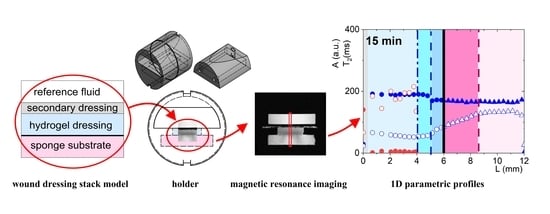
In Vitro Wound Dressing Stack Model as a First Step to Evaluate the Behavior of Dressing Materials in Wound Bed—An Assessment of Mass Transport Phenomena in Hydrogel Wound Dressings
Abstract
:1. Introduction
- to develop a simplified in vitro model simulating the place of application of a dressing to a wound area;
- to assess the potential of magnetic resonance imaging for studying changes in dressing properties when applied on various substrate environments.
2. Materials and Methods
2.1. Hydrogel Wound Dressing—Preparation
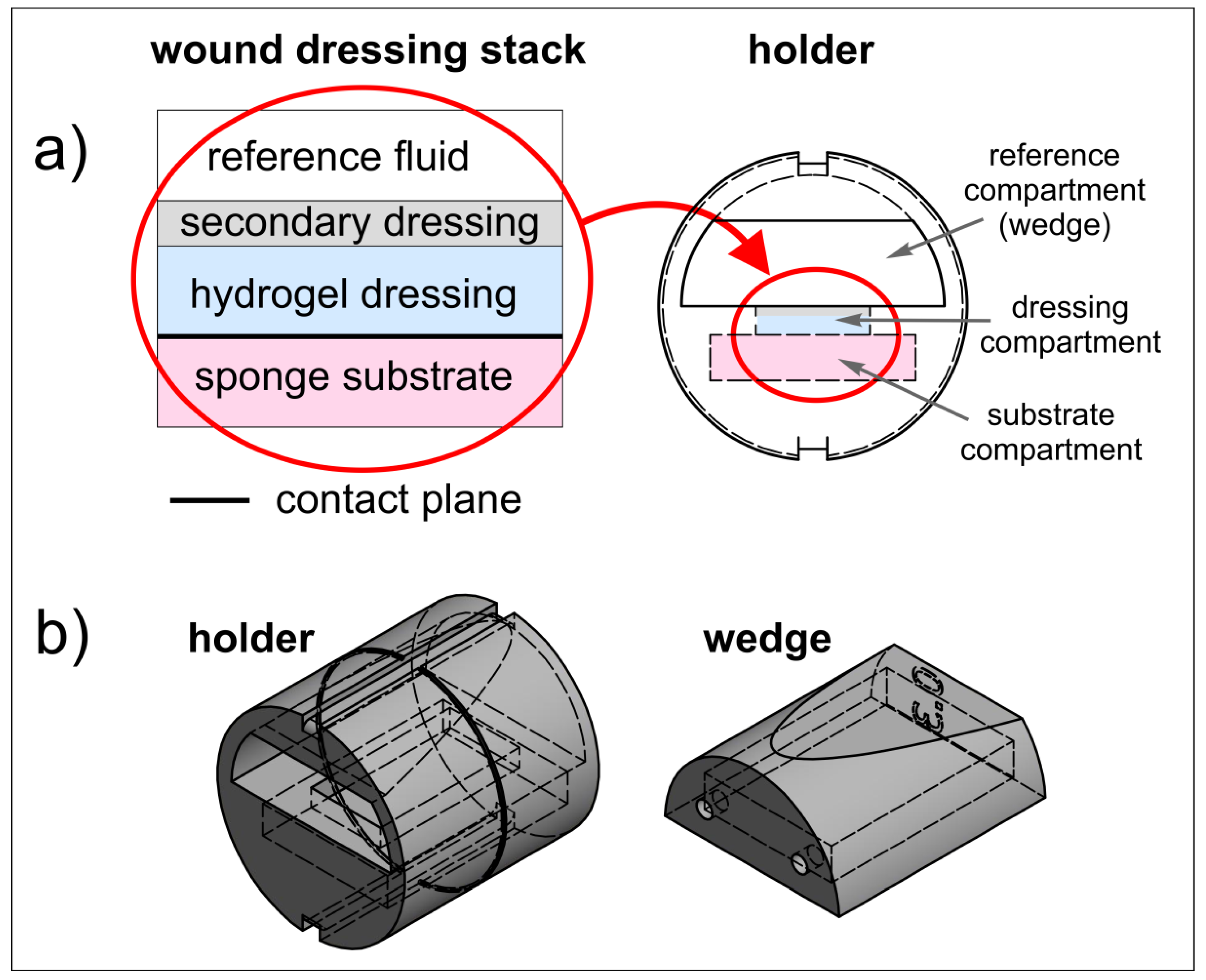
2.2. Wound Dressing Stack Model
2.3. 3D Printed Holder for Wound Dressing Testing
2.4. Magnetic Resonance Imaging and Image Analysis
3. Results and Discussion
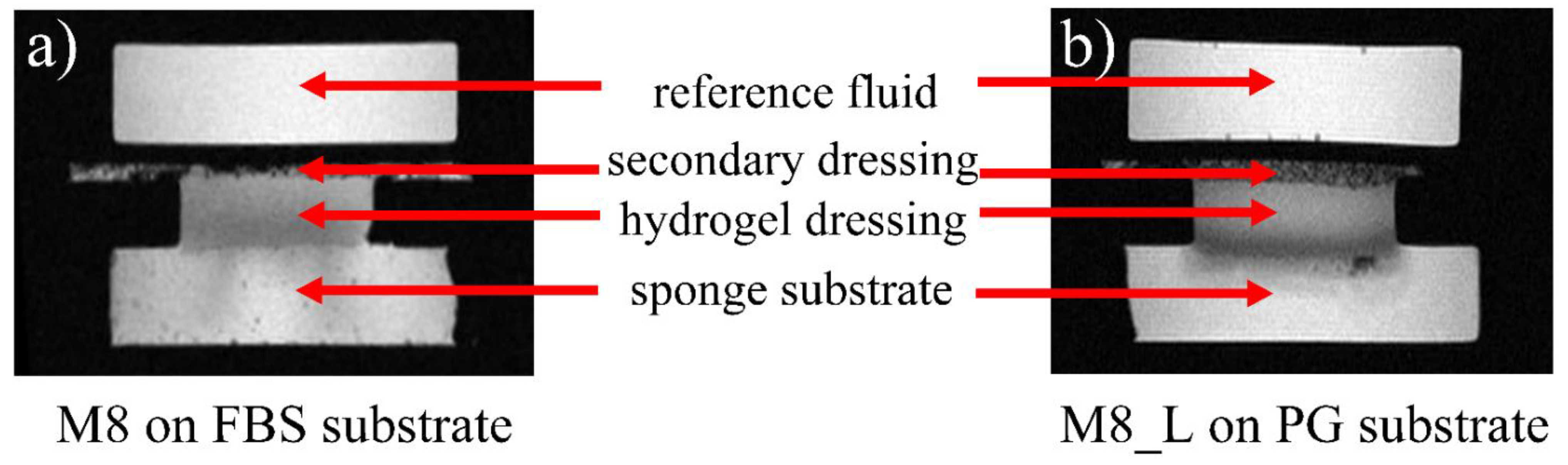
3.1. Qualitative Overview
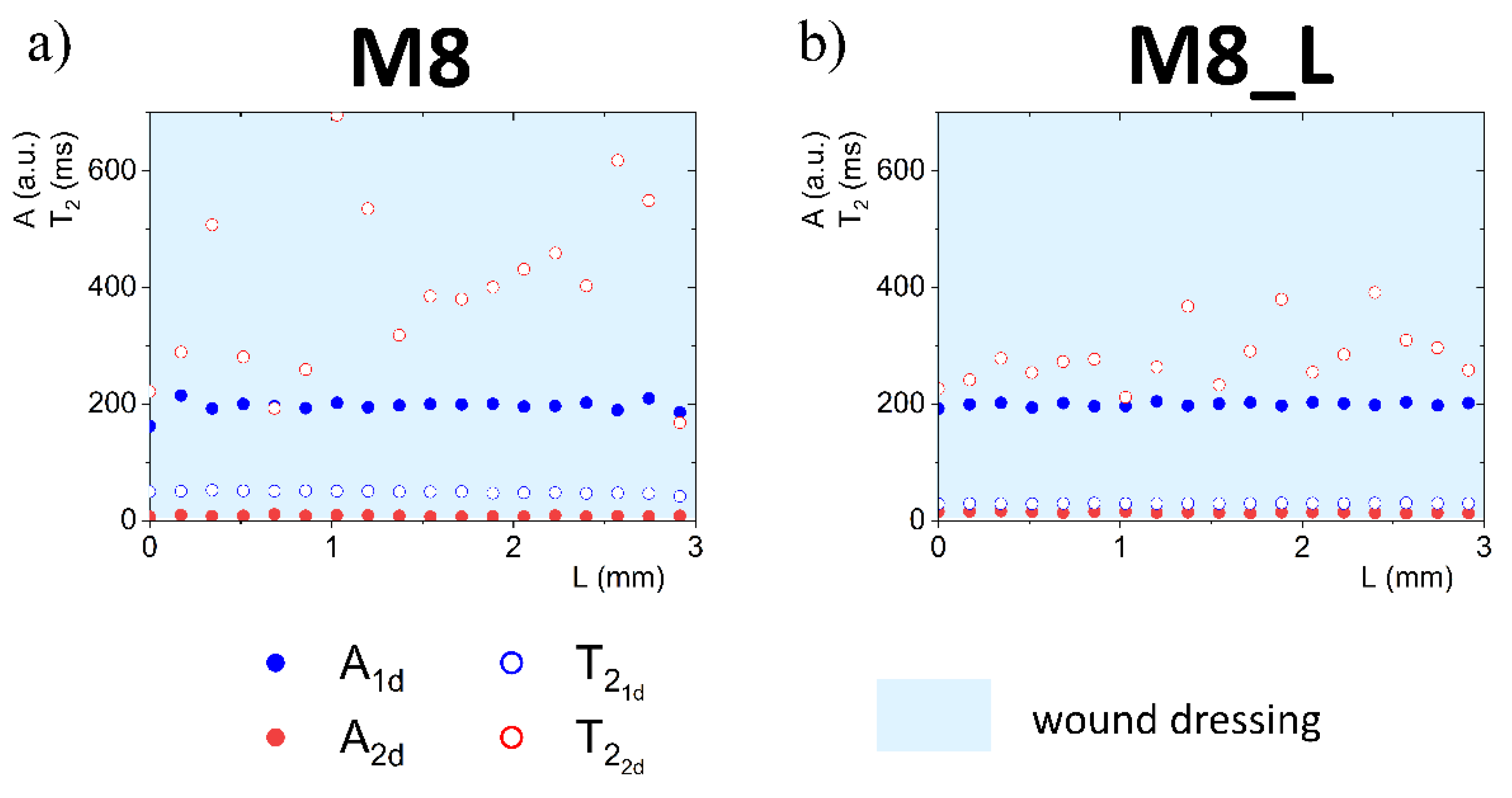
