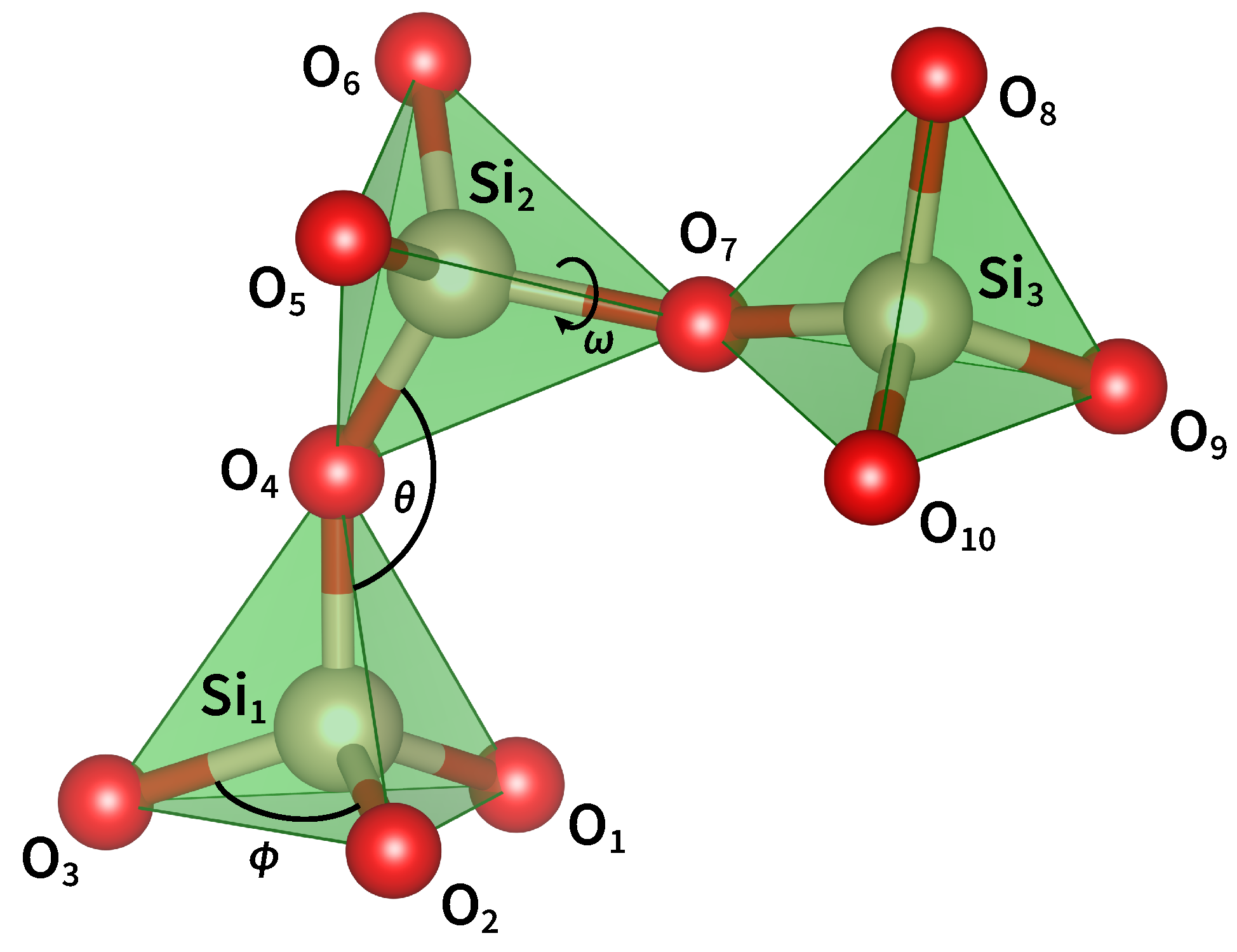
2. The Geometrical Properties of Silica
3. The Topology of Silica
4. Generation of Point Defects
4.1. E Centers
4.2. Non-Bridging Oxygen Hole Centers
- (i)
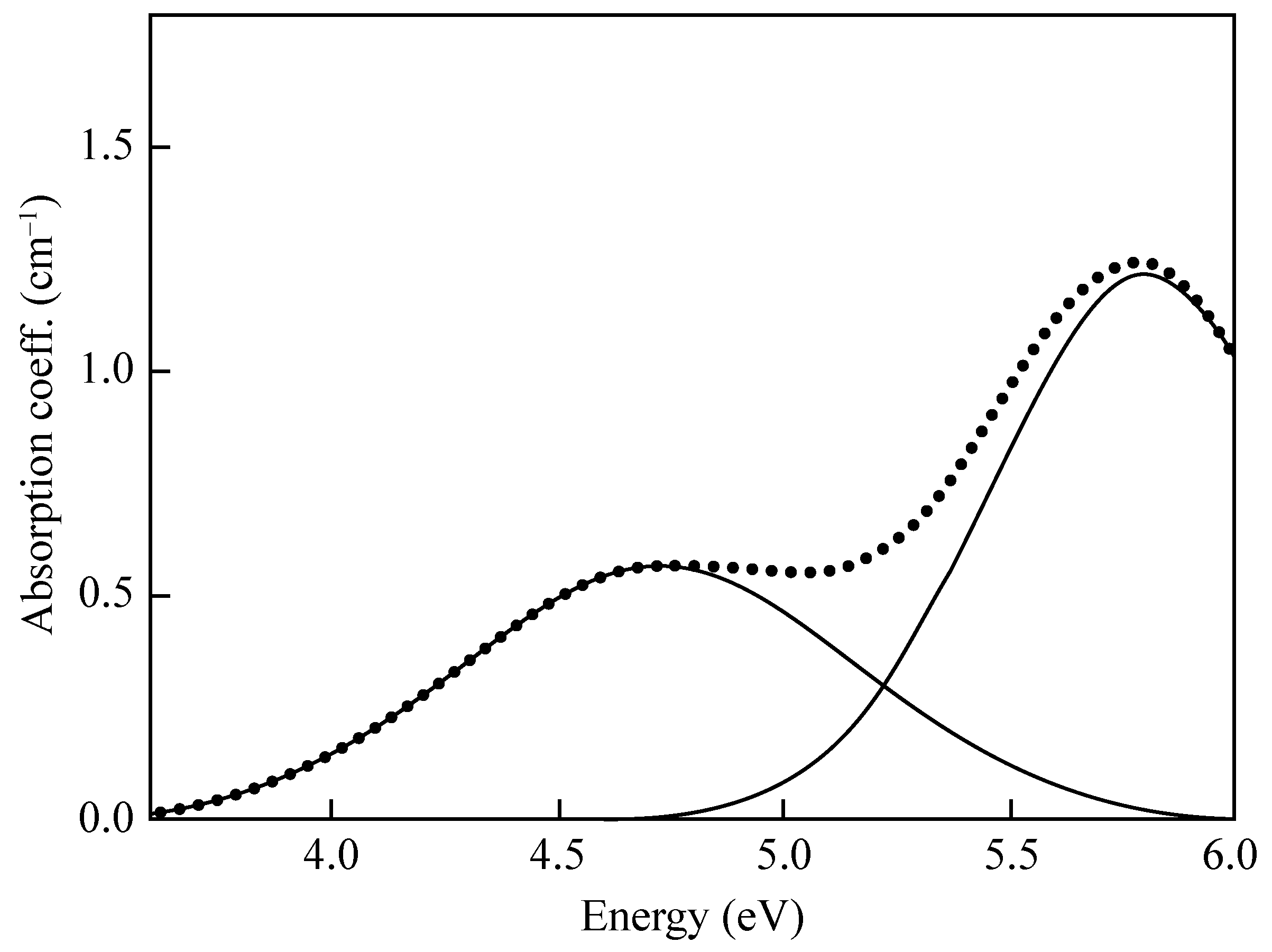
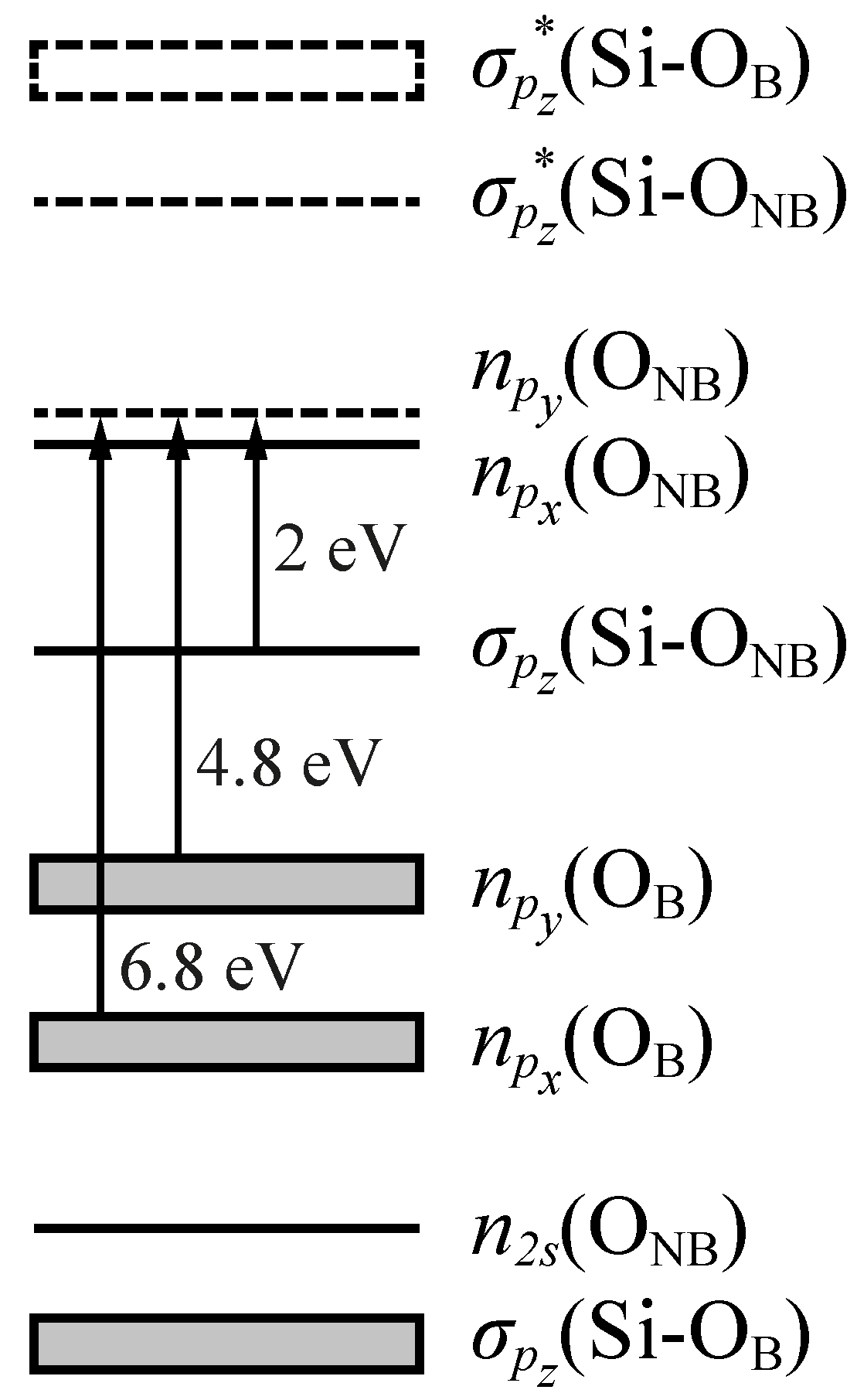
- An asymmetric Pekarian-shaped band peaked at 1.97 eV (FWHM = 0.17 eV, f ≈ ) attributed to the (Si-ONB)→npy(ONB) transition from the bonding orbital to the half-filled orbital of the non-bridging O atom (the HOMO of the cluster).
- (ii)
- A band centered at 4.8 eV (FWHM = 1.07 eV, f ≈ 0.05) originating from the npy(OB)→npy(ONB) transition between the OB lone-pair orbital perpendicular to the Si–O–Si plane and the HOMO.
- (iii)
- A band at 6.8 eV (FWHM ≈ 1.8 eV, f = 0.05) related to the npx(OB)→npy(ONB) transition from the OB lone-pair orbital lying in the Si–O–Si plane to the HOMO.
