The Precipitated Particle Refinement in High-Cr ODS Steels by Microalloying Element Addition
Abstract
:1. Introduction
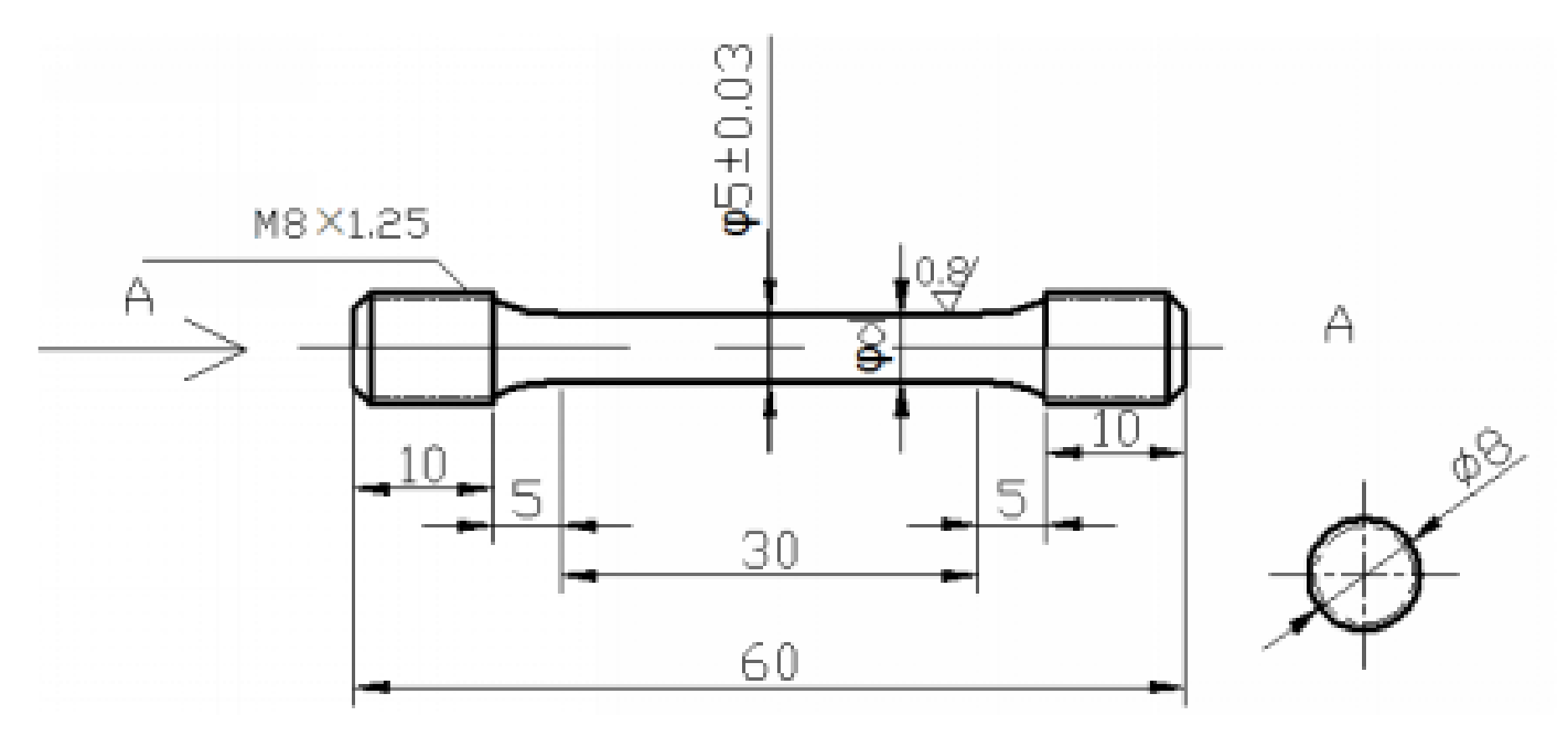
2. Materials and Experimental Details
3. Results and Discussion
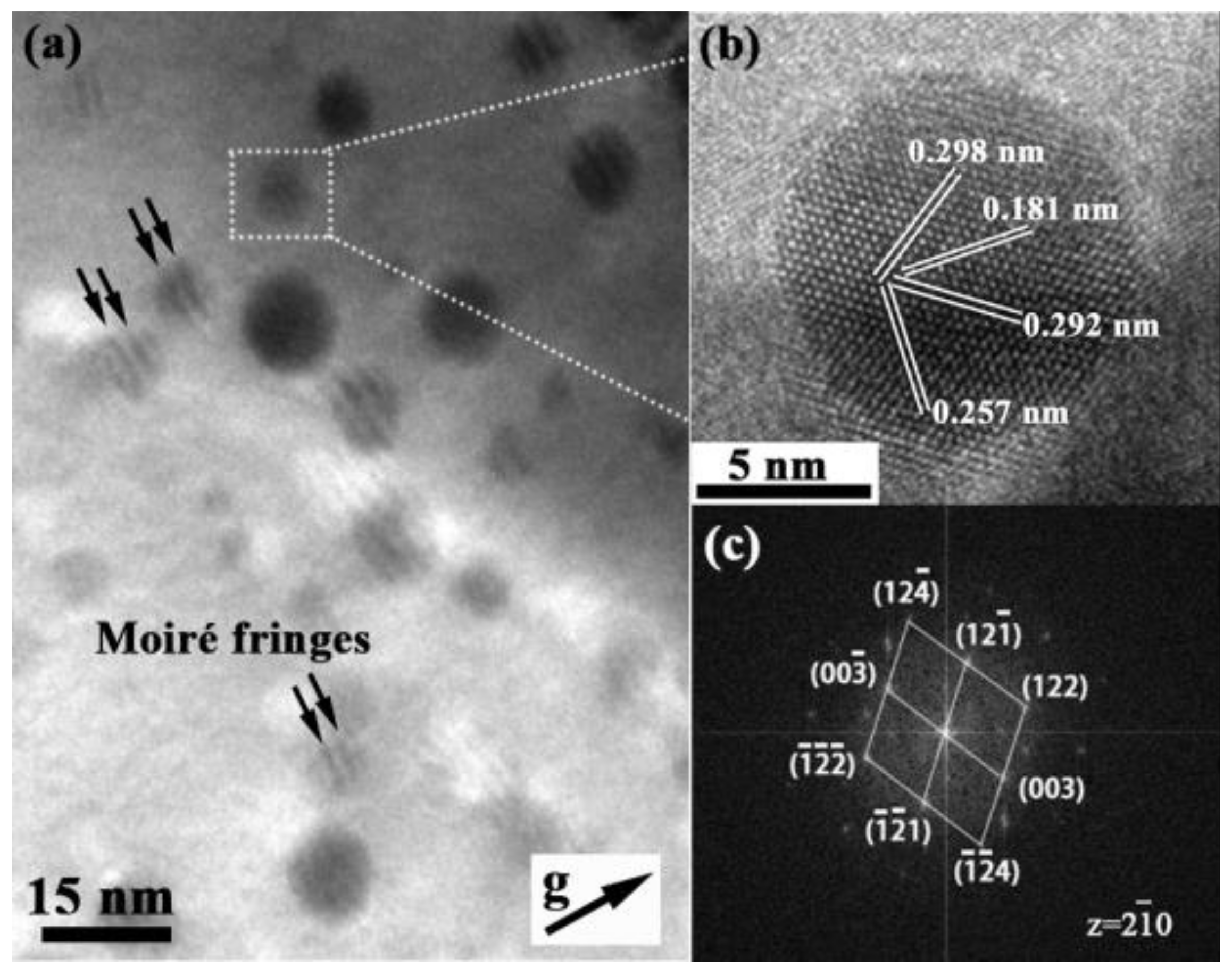
3.1. Effect of Microalloying Element Addition on Morphology and Distribution of Precipitated Particles in High-Cr ODS
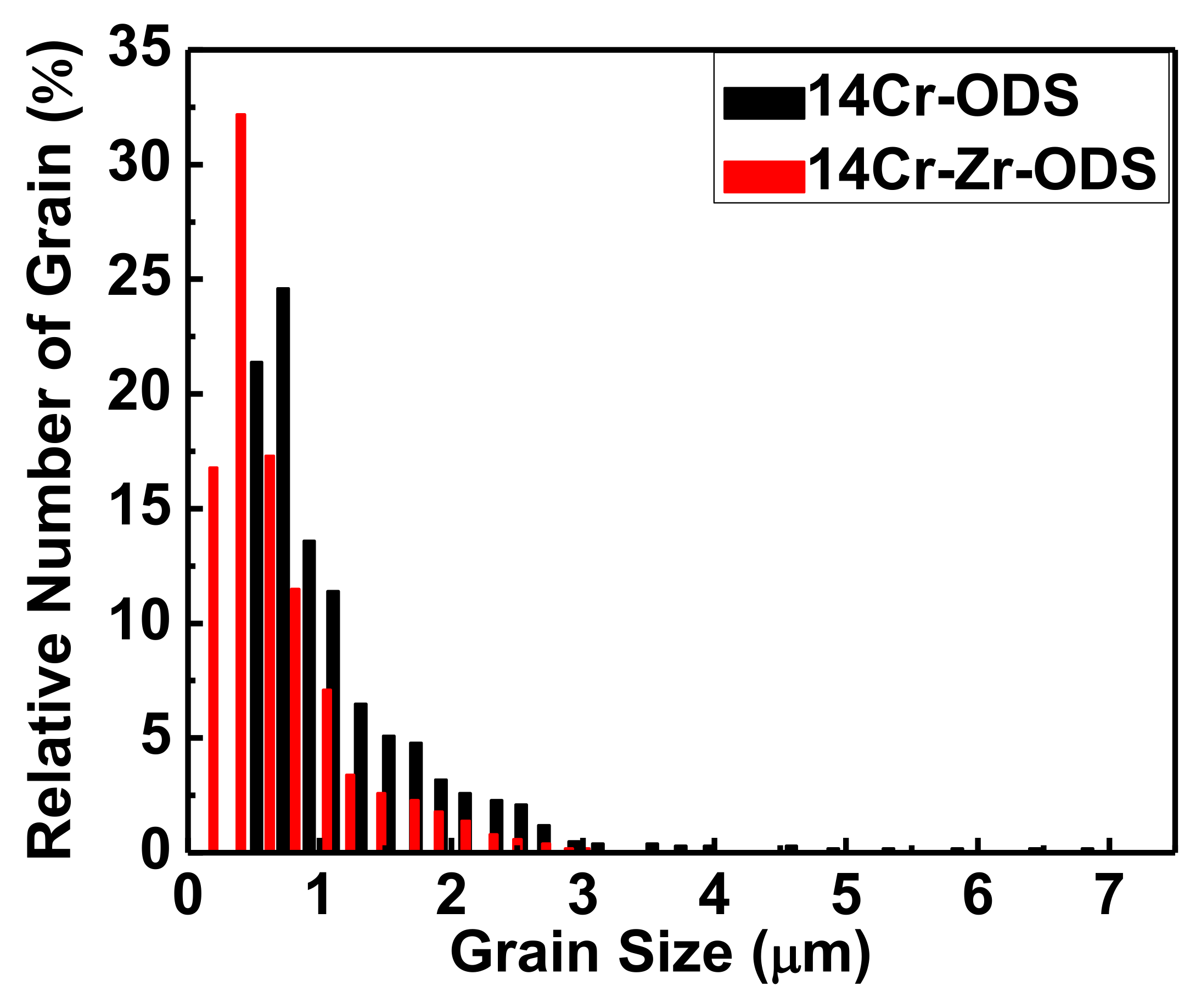
3.2. Effect of Microalloying Element Addition on Microstructure of High-Cr ODS
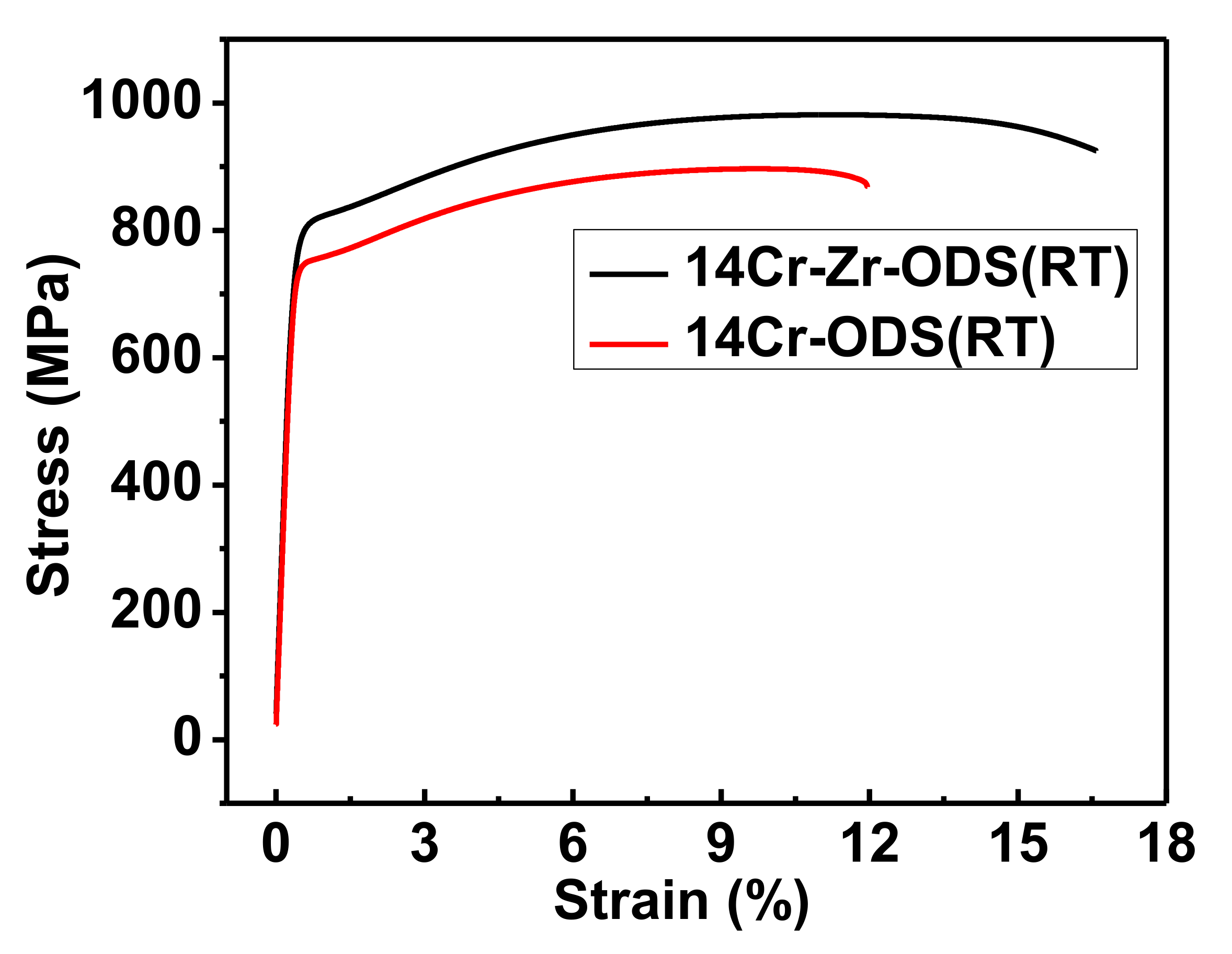
3.3. Mechanical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, Y.; Liu, Y.; Li, C.; Xia, X.; Wu, J.; Li, H. Coarsening behavior of γ′ precipitates in the γ’+γarea of a Ni3Al-based alloy. J. Alloys Compd. 2019, 771, 526–533. [Google Scholar] [CrossRef]
- Zhao, Q.; Yu, L.; Ma, Z.; Li, H.; Wang, Z.; Liu, Y. Hot deformation behavior and microstructure evolution of 14Cr ODS steel. Materials 2018, 11, 1044. [Google Scholar] [CrossRef] [Green Version]
- He, L.F.; Shirahata, J.; Nakayama, T.; Suzuki, T.; Suematsu, H.; Ihara, I.; Bao, Y.W.; Komatsu, T.; Niihara, K. Mechanical properties of Y2Ti2O7. Scr. Mater. 2011, 64, 548–551. [Google Scholar] [CrossRef]
- Fenghong, C.; Chang, C.; Zhenyu, W.; Muthuramalingam, T.; Anbuchezhiyan, G. Effects of silicon carbide and tungsten Carbide in aluminium metal matrix composites. Silicon-Neth. 2019, 11, 2625–2632. [Google Scholar] [CrossRef]