3.2. Quantitative Analysis
3.3. Dressings on FBS Wetted Substrate
3.4. Dressings on PG-Wetted Substrate
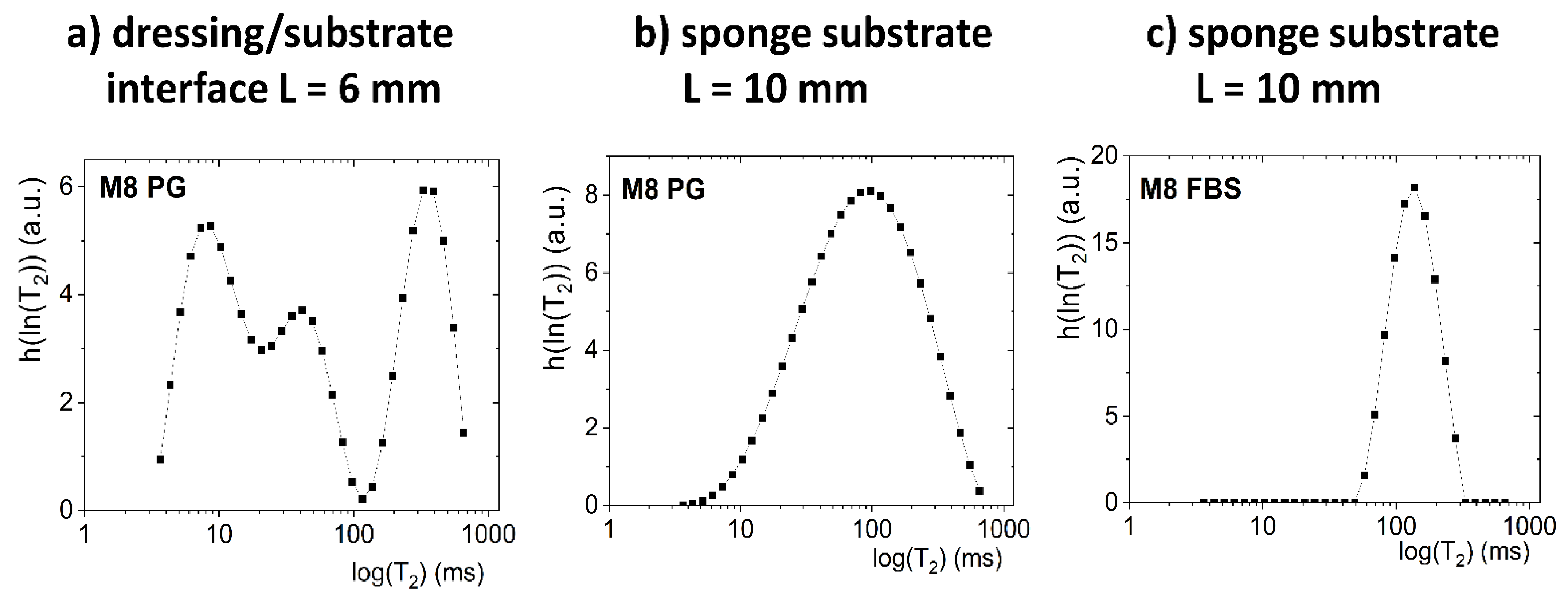
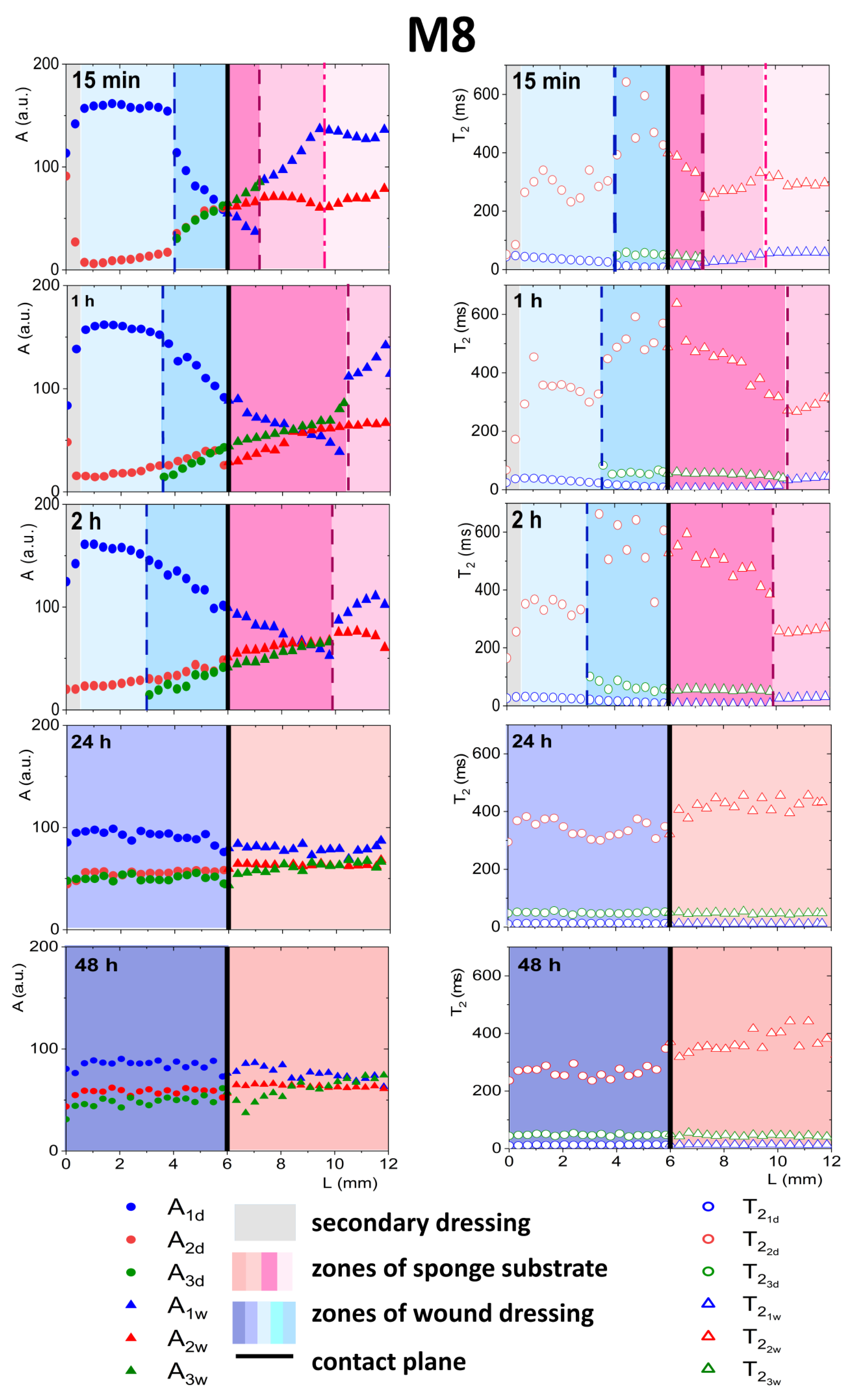
3.4.1. Detailed Description of M8 Formulation Results
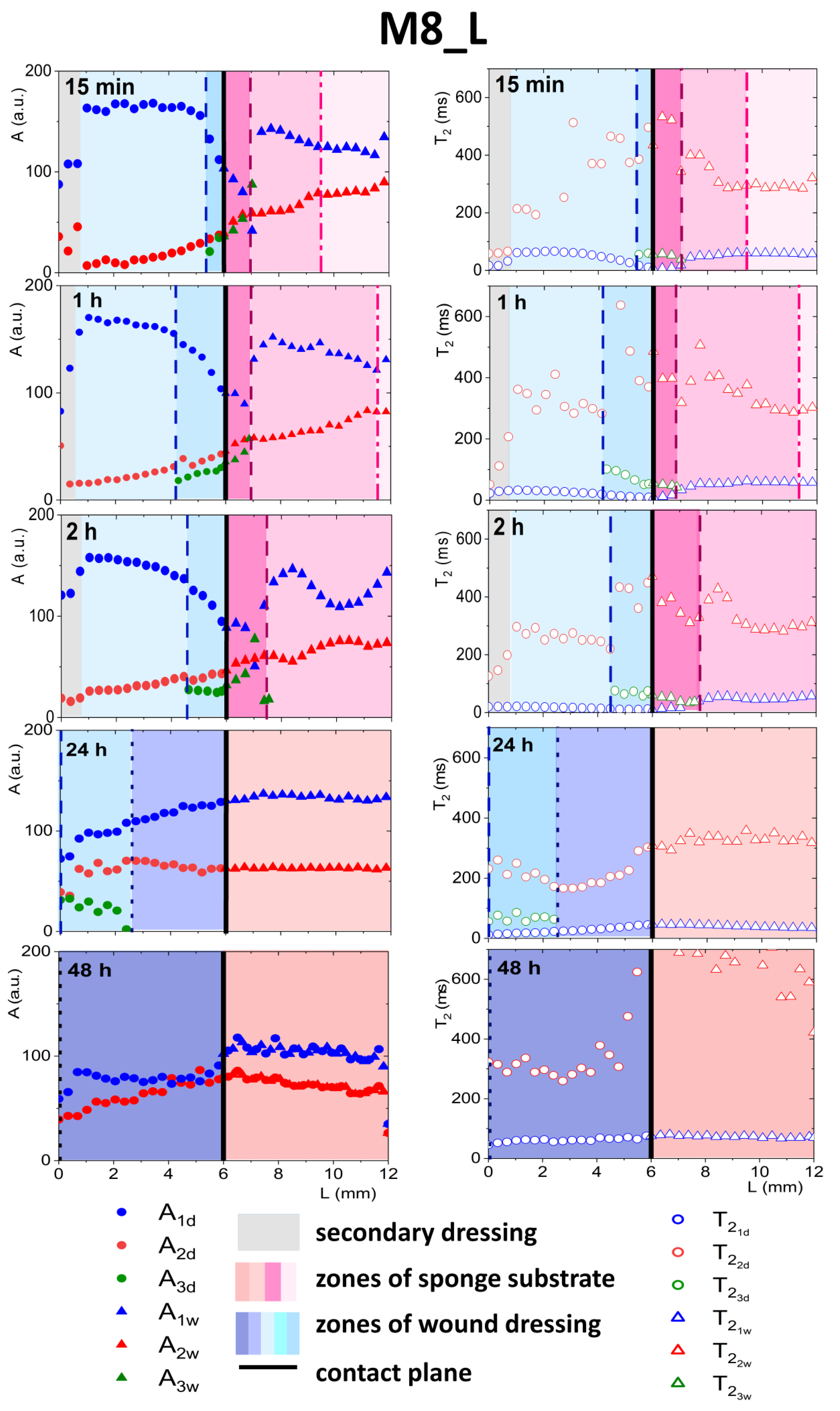
3.4.2. Detailed Description of M8_L Formulation Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Velnar, T.; Bailey, T.; Smrkoli, V. The Wound Healing Process: An Overview of the Cellular and Molecular Mechanisms. J. Int. Med Res. 2009, 37, 1528–1542. [Google Scholar] [CrossRef]
- Seaton, P.C.J.; Cant, R.P.; Trip, H.T. Quality indicators for a community-based wound care centre: An integrative review. Int. Wound J. 2020, 17, 587–600. [Google Scholar] [CrossRef]
- Obagi, Z.; Damiani, G.; Grada, A.; Falanga, V. Principles of Wound Dressings: A Review. Surg. Technol. Int.-Int. Dev. Surg. Surg. Res. 2019, 35, 8. [Google Scholar]
- Wilhelm, K.P.; Wilhelm, D.; Bielfeldt, S. Models of wound healing: An emphasis on clinical studies. Ski. Res. Technol. 2017, 23, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Test Methods for Primary Wound Dressings. 2002, BS EN 13726. Available online: https://www.en-standard.eu/csn-standards/85-health-service/8542-materials-for-sanitary-purposes/ (accessed on 15 November 2021).