4.3. Oxygen-Deficient Centers
5. Photodarkening in Optical Fibers
6. Summary and Future Directions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Griscom, D.L. Nature of Defects And Defect Generation in Optical Glasses. In Radiation Effects on Optical Materials; Levy, P.W., Ed.; SPIE: Bellingham, WA, USA, 1985. [Google Scholar] [CrossRef]
- Griscom, D.L. Point Defects in Amorphous SiO2: What Have We Learned from 30 Years of Experimentation? MRS Proc. 1985, 61, 213–221. [Google Scholar] [CrossRef]
- Griscom, D.L. A Minireview of the Natures of Radiation-Induced Point Defects in Pure and Doped Silica Glasses and Their Visible/Near-IR Absorption Bands, with Emphasis on Self-Trapped Holes and How They Can Be Controlled. Phys. Res. Int. 2013, 2013, 379041. [Google Scholar] [CrossRef]
- Imai, H.; Hirashima, H. Intrinsic- and extrinsic-defect formation in silica glasses by radiation. J. Non Cryst. Solids 1994, 179, 202–213. [Google Scholar] [CrossRef]
- Devine, R. Macroscopic and microscopic effects of radiation in amorphous SiO2. Nucl. Instruments Methods Phys. Res. Sect. B 1994, 91, 378–390. [Google Scholar] [CrossRef]
- Weeks, R.A. The many varieties of E′ centers: A review. J. Non Cryst. Solids 1994, 179, 1–9. [Google Scholar] [CrossRef]
- Boizot, B.; Agnello, S.; Reynard, B.; Boscaino, R.; Petite, G. Raman spectroscopy study of β-irradiated silica glass. J. Non Cryst. Solids 2003, 325, 22–28. [Google Scholar] [CrossRef]
- Kajihara, K.; Hirano, M.; Skuja, L.; Hosono, H. Intrinsic defect formation in amorphous SiO2 by electronic excitation: Bond dissociation versus Frenkel mechanisms. Phys. Rev. B 2008, 78, 094201. [Google Scholar] [CrossRef]
- Alessi, A.; Agnello, S.; Sporea, D.; Oproiu, C.; Brichard, B.; Gelardi, F. Formation of optically active oxygen deficient centers in Ge-doped SiO2 by γ- and β-ray irradiation. J. Non Cryst. Solids 2010, 356, 275–280. [Google Scholar] [CrossRef]
- Beaudier, A.; Wagner, F.R.; Natoli, J.Y. Using NBOHC fluorescence to predict multi-pulse laser-induced damage in fused silica. Opt. Commun. 2017, 402, 535–539. [Google Scholar] [CrossRef]
- Ollier, N.; Girard, S.; Peuget, S. Radiation Effects in Glass. In Encyclopedia of Glass Science, Technology, History, and Culture; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2021; pp. 405–414. [Google Scholar] [CrossRef]
- Kajihara, K.; Hirano, M.; Skuja, L.; Hosono, H. Frenkel defect process in amorphous silica. In Damage to VUV, EUV, and X-ray Optics III; Juha, L., Bajt, S., London, R.A., Eds.; SPIE: Bellingham, WA, USA, 2011. [Google Scholar] [CrossRef]
- Tsai, H.S.; Chao, D.S.; Wu, Y.H.; He, Y.T.; Chueh, Y.L.; Liang, J.H. Spectroscopic investigation of gamma radiation-induced coloration in silicate glass for nuclear applications. J. Nucl. Mater. 2014, 453, 233–238. [Google Scholar] [CrossRef]
- Brichard, B.; Agnello, S.; Nuccio, L.; Dusseau, L. Comparison Between Point Defect Generation by γ-rays in Bulk and Fibre Samples of High Purity Amorphous SiO2. IEEE Trans. Nucl. Sci. 2008, 55, 2121–2125. [Google Scholar] [CrossRef]
- Girard, S.; Ouerdane, Y.; Origlio, G.; Marcandella, C.; Boukenter, A.; Richard, N.; Baggio, J.; Paillet, P.; Cannas, M.; Bisutti, J.; et al. Radiation Effects on Silica-Based Preforms and Optical Fibers—I: Experimental Study With Canonical Samples. IEEE Trans. Nucl. Sci. 2008, 55, 3473–3482. [Google Scholar] [CrossRef]
- Girard, S.; Richard, N.; Ouerdane, Y.; Origlio, G.; Boukenter, A.; Martin-Samos, L.; Paillet, P.; Meunier, J.P.; Baggio, J.; Cannas, M.; et al. Radiation Effects on Silica-Based Preforms and Optical Fibers—II: Coupling Ab initio Simulations and Experiments. IEEE Trans. Nucl. Sci. 2008, 55, 3508–3514. [Google Scholar] [CrossRef]
- Girard, S.; Kuhnhenn, J.; Gusarov, A.; Brichard, B.; Uffelen, M.V.; Ouerdane, Y.; Boukenter, A.; Marcandella, C. Radiation Effects on Silica-Based Optical Fibers: Recent Advances and Future Challenges. IEEE Trans. Nucl. Sci. 2013, 60, 2015–2036. [Google Scholar] [CrossRef]
- Khalilov, V.K.; Klein, K.F.; Amosov, A.V. Influence of fiber drawing on optical UV attenuation of all-silica fibers with undoped core. In Surgical-Assist Systems; Bogner, M.S., Charles, S.T., Grundfest, W.S., Harrington, J.A., Katzir, A., Lome, L.S., Vannier, M.W., Hanwehr, R.V., Eds.; SPIE: Bellingham, WA, USA, 1998. [Google Scholar] [CrossRef]
- Girard, S.; Alessi, A.; Richard, N.; Martin-Samos, L.; De Michele, V.; Giacomazzi, L.; Agnello, S.; Di Francesca, D.; Morana, A.; Winkler, B.; et al. Overview of radiation induced point defects in silica-based optical fibers. Rev. Phys. 2019, 4, 100032. [Google Scholar] [CrossRef]
- De Michele, V.; Marcandella, C.; Morana, A.; Campanella, C.; Vidalot, J.; Paillet, P.; Gaillardin, M.; Marin, E.; Ouerdane, Y.; Boukenter, A.; et al. Pulsed X-Ray Radiation Response of Ultra-Low Loss Pure-Silica-Core Optical Fiber. Phys. Status Solidi 2021. [Google Scholar] [CrossRef]
- Campanella, C.; De Michele, V.; Morana, A.; Mélin, G.; Robin, T.; Marin, E.; Ouerdane, Y.; Boukenter, A.; Girard, S. Radiation Effects on Pure-Silica Multimode Optical Fibers in the Visible and Near-Infrared Domains: Influence of OH Groups. Appl. Sci. 2021, 11, 2991. [Google Scholar] [CrossRef]
- De Michele, V.; Sciortino, A.; Messina, F.; Cannas, M.; Boukenter, A.; Marin, E.; Girard, S.; Ouerdane, Y. NBOHCs’ photocycle revealed in synthetic silica by transient absorption measurements. In Proceedings of the The 22nd International Conference on Ultrafast Phenomena 2020, Online. 16–19 November 2020. [Google Scholar] [CrossRef]