- Gao, Q.; Liu, Z.; Li, H.; Zhang, H.; Jiang, C.; Hao, A.; Qu, F.; Lin, X. High-temperature oxidation behavior of modified 4Al alumina-forming austenitic steel: Effect of cold rolling. J. Mater. Sci. Technol. 2021, 68, 91–102. [Google Scholar] [CrossRef]
- Wu, J.; Li, C.; Liu, Y.; Wu, Y.; Guo, Q.; Li, H.; Wang, H. Effect of annealing treatment on microstructure evolution and creep behavior of a multiphase Ni3Al-based superalloy. Mater. Sci. Eng. A 2019, 743, 623–635. [Google Scholar] [CrossRef]
- Chauhan, A.; Hoffmann, J.; Litvinov, D.; Aktaa, J. High-temperature low-cycle fatigue behavior of a 9Cr-ODS steel: Part 1—Pure fatigue, microstructure evolution and damage characteristics. Mater. Sci. Eng. A 2017, 707, 207–220. [Google Scholar] [CrossRef]
- Ukai, S.; Ohtsuka, S. Low cycle fatigue properties of ODS ferritic–martensitic steels at high temperature. J. Nucl. Mater. 2007, 367, 234–238. [Google Scholar] [CrossRef]
- Chauhan, A.; Hoffmann, J.; Litvinov, D.; Aktaa, J. High-temperature low-cycle fatigue behavior of a 9Cr-ODS steel: Part 2—Hold time influence, microstructural evolution and damage characteristics. Mater. Sci. Eng. A 2018, 730, 197–206. [Google Scholar] [CrossRef]
- Chauhan, A.; Walter, M.; Aktaa, J. Towards improved ODS steels: A comparative high-temperature low-cycle fatigue study. Fatigue Fract. Eng. Mater. Struct. 2017, 40, 2128–2140. [Google Scholar] [CrossRef]
- Hilger, I.; Tegel, M.; Gorley, M.J.; Grant, P.S.; Weißgärber, T.; Kieback, B. The structural changes of Y2O3 in ferritic ODS alloys during milling. J. Nucl. Mater. 2014, 447, 242–247. [Google Scholar] [CrossRef]