- Mennini, N.; Greco, A.; Bellingeri, A.; De Vita, F.; Petrella, F. Quality of wound dressings: A first step in establishing shared criteria and objective procedures to evaluate their performance. J. Wound Care 2016, 25, 428–437. [Google Scholar] [CrossRef] [Green Version]
- Kucera, J.; Sojka, M.; Pavlik, V.; Szuszkiewicz, K.; Velebny, V.; Klein, P. Multispecies biofilm in an artificial wound bed-A novel model for in vitro assessment of solid antimicrobial dressings. J. Microbiol. Methods 2014, 103, 18–24. [Google Scholar] [CrossRef]
- Lipp, C.; Kirker, K.; Agostinho, A.; James, G.; Stewart, P. Testing wound dressings using an in vitro wound model. J. Wound Care 2010, 19, 220–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agostinho, A.M.; Hartman, A.; Lipp, C.; Parker, A.E.; Stewart, P.S.; James, G.A. An in vitro model for the growth and analysis of chronic wound MRSA biofilms. J. Appl. Microbiol. 2011, 111, 1275–1282. [Google Scholar] [CrossRef] [PubMed]
- Werthen, M.; Henriksson, L.; Jensen, P.O.; Sternberg, C.; Givskov, M.; Bjarnsholt, T. An in vitro model of bacterial infections in wounds and other soft tissues. Apmis 2010, 118, 156–164. [Google Scholar] [CrossRef]
- Greenman, J.; Thorn, R.M.; Saad, S.; Austin, A.J. In vitro diffusion bed, 3-day repeat challenge ’capacity’ test for antimicrobial wound dressings. Int. Wound J. 2006, 3, 322–329. [Google Scholar] [CrossRef]
- Li, S.X.; Mohamedi, A.H.; Senkowsky, J.; Nair, A.; Tang, L.P. Imaging in Chronic Wound Diagnostics. Adv. Wound Care 2020, 9, 245–263. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, Z.; Sammet, S.; Coffey, R.; Crockett, A.; Yuh, W.T.C.; Miller, S. Assessing the safety and compatibility of silver based wound dressings in a magnetic resonance environment. Burns 2009, 35, 1080–1085. [Google Scholar] [CrossRef]
- Bailey, J.K.; Sammet, S.; Overocker, J.; Craft-Coffman, B.; Acevedo, C.M.; Cowan, M.E.; Powell, H.M. MRI compatibility of silver based wound dressings. Burns 2018, 44, 1940–1946. [Google Scholar] [CrossRef]
- Gorska, A.; Dorozynski, P.; Weglarz, W.P.; Jasinski, K.; Kurek, M.; Jachowicz, R.; Klaja, J.; Kulinowski, P. Spatiotemporal characterization of hydration process of asymmetric polymeric wound dressings for decubitus ulcers. J. Biomed. Mater. Res. Part B-Appl. Biomater. 2018, 106, 843–853. [Google Scholar] [CrossRef]
- Gorska, A.; Krupa, A.; Majda, D.; Kulinowski, P.; Kurek, M.; Weglarz, W.P.; Jachowicz, R. Poly(Vinyl Alcohol) Cryogel Membranes Loaded with Resveratrol as Potential Active Wound Dressings. AAPS PharmSciTech 2021, 22, 14. [Google Scholar] [CrossRef] [PubMed]
- Milne, S.D.; Connolly, P. The influence of different dressings on the pH of the wound environment. J. Wound Care 2014, 23, 53–57. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, A.S.; Seidi, O.; Ribeiro, N.; Colaco, R.; Serro, A.P. Tribomechanical Comparison between PVA Hydrogels Obtained Using Different Processing Conditions and Human Cartilage. Materials 2019, 12, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavalu, S.; Fritea, L.; Brocks, M.; Barbaro, K.; Murvai, G.; Costea, T.O.; Antoniac, I.; Verona, C.; Romani, M.; Latini, A.; et al. Novel Hybrid Composites Based on PVA/SeTiO2 Nanoparticles and Natural Hydroxyapatite for Orthopedic Applications: Correlations between Structural, Morphological and Biocompatibility Properties. Materials 2020, 13, 20. [Google Scholar] [CrossRef]