- Ringdalen, E. Changes in Quartz During Heating and the Possible Effects on Si Production. JOM 2014, 67, 484–492. [Google Scholar] [CrossRef] [Green Version]
- Drees, L.R.; Wilding, L.P.; Smeck, N.E.; Senkayi, A.L. Silica in Soils: Quartz and Disordered Silica Polymorphs. In Minerals in Soil Environments; Dixon, J.B., Weed, S.B., Eds.; Soil Science Society of America: Madison, WI, USA, 2018; pp. 913–974. [Google Scholar] [CrossRef]
- Raman, C.V.; Nedungadi, T.M.K. The α–β Transformation of Quartz. Nature 1940, 145, 147. [Google Scholar] [CrossRef]
- Flörke, O.W.; Graetsch, H.A.; Brunk, F.; Benda, L.; Paschen, S.; Bergna, H.E.; Roberts, W.O.; Welsh, W.A.; Libanati, C.; Ettlinger, M. Silica. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley: Hoboken, NJ, USA, 2000. [Google Scholar] [CrossRef]
- Newton, M.D.; Gibbs, G.V. Ab initio calculated geometries and charge distributions for H4SiO4 and H6Si2O7 compared with experimental values for silicates and siloxanes. Phys. Chem. Miner. 1980, 6, 221–246. [Google Scholar] [CrossRef]
- Noritake, F.; Kawamura, K. The Nature of Si-O-Si Bonding via Molecular Orbital Calculations. J. Comput. Chem. 2015, 14, 124–130. [Google Scholar] [CrossRef] [Green Version]
- Pauling, L. The nature of silicon–oxygen bonds. Am. Mineral. 1980, 65, 321–323. [Google Scholar]
- Awazu, K.; Kawazoe, H. Strained Si–O–Si bonds in amorphous SiO2 materials: A family member of active centers in radio, photo, and chemical responses. J. Appl. Phys. 2003, 94, 6243–6262. [Google Scholar] [CrossRef]
- Rino, J.P.; Ebbsjö, I.; Kalia, R.K.; Nakano, A.; Vashishta, P. Structure of rings in vitreous SiO2. Phys. Rev. B 1993, 47, 3053–3062. [Google Scholar] [CrossRef]
- Wright, A.C. Neutron scattering from vitreous silica. V. The structure of vitreous silica: What have we learned from 60 years of diffraction studies? J. Non Cryst. Solids 1994, 179, 84–115. [Google Scholar] [CrossRef]
- Charpentier, T.; Kroll, P.; Mauri, F. First-Principles Nuclear Magnetic Resonance Structural Analysis of Vitreous Silica. J. Phys. Chem. C 2009, 113, 7917–7929. [Google Scholar] [CrossRef]
- Sternberg, U. The bond angle dependence of the asymmetry parameter of the oxygen-17 electric field gradient tensor. Solid State Nucl. Magn. Reson. 1993, 2, 181–190. [Google Scholar] [CrossRef]
- Clark, T.M.; Grandinetti, P.J. Dependence of bridging oxygen 17O quadrupolar coupling parameters on Si–O distance and Si–O–Si angle. J. Phys. Condens. Matter 2003, 15, S2387–S2395. [Google Scholar] [CrossRef] [Green Version]
- Smith, J.V.; Blackwell, C.S. Nuclear magnetic resonance of silica polymorphs. Nature 1983, 303, 223–225. [Google Scholar] [CrossRef]
- Clark, T.M.; Grandinetti, P.J.; Florian, P.; Stebbins, J.F. Correlated structural distributions in silica glass. Phys. Rev. B 2004, 70, 064202. [Google Scholar] [CrossRef] [Green Version]
- Yuan, X.; Cormack, A. Si–O–Si bond angle and torsion angle distribution in vitreous silica and sodium silicate glasses. J. Non Cryst. Solids 2003, 319, 31–43. [Google Scholar] [CrossRef]
- Malfait, W.J.; Halter, W.E.; Verel, R. 29Si NMR spectroscopy of silica glass: T1 relaxation and constraints on the Si–O–Si bond angle distribution. Chem. Geol. 2008, 256, 269–277. [Google Scholar] [CrossRef]
- Wright, A.C. Diffraction studies of glass structure. J. Non Cryst. Solids 1990, 123, 129–148. [Google Scholar] [CrossRef]
- Ginhoven, R.M.V.; Jónsson, H.; Corrales, L.R. Silica glass structure generation for ab initio calculations using small samples of amorphous silica. Phys. Rev. B 2005, 71. [Google Scholar] [CrossRef] [Green Version]
- King, S.V. Ring Configurations in a Random Network Model of Vitreous Silica. Nature 1967, 213, 1112–1113. [Google Scholar] [CrossRef]
- Guttman, L. Ring structure of the crystalline and amorphous forms of silicon dioxide. J. Non Cryst. Solids 1990, 116, 145–147. [Google Scholar] [CrossRef]
- Franzblau, D.S. Computation of ring statistics for network models of solids. Phys. Rev. B 1991, 44, 4925–4930. [Google Scholar] [CrossRef] [PubMed]
- Görlich, E. The structure of SiO2 — Current views. Ceram. Int. 1982, 8, 3–16. [Google Scholar] [CrossRef]
- Papike, J.J. Chemistry of the rock-forming silicates: Multiple-chain, sheet, and framework structures. Rev. Geophys. 1988, 26, 407–444. [Google Scholar] [CrossRef] [Green Version]
- Sarnthein, J.; Pasquarello, A.; Car, R. Structural and Electronic Properties of Liquid and Amorphous SiO2: An Ab Initio Molecular Dynamics Study. Phys. Rev. Lett. 1995, 74, 4682–4685. [Google Scholar] [CrossRef]
- Pasquarello, A.; Car, R. Identification of Raman Defect Lines as Signatures of Ring Structures in Vitreous Silica. Phys. Rev. Lett. 1998, 80, 5145–5147. [Google Scholar] [CrossRef]
- Giacomazzi, L.; Umari, P.; Pasquarello, A. Medium-range structure of vitreous SiO2 obtained through first-principles investigation of vibrational spectra. Phys. Rev. B 2009, 79. [Google Scholar] [CrossRef]
- Conradt, R. Thermodynamics and Kinetics of Glass. In Springer Handbook of Glass; Springer International Publishing: Berlin/Heidelberg, Germany, 2019; pp. 51–77. [Google Scholar] [CrossRef]
- Karmakar, B. Fundamentals of Glass and Glass Nanocomposites. In Glass Nanocomposites; Elsevier: Amsterdam, The Netherlands, 2016; pp. 3–53. [Google Scholar] [CrossRef]
- Lopes, M. Introduction to Glass Science and Technology; The Royal Society of Chemistry: London, UK, 2005. [Google Scholar] [CrossRef]
- Hosono, H.; Ikuta, Y.; Kinoshita, T.; Kajihara, K.; Hirano, M. Physical Disorder and Optical Properties in the Vacuum Ultraviolet Region of amorphous SiO2. Phys. Rev. Lett. 2001, 87. [Google Scholar] [CrossRef]