- Yutani, K.; Kishimoto, H.; Kasada, R.; Kimura, A. Evaluation of Helium effects on swelling behavior of oxide dispersion strengthened ferritic steels under ion irradiation. J. Nucl. Mater. 2007, 367–370, 423–427. [Google Scholar] [CrossRef]
- Li, Z.; Chen, L.; Zhang, H.; Liu, S. High-temperature oxidation properties and microstructural evolution of nanostructure Fe-Cr-Al ODS alloys. Materials 2021, 14, 526. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.A.; Unifantowicz, P.; Baluc, N.; Smith, G.D.W.; Marquis, E.A. The formation and evolution of oxide particles in oxide-dispersion-strengthened ferritic steels during processing. Acta Mater. 2013, 61, 2219–2235. [Google Scholar] [CrossRef]
- Na, X.; Wenqing, L.; Liu, Z.; Muthuramalingam, T. Effect of scandium in Al–Sc and Al–Sc–Zr alloys under precipitation strengthening mechanism at 350 °C aging. Met. Mater. Int. 2020, 27, 5145–5153. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Y.; Muthuramalingam, T.; Anbuchezhiyan, G. Effect of B4C and MOS2 reinforcement on micro structure and wear properties of aluminum hybrid composite for automotive applications. Compos. Part B Eng. 2019, 176, 107329. [Google Scholar] [CrossRef]
- Gong, M.; Zhou, Z.; Hu, H.; Zhang, G.; Li, S.; Wang, M. Effects of aluminum on microstructure and mechanical behavior of 14Cr–ODS steels. J. Nucl. Mater. 2015, 462, 502–507. [Google Scholar] [CrossRef]
- Li, Z.; Chen, L.; Zhang, H.; Zhang, S.; Zhang, Z.; Liu, S. Effect of hot rolling on the microstructure and properties of nanostructured 15Cr ODS alloys with Al and Zr addition. Materials 2020, 13, 3695. [Google Scholar] [CrossRef]