- Chaturvedi, A.; Bajpai, A.K.; Bajpai, J.; Singh, S.K. Evaluation of poly(vinyl alcohol) based cryogel-zinc oxide nanocomposites for possible applications as wound dressing materials. Mater. Sci. Eng. C-Mater. Biol. Appl. 2016, 65, 408–418. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ma, X.; Li, Y.; Fan, D. Preparation of smooth and macroporous hydrogel via hand-held blender for wound healing applications: In vitro and in vivo evaluations. Biomed. Mater. 2020, 15, 055032. [Google Scholar] [CrossRef]
- Sakai, S.; Tsumura, M.; Inoue, M.; Koga, Y.; Fukano, K.; Taya, M. Polyvinyl alcohol-based hydrogel dressing gellable on-wound via a co-enzymatic reaction triggered by glucose in the wound exudate. J. Mater. Chem. B 2013, 1, 5067–5075. [Google Scholar] [CrossRef]
- Gao, T.L.; Jiang, M.H.; Liu, X.Q.; You, G.J.; Wang, W.Y.; Sun, Z.H.; Ma, A.G.; Chen, J. Patterned Polyvinyl Alcohol Hydrogel Dressings with Stem Cells Seeded for Wound Healing. Polymers 2019, 11, 12. [Google Scholar] [CrossRef] [Green Version]
- Hoare, T.R.; Kohane, D.S. Hydrogels in drug delivery: Progress and challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef] [Green Version]
- Nurzynska, A.; Klimek, K.; Palka, K.; Szajnecki, L.; Ginalska, G. Curdlan-Based Hydrogels for Potential Application as Dressings for Promotion of Skin Wound Healing-Preliminary In Vitro Studies. Materials 2021, 14, 17. [Google Scholar] [CrossRef] [PubMed]
- Millon, L.E.; Nieh, M.P.; Hutter, J.L.; Wan, W.K. SANS characterization of an anisotropic poly(vinyl alcohol) hydrogel with vascular applications. Macromolecules 2007, 40, 3655–3662. [Google Scholar] [CrossRef] [Green Version]
- Hickey, A.S.; Peppas, N.A. Mesh size and diffusive characteristics of semicrystalline poly(vinyl alcohol) membranes prepared by freezing-thawing techniques. J. Membr. Sci. 1995, 107, 229–237. [Google Scholar] [CrossRef]
- Wan, W.K.; Bannerman, A.D.; Yang, L.F.; Mak, H. Poly(Vinyl Alcohol) Cryogels for Biomedical Applications. In Polymeric Cryogels: Macroporous Gels with Remarkable Properties; Okay, O., Ed.; Advances in Polymer Science; Springer: Berlin/Heidelberg, Germany, 2014; Volume 263, pp. 283–321. [Google Scholar]
- Tavakoli, S.; Klar, A.S. Advanced Hydrogels as Wound Dressings. Biomolecules 2020, 10, 20. [Google Scholar] [CrossRef]
- Zhou, Y.; Hu, K.; Shen, J.F.; Wu, X.J.; Cheng, G.Z. Microstructure variations with concentration of propylene glycol-water solution probed by NMR. J. Mol. Struct. 2009, 921, 150–155. [Google Scholar] [CrossRef]
- Rhys, N.H.; Gillams, R.J.; Collins, L.E.; Callear, S.K.; Lawrence, M.J.; McLain, S.E. On the structure of an aqueous propylene glycol solution. J. Chem. Phys. 2016, 145, 12. [Google Scholar] [CrossRef]
- Kulinowski, P.; Dorozynski, P.; Jachowicz, R.; Jasinski, A. Magnetic resonance imaging analysis of moving fronts in floating dosage forms. Acta Phys. Pol.-Ser. A Gen. Phys. 2005, 108, 155–160. [Google Scholar] [CrossRef]
- Kulinowski, P.; Dorozynski, P.; Mlynarczyk, A.; Weglarz, W.P. Magnetic Resonance Imaging and Image Analysis for Assessment of HPMC Matrix Tablets Structural Evolution in USP Apparatus 4. Pharm. Res. 2011, 28, 1065–1073. [Google Scholar] [CrossRef] [Green Version]
- Kulinowski, P.; Mlynarczyk, A.; Dorozynski, P.; Jasinski, K.; Gruwel, M.L.H.; Tomanek, B.; Weglarz, W.P. Magnetic Resonance Microscopy for Assessment of Morphological Changes in Hydrating Hydroxypropylmethyl Cellulose Matrix Tablets In Situ. Pharm. Res. 2012, 29, 3420–3433. [Google Scholar] [CrossRef] [PubMed]