- Jones, F.; Huff, N. The structure and properties of glass fibres. In Handbook of Textile Fibre Structure; Elsevier: Amsterdam, The Netherlands, 2009; pp. 307–352. [Google Scholar] [CrossRef]
- Galeener, F. Planar rings in glasses. Solid State Commun. 1982, 44, 1037–1040. [Google Scholar] [CrossRef]
- Barrio, R.A.; Galeener, F.L.; Martínez, E.; Elliott, R.J. Regular ring dynamics in AX2 tetrahedral glasses. Phys. Rev. B 1993, 48, 15672–15689. [Google Scholar] [CrossRef] [PubMed]
- Hibino, Y.; Hanawa, F.; Horiguchi, M. Drawing-induced residual stress effects on optical characteristics in pure-silica-core single-mode fibers. J. Appl. Phys. 1989, 65, 30–34. [Google Scholar] [CrossRef]
- Lancry, M.; Régnier, E.; Poumellec, B. Fictive temperature in silica-based glasses and its application to optical fiber manufacturing. Prog. Mater. Sci. 2012, 57, 63–94. [Google Scholar] [CrossRef]
- Friebele, E.J.; Sigel, G.H.; Griscom, D.L. Drawing-induced defect centers in a fused silica core fiber. Appl. Phys. Lett. 1976, 28, 516–518. [Google Scholar] [CrossRef]
- Alessi, A.; Girard, S.; Cannas, M.; Agnello, S.; Boukenter, A.; Ouerdane, Y. Influence of Drawing Conditions on the Properties and Radiation Sensitivities of Pure-Silica-Core Optical Fibers. J. Light. Technol. 2012, 30, 1726–1732. [Google Scholar] [CrossRef]
- Song, K.S.; Williams, R.T. Silicon Dioxide. In Self-Trapped Excitons; Song, K.S., Williams, R.T., Eds.; Springer: Berlin/Heidelberg, Germany, 1993. [Google Scholar] [CrossRef]
- Ginhoven, R.M.V.; Jónsson, H.; Corrales, L.R. Characterization of exciton self-trapping in amorphous silica. J. Non Cryst. Solids 2006, 352, 2589–2595. [Google Scholar] [CrossRef]
- Ismail-Beigi, S.; Louie, S.G. Self-Trapped Excitons in Silicon Dioxide: Mechanism and Properties. Phys. Rev. Lett. 2005, 95. [Google Scholar] [CrossRef]
- Shluger, A.L. The model of a triplet self-trapped exciton in crystalline SiO2. J. Phys. C Solid State Phys. 1988, 21, L431–L434. [Google Scholar] [CrossRef]
- Stapelbroek, M.; Griscom, D.; Friebele, E.; Sigel, G. Oxygen-associated trapped-hole centers in high-purity fused silicas. J. Non Cryst. Solids 1979, 32, 313–326. [Google Scholar] [CrossRef]
- Tsai, T.E.; Griscom, D.L. Experimental evidence for excitonic mechanism of defect generation in high-purity silica. Phys. Rev. Lett. 1991, 67, 2517–2520. [Google Scholar] [CrossRef]
- Hosono, H.; Kawazoe, H.; Matsunami, N. Experimental Evidence for Frenkel Defect Formation in Amorphous SiO2 by Electronic Excitation. Phys. Rev. Lett. 1998, 80, 317–320. [Google Scholar] [CrossRef]
- Itoh, C.; Suzuki, T.; Itoh, N. Luminescence and defect formation in undensified and densified amorphous SiO2. Phys. Rev. B 1990, 41, 3794–3799. [Google Scholar] [CrossRef] [PubMed]
- Devine, R.A.B. Radiation-sensitivity enhancement and annealing variation in densified, amorphous SiO2. Phys. Rev. B 1987, 35, 9783–9789. [Google Scholar] [CrossRef]
- Griscom, D.L. Trapped-electron centers in pure and doped glassy silica: A review and synthesis. J. Non Cryst. Solids 2011, 357, 1945–1962. [Google Scholar] [CrossRef]
- Agnello, S.; Boscaino, R.; Buscarino, G.; Cannas, M.; Gelardi, F.M. Structural relaxation of E centers in amorphous silica. Phys. Rev. B 2002, 66, 113201. [Google Scholar] [CrossRef] [Green Version]
- Devine, R.A.B. Defect Creation and Two-Photon Absorption in Amorphous SiO2. Phys. Rev. Lett. 1989, 62, 340. [Google Scholar] [CrossRef]
- Glinka, Y.; Lin, S.H.; Chen, Y.T. Two-photon-excited luminescence and defect formation in SiO2 nanoparticles induced by 6.4-eV ArF laser light. Phys. Rev. B 2000, 62, 4733–4743. [Google Scholar] [CrossRef] [Green Version]
- Kajihara, K.; Ikuta, Y.; Hirano, M.; Hosono, H. Power dependence of defect formation in SiO2 glass by F2 laser irradiation. Appl. Phys. Lett. 2002, 81, 3164–3166. [Google Scholar] [CrossRef]
- Imai, H.; Arai, K.; Imagawa, H.; Hosono, H.; Abe, Y. Two types of oxygen-deficient centers in synthetic silica glass. Phys. Rev. B 1988, 38, 12772–12775. [Google Scholar] [CrossRef] [PubMed]
- Kajihara, K.; Ikuta, Y.; Oto, M.; Hirano, M.; Skuja, L.; Hosono, H. UV–VUV laser induced phenomena in SiO2 glass. Nucl. Instruments Methods Phys. Res. Sect. B 2004, 218, 323–331. [Google Scholar] [CrossRef]
- Griscom, D.L. Characterization of three E′-center variants in X- and γ-irradiated high purity a-SiO2. Nucl. Instruments Methods Phys. Res. Sect. 1984, 1, 481–488. [Google Scholar] [CrossRef]
- Griscom, D.L. Optical Properties and Structure of Defects in Silica Glass. J. Ceram. Soc. 1991, 99, 923–942. [Google Scholar] [CrossRef]
- Kajihara, K.; Skuja, L.; Hirano, M.; Hosono, H. In situobservation of the formation, diffusion, and reactions of hydrogenous species in F2-laser-irradiated SiO2 glass using a pump-and-probe technique. Phys. Rev. B 2006, 74. [Google Scholar] [CrossRef]
- Skuja, L. Optical properties of defects in silica. In Defects in SiO2 and Related Dielectrics: Science and Technology; Pacchioni, G., Skuja, L., Griscom, D.L., Eds.; Springer: Berlin/Heidelberg, Germany, 2000; pp. 73–116. [Google Scholar] [CrossRef]
- Buscarino, G.; Boscaino, R.; Agnello, S.; Gelardi, F.M. Optical absorption and electron paramagnetic resonance of the E center in amorphous silicon dioxide. Phys. Rev. B 2008, 77, 155214. [Google Scholar] [CrossRef] [Green Version]
- Agnello, S.; Buscarino, G.; Gelardi, F.M.; Boscaino, R. Optical absorption band at 5.8 eV associated with the E centers in amorphous silicon dioxide: Optical absorption and EPR measurements. Phys. Rev. B 2008, 77, 195206. [Google Scholar] [CrossRef]