- Ren, J.; Yu, L.; Liu, Y.; Liu, C.; Li, H.; Wu, J. Effects of Zr addition on strengthening mechanisms of Al-alloyed high-Cr ODS steels. Materials 2018, 11, 118. [Google Scholar] [CrossRef] [Green Version]
- Kimura, A.; Kasada, R.; Iwata, N.; Kishimoto, H.; Zhang, C.H.; Isselin, J.; Dou, P.; Lee, J.H.; Muthukumar, N.; Okuda, T.; et al. Development of Al added high-Cr ODS steels for fuel cladding of next generation nuclear systems. J. Nucl. Mater. 2011, 417, 176–179. [Google Scholar] [CrossRef]
- Hättestrand, M.; Nilsson, J.; Stiller, K.; Liu, P.; Andersson, M. Precipitation hardening in a 12%Cr–9%Ni–4%Mo–2%Cu stainless steel. Acta Mater. 2004, 52, 1023–1037. [Google Scholar] [CrossRef]
- Pešička, J.; Kužel, R.; Dronhofer, A.; Eggeler, G. The evolution of dislocation density during heat treatment and creep of tempered martensite ferritic steels. Acta Mater. 2003, 51, 4847–4862. [Google Scholar] [CrossRef]
- Zhang, H.; Gorley, M.J.; Chong, K.B.; Fitzpatrick, M.E.; Roberts, S.G.; Grant, P.S. An in situ powder neutron diffraction study of nano-precipitate formation during processing of oxide-dispersion-strengthened ferritic steels. J. Alloys Compd. 2014, 582, 769–773. [Google Scholar] [CrossRef] [Green Version]
- Alinger, M.J.; Odette, G.R.; Hoelzer, D.T. On the role of alloy composition and processing parameters in nanocluster formation and dispersion strengthening in nanostuctured ferritic alloys. Acta Mater. 2009, 57, 392–406. [Google Scholar] [CrossRef]