- Kulinowski, P.; Mlynarczyk, A.; Jasinski, K.; Talik, P.; Gruwel, M.L.H.; Tomanek, B.; Weglarz, W.P.; Dorozynski, P. Magnetic Resonance Microscopy for Assessment of Morphological Changes in Hydrating Hydroxypropylmethylcellulose Matrix Tablets In Situ—Is it Possible to Detect Phenomena Related to Drug Dissolution Within the Hydrated Matrices? Pharm. Res. 2014, 31, 2383–2392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juszczyk, E.; Kulinowski, P.; Baran, E.; Birczyński, A.; Majda, D.; García-Montoya, E.; Pérez-Lozano, P.; Suñé-Negre, J.M.; Węglarz, W.P.; Dorożyński, P. Spatiotemporal analysis of hydration mechanism in sodium alginate matrix tablets. Materials 2021, 14, 646. [Google Scholar] [CrossRef]
- Kulinowski, P.; Woyna-Orlewicz, K.; Rappen, G.-M.; Haznar-Garbacz, D.; Weglarz, W.P.; Dorozynski, P.P. An understanding of modified release matrix tablets behavior during drug dissolution as the key for prediction of pharmaceutical product performance—Case study of multimodal characterization of quetiapine fumarate tablets. Int. J. Pharm. 2015, 484, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Kulinowski, P.; Woyna-Orlewicz, K.; Obral, J.; Rappen, G.M.; Haznar-Garbacz, D.; Weglarz, W.P.; Jachowicz, R.; Wyszogrodzka, G.; Klaja, J.; Dorozynski, P.P. Multimodal approach to characterization of hydrophilic matrices manufactured by wet and dry granulation or direct compression methods. Int. J. Pharm. 2016, 499, 263–270. [Google Scholar] [CrossRef]
- Kulinowski, P.; Hudy, W.; Mendyk, A.; Juszczyk, E.; Weglarz, W.P.; Jachowicz, R.; Dorozynski, P. The Relationship between the Evolution of an Internal Structure and Drug Dissolution from Controlled-Release Matrix Tablets. AAPS PharmSciTech 2016, 17, 735–742. [Google Scholar] [CrossRef] [Green Version]

| A1d (a.u.) | A2d (a.u.) | |||
|---|---|---|---|---|
| M8 | 195± 12 | 48.9 ± 2.6 | 8.0± 1.1 | 394 ± 157 |
| M8_L | 199 ± 3.6 | 29.7 ± 0.4 | 14.2 ± 1.1 | 296 ± 59 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baran, E.; Górska, A.; Birczyński, A.; Hudy, W.; Kulinowski, W.; Jamróz, W.; Węglarz, W.P.; Kulinowski, P. In Vitro Wound Dressing Stack Model as a First Step to Evaluate the Behavior of Dressing Materials in Wound Bed—An Assessment of Mass Transport Phenomena in Hydrogel Wound Dressings. Materials 2021, 14, 7702. https://doi.org/10.3390/ma14247702
Baran E, Górska A, Birczyński A, Hudy W, Kulinowski W, Jamróz W, Węglarz WP, Kulinowski P. In Vitro Wound Dressing Stack Model as a First Step to Evaluate the Behavior of Dressing Materials in Wound Bed—An Assessment of Mass Transport Phenomena in Hydrogel Wound Dressings. Materials. 2021; 14(24):7702. https://doi.org/10.3390/ma14247702
Chicago/Turabian StyleBaran, Ewelina, Anna Górska, Artur Birczyński, Wiktor Hudy, Wojciech Kulinowski, Witold Jamróz, Władysław P. Węglarz, and Piotr Kulinowski. 2021. "In Vitro Wound Dressing Stack Model as a First Step to Evaluate the Behavior of Dressing Materials in Wound Bed—An Assessment of Mass Transport Phenomena in Hydrogel Wound Dressings" Materials 14, no. 24: 7702. https://doi.org/10.3390/ma14247702
APA StyleBaran, E., Górska, A., Birczyński, A., Hudy, W., Kulinowski, W., Jamróz, W., Węglarz, W. P., & Kulinowski, P. (2021). In Vitro Wound Dressing Stack Model as a First Step to Evaluate the Behavior of Dressing Materials in Wound Bed—An Assessment of Mass Transport Phenomena in Hydrogel Wound Dressings. Materials, 14(24), 7702. https://doi.org/10.3390/ma14247702