- Giordano, L.; Sushko, P.V.; Pacchioni, G.; Shluger, A.L. Optical and EPR properties of point defects at a crystalline silica surface: Ab initio embedded-cluster calculations. Phys. Rev. B 2007, 75, 024109. [Google Scholar] [CrossRef]
- Cannas, M.; Leone, M. Photoluminescence at 1.9 eV in synthetic wet silica. J. Non Cryst. Solids 2001, 280, 183–187. [Google Scholar] [CrossRef]
- Nishikawa, H.; Nakamura, R.; Ohki, Y.; Hama, Y. Correlation of preexisting diamagnetic defect centers with induced paramagnetic defect centers by ultraviolet or vacuum-ultraviolet photons in high-purity silica glasses. Phys. Rev. B 1993, 48, 15584–15594. [Google Scholar] [CrossRef]
- Suzuki, T.; Skuja, L.; Kajihara, K.; Hirano, M.; Kamiya, T.; Hosono, H. Electronic Structure of Oxygen Dangling Bond in Glassy SiO2: The Role of Hyperconjugation. Phys. Rev. Lett. 2003, 90, 186404. [Google Scholar] [CrossRef]
- Skuja, L.; Kajihara, K.; Hirano, M.; Hosono, H. Visible to vacuum-UV range optical absorption of oxygen dangling bonds in amorphous SiO2. Phys. Rev. B 2011, 84, 205206. [Google Scholar] [CrossRef]
- Hosono, H.; Kajihara, K.; Suzuki, T.; Ikuta, Y.; Skuja, L.; Hirano, M. Vacuum ultraviolet optical absorption band of non-bridging oxygen hole centers in SiO2 glass. Solid State Commun. 2002, 122, 117–120. [Google Scholar] [CrossRef]
- Sakurai, Y. Low temperature dependence of photoluminescence band near 2.0 eV in silica glass. J. Appl. Phys. 2000, 87, 755–759. [Google Scholar] [CrossRef]
- Skuja, L. The origin of the intrinsic 1.9 eV luminescence band in glassy SiO2. J. Non Cryst. Solids 1994, 179, 51–69. [Google Scholar] [CrossRef]
- Vaccaro, L.; Cannas, M.; Boscaino, R. Phonon coupling of non-bridging oxygen hole center with the silica environment: Temperature dependence of the 1.9 eV emission spectra. J. Lumin. 2008, 128, 1132–1136. [Google Scholar] [CrossRef]
- Skuja, L.; Suzuki, T.; Tanimura, K. Site-selective laser-spectroscopy studies of the intrinsic 1.9-eV luminescence center in glassy SiO2. Phys. Rev. B 1995, 52, 15208–15216. [Google Scholar] [CrossRef]
- Amossov, A.; Rybaltovsky, A. Oxygen-deficient centers in silica glasses: A review of their properties and structure. J. Non Cryst. Solids 1994, 179, 75–83. [Google Scholar] [CrossRef]
- Garino-Canina, V. La luminescence de la silice vitreuse en fonction de la temperature. Comptes Rendus de L’academie des Sci. 1954, 238, 1577. [Google Scholar]
- Mitchell, E.W.J.; Paige, E.G.S. CXI. The optical effects of radiation induced atomic damage in quartz. Philos. Mag. 1956, 1, 1085–1115. [Google Scholar] [CrossRef]
- Cohen, A.J. Neutron Specific Color Center in Fused Silica and an Impurity Band of Identical Wavelength. Phys. Rev. 1957, 105, 1151–1155. [Google Scholar] [CrossRef]
- O’Reilly, E.P.; Robertson, J. Theory of defects in vitreous silicon dioxide. Phys. Rev. B 1983, 27, 3780–3795. [Google Scholar] [CrossRef]
- Hosono, H.; Abe, Y.; Imagawa, H.; Imai, H.; Arai, K. Experimental evidence for the Si–Si bond model of the 7.6-eV band in SiO2 glass. Phys. Rev. B 1991, 44, 12043–12045. [Google Scholar] [CrossRef]
- Imagawa, H.; Arai, T.; Hosono, H.; Imai, H.; Arai, K. Reaction kinetics of oxygen-deficient centers with diffusing oxygen molecules in silica glass. J. Non Cryst. Solids 1994, 179, 70–74. [Google Scholar] [CrossRef]
- Imai, H.; Arai, K.; Hosono, H.; Abe, Y.; Arai, T.; Imagawa, H. Dependence of defects induced by excimer laser on intrinsic structural defects in synthetic silica glasses. Phys. Rev. B 1991, 44, 4812–4818. [Google Scholar] [CrossRef] [PubMed]
- Skuja, L.; Streletsky, A.; Pakovich, A. A new intrinsic defect in amorphous SiO2: Twofold coordinated silicon. Solid State Commun. 1984, 50, 1069–1072. [Google Scholar] [CrossRef]
- Tsai, T.E.; Griscom, D.L. On the structures of hydrogen-associated defect centers in irradiated high-purity a-SiO2:OH. J. Non Cryst. Solids 1987, 91, 170–179. [Google Scholar] [CrossRef]
- Radtsig, V. Paramagnetic centers on fresh surfaces of quartz. Interactions with molecules of H2 and D2. Kinet. Katal. 1979, 20, 456–464. [Google Scholar]
- Amosov, A.; Malyshkin, S. Role of oxygen vacancy type defects in formation of radiation-induced color centers in quartz glasses. Fizika i Khimiya Stekla 1984, 10, 305–309. [Google Scholar]
- Nishikawa, H.; Nakamura, R.; Ohki, Y.; Hama, Y. Enhanced photogeneration of E′ centers from neutral oxygen vacancies in the presence of hydrogen in high-purity silica glass. Phys. Rev. B 1993, 48, 2968–2973. [Google Scholar] [CrossRef]
- Radzig, V.A.; Bagratashvili, V.N.; Tsypina, S.I.; Chernov, P.V.; Rybaltovskii, A.O. Photoinduced Reactions of Oxygen-Deficient Centers with Molecular Hydrogen in Silica Glasses. J. Phys. Chem. 1995, 99, 6640–6647. [Google Scholar] [CrossRef]
- Edwards, A.H.; Germann, G. Interaction of hydrogenated molecules with intrinsic defects in a-SiO2. Nucl. Instruments Methods Phys. Res. Sect. B 1988, 32, 238–247. [Google Scholar] [CrossRef]
- Tohmon, R.; Mizuno, H.; Ohki, Y.; Sasagane, K.; Nagasawa, K.; Hama, Y. Correlation of the 5.0- and 7.6-eV absorption bands in SiO2 with oxygen vacancy. Phys. Rev. B 1989, 39, 1337–1345. [Google Scholar] [CrossRef]
- Kohketsu, M.; Awazu, K.; Kawazoe, H.; Yamane, M. Photoluminescence Centers in VAD SiO2 Glasses Sintered under Reducing or Oxidizing Atmospheres. Jpn. J. Appl. Phys. 1989, 28, 615–621. [Google Scholar] [CrossRef]
- Skuja, L.N.; Silin, A.R.; Mareš, J. Decay time and polarization properties of luminescence centers in vitreous silica. Phys. Status Solidi 1978, 50, K149–K152. [Google Scholar] [CrossRef]