- Toualbi, L.; Ratti, M.; André, G.; Onimus, F.; de Carlan, Y. Use of neutron and X-ray diffraction to study the precipitation mechanisms of oxides in ODS materials. J. Nucl. Mater. 2011, 417, 225–228. [Google Scholar] [CrossRef]
- Gale, W.F.; Totemeier, T.C. Equilibrium diagrams. In Smithells Metals Reference Book, 8th ed.; Butterworth-Heinemann: Oxford, UK, 2004; pp. 228–299. [Google Scholar] [CrossRef]
- Hin, C.; Wirth, B.D. Formation of oxide nanoclusters in nanostructured ferritic alloys during anisothermal heat treatment: A kinetic Monte Carlo study. Mater. Sci. Eng. A 2011, 528, 2056–2061. [Google Scholar] [CrossRef]
- Miao, P.; Odette, G.R.; Yamamoto, T.; Alinger, M.; Klingensmith, D. Thermal stability of nano-structured ferritic alloy. J. Nucl. Mater. 2008, 377, 59–64. [Google Scholar] [CrossRef]
- Sallez, N.; Boulnat, X.; Borbély, A.; Béchade, J.L.; Fabrègue, D.; Perez, M.; de Carlan, Y.; Hennet, L.; Mocuta, C.; Thiaudière, D.; et al. In Situ characterization of microstructural instabilities: Recovery, recrystallization and abnormal growth in nanoreinforced steel powder. Acta Mater. 2015, 87, 377–389. [Google Scholar] [CrossRef]
- Sallez, N.; Hatzoglou, C.; Delabrouille, F.; Sornin, D.; Chaffron, L.; Blat-Yrieix, M.; Radiguet, B.; Pareige, P.; Donnadieu, P.; Bréchet, Y. Precipitates and boundaries interaction in ferritic ODS steels. J. Nucl. Mater. 2016, 472, 118–126. [Google Scholar] [CrossRef]
- Krol, T.; Baither, D.; Nembach, E. The formation of precipitate free zones along grain boundaries in a superalloy and the ensuing effects on its plastic deformation. Acta Mater. 2004, 52, 2095–2108. [Google Scholar] [CrossRef]