- Stathis, J.H.; Kastner, M.A. Time-resolved photoluminescence in amorphous silicon dioxide. Phys. Rev. B 1987, 35, 2972–2979. [Google Scholar] [CrossRef] [PubMed]
- Skuja, L. Isoelectronic series of twofold coordinated Si, Ge, and Sn atoms in glassy SiO2: A luminescence study. J. Non Cryst. Solids 1992, 149, 77–95. [Google Scholar] [CrossRef]
- Anedda, A.; Congiu, F.; Raga, F.; Corazza, A.; Martini, M.; Spinolo, G.; Vedda, A. Time resolved photoluminescence of a centers in neutron irradiated SiO2. Nucl. Instruments Methods Phys. Res. Sect. B 1994, 91, 405–409. [Google Scholar] [CrossRef]
- Skuja, L. Direct singlet-to-triplet optical absorption and luminescence excitation band of the twofold-coordinated silicon center in oxygen-deficient glassy SiO2. J. Non Cryst. Solids 1994, 167, 229–238. [Google Scholar] [CrossRef]
- Skuja, L.N.; Trukhin, A.N.; Plaudis, A.E. Luminescence in Germanium-Doped Glassy SiO2. Phys. Status Solidi 1984, 84, K153–K157. [Google Scholar] [CrossRef]
- Zhang, B.L.; Raghavachari, K. Photoabsorption and photoluminescence of divalent defects in silicate and germanosilicate glasses: First-principles calculations. Phys. Rev. B 1997, 55, R15993–R15996. [Google Scholar] [CrossRef]
- Pacchioni, G.; Ferrario, R. Optical transitions and EPR properties of two-coordinated Si, Ge, Sn and related H(I), H(II), and H(III) centers in pure and doped silica from ab initio calculations. Phys. Rev. B 1998, 58, 6090–6096. [Google Scholar] [CrossRef]
- Pacchioni, G. Ab initio theory of point defects in SiO2. In Defects in SiO2 and Related Dielectrics: Science and Technology; Pacchioni, G., Skuja, L., Griscom, D.L., Eds.; Springer: Berlin/Heidelberg, Germany, 2000; pp. 161–195. [Google Scholar] [CrossRef]
- Sokolov, V.O.; Sulimov, V.B. Theory of Twofold Coordinated Silicon and Germanium Atoms in Solid Silicon Dioxide. Phys. Status Solidi 1994, 186, 185–198. [Google Scholar] [CrossRef]
- Agnello, S.; Boscaino, R.; Cannas, M.; Gelardi, F.M.; Leone, M. γ-ray-induced bleaching in silica: Conversion from optical to paramagnetic defects. Phys. Rev. B 2000, 61, 1946–1951. [Google Scholar] [CrossRef]
- Gelardi, F.; Agnello, S. Gamma rays induced conversion of native defects in natural silica. In Defects in SiO2 and Related Dielectrics: Science and Technology; Pacchioni, G., Skuja, L., Griscom, D.L., Eds.; Springer: Berlin/Heidelberg, Germany, 2000; pp. 285–305. [Google Scholar] [CrossRef]
- Agnello, S.; Boscaino, R.; Mattina, F.L.; Grandi, S.; Magistris, A. Hydrogen-Related Paramagnetic Centers in Ge-Doped Sol-Gel Silica Induced by γ-Ray Irradiation. J. Sol-Gel Sci. Technol. 2006, 37, 63–68. [Google Scholar] [CrossRef]
- Morana, A.; Girard, S.; Cannas, M.; Marin, E.; Marcandella, C.; Paillet, P.; Périsse, J.; Macé, J.R.; Boscaino, R.; Nacir, B.; et al. Influence of neutron and gamma-ray irradiations on rad-hard optical fiber. Opt. Mater. Express 2015, 5, 898–911. [Google Scholar] [CrossRef]
- Girard, S.; Marcandella, C.; Origlio, G.; Ouerdane, Y.; Boukenter, A.; Meunier, J.P. Radiation-induced defects in fluorine-doped silica-based optical fibers: Influence of a pre-loading with H2. J. Non Cryst. Solids 2009, 355, 1089–1091. [Google Scholar] [CrossRef]
- Lo Piccolo, G.M.; Morana, A.; Alessi, A.; Boukenter, A.; Girard, S.; Ouerdane, Y.; Gelardi, F.M.; Agnello, S.; Cannas, M. Ultraviolet–visible light-induced solarisation in silica-based optical fibres for indoor solar applications. J. Non Cryst. Solids 2021, 552, 120458. [Google Scholar] [CrossRef]
- Agrawal, G. Fiber-Optic Communication Systems; Wiley: Hoboken, NJ, USA, 2021. [Google Scholar]
- Hartog, A.H. An Introduction to Distributed Optical Fibre Sensors; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar] [CrossRef]
- Bao, X.; Chen, L. Recent Progress in Distributed Fiber Optic Sensors. Sensors 2012, 12, 8601–8639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, P.; Lalam, N.; Badar, M.; Liu, B.; Chorpening, B.T.; Buric, M.P.; Ohodnicki, P.R. Distributed optical fiber sensing: Review and perspective. Appl. Phys. Rev. 2019, 6, 041302. [Google Scholar] [CrossRef]
- Gilliam, W.; Fleming, B.T.; Vorobiev, D.; Winter, B.; Wadsworth, W.; Birks, T. Far-ultraviolet optical fibers for instrumentation in the sub-200 nm regime. In UV/Optical/IR Space Telescopes and Instruments: Innovative Technologies and Concepts X; Breckinridge, J.B., Stahl, H.P., Barto, A.A., Eds.; SPIE: Bellingham, WA, USA, 2021. [Google Scholar] [CrossRef]
- Waldo, W. Techniques and Tools for Optical Lithography. In Handbook of VLSI Microlithography; Elsevier: Amsterdam, The Netherlands, 2001; pp. 472–643. [Google Scholar] [CrossRef]
- Makarov, N.S.; Ramasamy, K.; Jackson, A.; Velarde, A.; Castaneda, C.; Archuleta, N.; Hebert, D.; Bergren, M.R.; McDaniel, H. Fiber-Coupled Luminescent Concentrators for Medical Diagnostics, Agriculture, and Telecommunications. ACS Nano 2019, 13, 9112–9121. [Google Scholar] [CrossRef]
- Wang, J.; Dong, J. Optical Waveguides and Integrated Optical Devices for Medical Diagnosis, Health Monitoring and Light Therapies. Sensors 2020, 20, 3981. [Google Scholar] [CrossRef] [PubMed]
- Artyushenko, V.G. Fiber solutions from 355 nm to 16 μm in advanced diagnostics and laser surgery (Conference Presentation). In Optical Fibers and Sensors for Medical Diagnostics and Treatment Applications XX; Gannot, I., Ed.; SPIE: Bellingham, WA, USA, 2020. [Google Scholar] [CrossRef]
- Ullah, I.; Whang, A. Development of Optical Fiber-Based Daylighting System and Its Comparison. Energies 2015, 8, 7185–7201. [Google Scholar] [CrossRef] [Green Version]
- Antón, I.; Silva, D.; Sala, G.; Bett, A.; Siefer, G.; Luque-Heredia, I.; Trebst, T. The PV-FIBRE concentrator: A system for indoor operation of 1000x MJ solar cells. Prog. Photovolt. Res. Appl. 2007, 15, 431–447. [Google Scholar] [CrossRef]
- Núñez, R.; Antón, I.; Sala, G. Hybrid lighting-CPV, a new efficient concept mixing illumination with CPV. Opt. Express 2013, 21, 4864–4874. [Google Scholar] [CrossRef]
- Núñez, R.; Antón, I.; Sala, G. Proof-of-concept of a building-integrated hybrid concentrator photovoltaics-lighting system. Light. Res. Technol. 2017, 50, 1082–1090. [Google Scholar] [CrossRef]
- Aslian, A.; Asli, B.H.S.; Tan, C.J.; Adikan, F.R.M.; Toloei, A. Design and Analysis of an Optical Coupler for Concentrated Solar Light Using Optical Fibers in Residential Buildings. Int. J. Photoenergy 2016, 2016, 3176052. [Google Scholar] [CrossRef]
- Vu, N.H.; Shin, S. Flat Optical Fiber Daylighting System with Lateral Displacement Sun-Tracking Mechanism for Indoor Lighting. Energies 2017, 10, 1679. [Google Scholar] [CrossRef] [Green Version]
- Vu, N.H.; Pham, T.T.; Shin, S. Modified optical fiber daylighting system with sunlight transportation in free space. Opt. Express 2016, 24, A1528–A1545. [Google Scholar] [CrossRef] [PubMed]
- Henschel, H.; Kohn, O.; Weinand, U. Radiation hardening of pure silica optical fibres by high pressure hydrogen treatment. In Proceedings of the 2001 6th European Conference on Radiation and Its Effects on Components and Systems (Cat. No.01TH8605), Grenoble, France, 10–14 September 2001. [Google Scholar] [CrossRef]
- Karlitschek, P.; Hillrichs, G.; Klein, K.F. Influence of hydrogen on the colour center formation in optical fibers induced by pulsed UV-laser radiation. Part 1: All silica fibers with high-OH undoped core. Opt. Commun. 1998, 155, 376–385. [Google Scholar] [CrossRef]
- Karlitschek, P.; Hillrichs, G.; Klein, K.F. Influence of hydrogen on the colour center formation in optical fibers induced by pulsed UV-laser radiation. Opt. Commun. 1998, 155, 386–397. [Google Scholar] [CrossRef]
- Oto, M.; Kikugawa, S.; Sarukura, N.; Hirano, M.; Hosono, H. Optical fiber for deep ultraviolet light. IEEE Photonics Technol. Lett. 2001, 13, 978–980. [Google Scholar] [CrossRef]
- Brichard, B.; Fernandez, A.; Ooms, H.; Borgermans, P.; Berghmans, F. Dependence of the POR and NBOHC defects as function of the dose in hydrogen-treated and untreated KU1 glass fibers. IEEE Trans. Nucl. Sci. 2003, 50, 2024–2029. [Google Scholar] [CrossRef]
- Girard, S.; Vivona, M.; Laurent, A.; Cadier, B.; Marcandella, C.; Robin, T.; Pinsard, E.; Boukenter, A.; Ouerdane, Y. Radiation hardening techniques for Er/Yb doped optical fibers and amplifiers for space application. Opt. Express 2012, 20, 8457–8465. [Google Scholar] [CrossRef] [PubMed]
- Zotov, K.V.; Likhachev, M.E.; Tomashuk, A.L.; Bubnov, M.M.; Yashkov, M.V.; Gur’yanov, A.N. Radiation-resistant erbium-doped silica fibre. Quantum Electron. 2007, 37, 946–949. [Google Scholar] [CrossRef]
- Zotov, K.; Likhachev, M.; Tomashuk, A.; Bubnov, M.; Yashkov, M.; Guryanov, A.; Klyamkin, S. Radiation-resistant erbium-doped fiber for spacecraft applications. In Proceedings of the 2007 9th European Conference on Radiation and Its Effects on Components and Systems, Deauville, France, 10–14 September 2007. [Google Scholar] [CrossRef]
- Lemaire, P.J.; Lindholm, E.A. Hermetic Optical Fibers. In Specialty Optical Fibers Handbook; Elsevier: Amsterdam, The Netherlands, 2007; pp. 453–490. [Google Scholar] [CrossRef]
- Bogatyrev, V.A.; Semjonov, S. Metal-Coated Fibers. In Specialty Optical Fibers Handbook; Elsevier: Amsterdam, The Netherlands, 2007; pp. 491–512. [Google Scholar] [CrossRef]
- Hartung, A.; Kobelke, J.; Schwuchow, A.; Wondraczek, K.; Bierlich, J.; Popp, J.; Frosch, T.; Schmidt, M.A. Double antiresonant hollow core fiber–guidance in the deep ultraviolet by modified tunneling leaky modes. Opt. Express 2014, 22, 19131–19140. [Google Scholar] [CrossRef] [PubMed]
- White, T.P.; McPhedran, R.C.; de Sterke, C.M.; Litchinitser, N.M.; Eggleton, B.J. Resonance and scattering in microstructured optical fibers. Opt. Lett. 2002, 27, 1977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Litchinitser, N.M.; Abeeluck, A.K.; Headley, C.; Eggleton, B.J. Antiresonant reflecting photonic crystal optical waveguides. Opt. Lett. 2002, 27, 1592–1594. [Google Scholar] [CrossRef] [PubMed]
- Debord, B.; Amrani, F.; Vincetti, L.; Gérôme, F.; Benabid, F. Hollow-Core Fiber Technology: The Rising of “Gas Photonics”. Fibers 2019, 7, 16. [Google Scholar] [CrossRef] [Green Version]
- Ni, W.; Yang, C.; Luo, Y.; Xia, R.; Lu, P.; Hu, D.J.J.; Danto, S.; Shum, P.P.; Wei, L. Recent Advancement of Anti-Resonant Hollow-Core Fibers for Sensing Applications. Photonics 2021, 8, 128. [Google Scholar] [CrossRef]
- Yu, F.; Knight, J.C. Low loss anti-resonant hollow-core fibers and applications. In Proceedings of the 2017 19th International Conference on Transparent Optical Networks (ICTON), Girona, Spain, 2–6 July 2017. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lo Piccolo, G.M.; Cannas, M.; Agnello, S. Intrinsic Point Defects in Silica for Fiber Optics Applications. Materials 2021, 14, 7682. https://doi.org/10.3390/ma14247682
Lo Piccolo GM, Cannas M, Agnello S. Intrinsic Point Defects in Silica for Fiber Optics Applications. Materials. 2021; 14(24):7682. https://doi.org/10.3390/ma14247682
Chicago/Turabian StyleLo Piccolo, Giuseppe Mattia, Marco Cannas, and Simonpietro Agnello. 2021. "Intrinsic Point Defects in Silica for Fiber Optics Applications" Materials 14, no. 24: 7682. https://doi.org/10.3390/ma14247682