- Grönhagen, K.; Ågren, J. Grain-boundary segregation and dynamic solute drag theory—A phase-field approach. Acta Mater. 2007, 55, 955–960. [Google Scholar] [CrossRef]
- Bouchaud, E.; Bouchaud, J.P.; Naka, S.; Lapasset, G.; Octor, H. Dissolution of precipitates by migrating grain boundaries. Acta Metall. Mater. 1992, 40, 3451–3458. [Google Scholar] [CrossRef]
- Zhao, N.; Feng, Y. Solid Phase Transformations in Alloys, 1st ed.; Central South University Press: Changsha, China, 2008; pp. 81–82. [Google Scholar]
- Dou, P.; Kimura, A.; Okuda, T.; Inoue, M.; Ukai, S.; Ohnuki, S.; Fujisawa, T.; Abe, F. Effects of extrusion temperature on the nano-mesoscopic structure and mechanical properties of an Al-alloyed high-Cr ODS ferritic steel. J. Nucl. Mater. 2011, 417, 166–170. [Google Scholar] [CrossRef]
- Ribis, J.; de Carlan, Y. Interfacial strained structure and orientation relationships of the nanosized oxide particles deduced from elasticity-driven morphology in oxide dispersion strengthened materials. Acta Mater. 2012, 60, 238–252. [Google Scholar] [CrossRef]
- Mao, X.; Oh, K.H.; Kang, S.H.; Kim, T.K.; Jang, J. On the coherency of Y2Ti2O7 particles with austenitic matrix of oxide dispersion strengthened steel. Acta Mater. 2015, 89, 141–152. [Google Scholar] [CrossRef]
- Ashby, M.F.; Brown, L.M. Diffraction contrast from spherically symmetrical coherency strains. Philos. Mag. 1963, 8, 1083–1103. [Google Scholar] [CrossRef]
- Zhang, L.; Yu, L.; Liu, Y.; Liu, C.; Li, H.; Wu, J. Influence of Zr addition on the microstructures and mechanical properties of 14Cr ODS steels. Mater. Sci. Eng. A 2017, 695, 66–73. [Google Scholar] [CrossRef]
- Dou, P.; Kimura, A.; Kasada, R.; Okuda, T.; Inoue, M.; Ukai, S.; Ohnuki, S.; Fujisawa, T.; Abe, F. TEM and HRTEM study of oxide particles in an Al-alloyed high-Cr oxide dispersion strengthened steel with Zr addition. J. Nucl. Mater. 2014, 444, 441–453. [Google Scholar] [CrossRef]
- Xu, H.; Li, W.; Sha, X.; Meng, J.; Kang, C.; Wang, W.; Zang, X.; Wang, Z. Effects of Zr addition on the microstructural stability of 15Cr-ODS steels under elevated-temperature annealing. Fusion Eng. Des. 2019, 138, 231–238. [Google Scholar] [CrossRef]
- Chauhan, A.; Bergner, F.; Etienne, A.; Aktaa, J.; de Carlan, Y.; Heintze, C.; Litvinov, D.; Hernandez-Mayoral, M.; Radiguet, B.; Ulbricht, A. Microstructure characterization and strengthening mechanisms of oxide dispersion strengthened (ODS) Fe-9%Cr and Fe-14%Cr extruded bars. J. Nucl. Mater. 2017, 495, 6–19. [Google Scholar] [CrossRef]
- Dadé, M.; Malaplate, J.; Garnier, J.; De Geuser, F.; Barcelo, F.; Wident, P.; Deschamps, A. Influence of microstructural parameters on the mechanical properties of oxide dispersion strengthened Fe-14Cr steels. Acta Mater. 2017, 127, 165–177. [Google Scholar] [CrossRef] [Green Version]
- Praud, M.; Mompiou, F.; Malaplate, J.; Caillard, D.; Garnier, J.; Steckmeyer, A.; Fournier, B. Study of the deformation mechanisms in a Fe–14% Cr ODS alloy. J. Nucl. Mater. 2012, 428, 90–97. [Google Scholar] [CrossRef]
- Ren, J.; Yu, L.; Liu, Y.; Ma, Z.; Liu, C.; Li, H.; Wang, H. Microstructure evolution and tensile properties of an Al added high-Cr ODS steel during thermal aging at 650 °C. Fusion Eng. Des. 2020, 157, 111700. [Google Scholar] [CrossRef]
- Kim, J.H.; Byun, T.S.; Hoelzer, D.T.; Park, C.H.; Yeom, J.T.; Hong, J.K. Temperature dependence of strengthening mechanisms in the nanostructured ferritic alloy 14YWT: Part II—Mechanistic models and predictions. Mater. Sci. Eng. A 2013, 559, 111–118. [Google Scholar] [CrossRef]
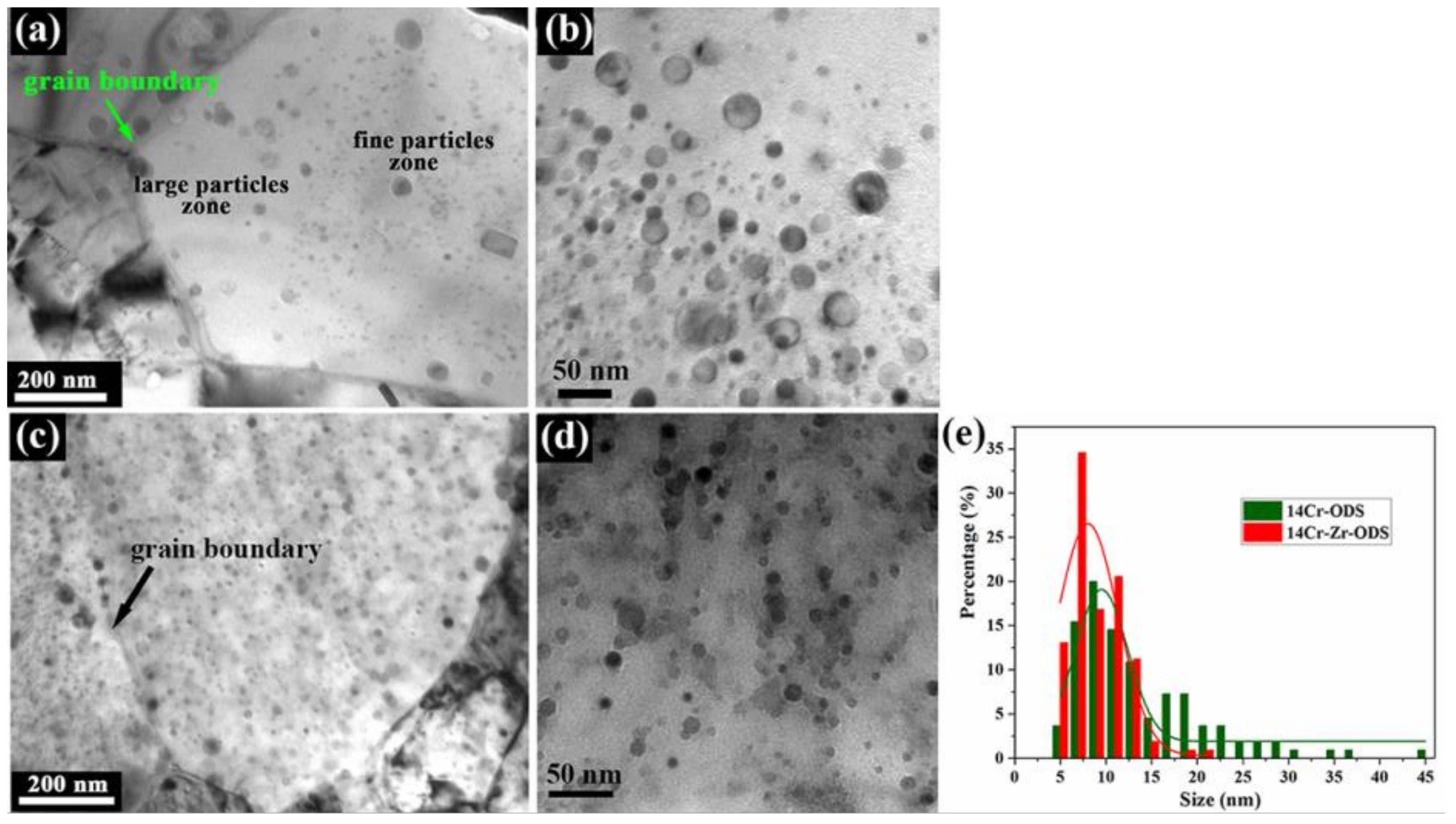



| dp (nm) | λ (nm) | Np (1022 m−3) | |
|---|---|---|---|
| 14Cr-ODS | 9.032 ± 1.601 | 19.851 ± 1.531 | 2.75 ± 0.21 |
| 14Cr-Zr-ODS | 7.583 ± 0.825 | 15.987 ± 0.603 | 4.13 ± 0.15 |
| YS (MPa) | UTS (MPa) | UE (%) | |
|---|---|---|---|
| 14Cr-ODS | 752.0 ± 22.5 | 892.3 ± 18.9 | 11.8 ± 2.1 |
| 14Cr-Zr-ODS | 816.6 ± 23.1 | 980.6 ± 20.7 | 16.7 ± 1.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Zhang, L.; Long, D.; Yu, L.; Li, H. The Precipitated Particle Refinement in High-Cr ODS Steels by Microalloying Element Addition. Materials 2021, 14, 7767. https://doi.org/10.3390/ma14247767
Li Y, Zhang L, Long D, Yu L, Li H. The Precipitated Particle Refinement in High-Cr ODS Steels by Microalloying Element Addition. Materials. 2021; 14(24):7767. https://doi.org/10.3390/ma14247767
Chicago/Turabian StyleLi, Yingying, Liye Zhang, Dijun Long, Liming Yu, and Huijun Li. 2021. "The Precipitated Particle Refinement in High-Cr ODS Steels by Microalloying Element Addition" Materials 14, no. 24: 7767. https://doi.org/10.3390/ma14247767





