Custom-Made Zirconium Dioxide Implants for Craniofacial Bone Reconstruction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cytotoxicity Study
2.2. Genotoxicity Study
2.3. Clinical Application of the Zirconium Dioxide Implant
3. Results
3.1. Cytotoxicity Results
3.2. Genotoxicity Results
3.3. Clinical Treatment with a Zirconium Implant in Patients
4. Discussion
- Anatomical symmetry
- No donor site morbidity
- Easy positioning—i.e., no need for long-lasting maneuvers inside the operating field
- No resorbable implant—i.e., long-time stale shape
- Stiff reconstructions
- Not only surface, but volume-restoring implant
- Osseointegration
- Better thermal conductivity than titanium
- Visible in roentgenological imaging
- Not affect magnetic resonance imaging (excluding titanium screw artifacts)
- Time needed for design and manufacture
- Surgeon involvement in design
- Limited subsequent implant modification during surgery
- CT artifacts because the implant is thick on follow-up examination
- Limited volume of the substrate material block for cranioplasty.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoffmann, J.; Cornelius, C.P.; Groten, M.; Probster, L.; Pfannenberg, C.; Schwenzer, N. Orbital reconstruction with individually copy-milled ceramic implants. Plast. Reconstr. Surg. 1998, 101, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Metzger, M.C.; Schon, R.; Weyer, N.; Rafii, A.; Gellrich, N.C.; Schmelzeisen, R.; Strong, B.E. Anatomical 3-dimensional pre-bent titanium implant for orbital floor fractures. Ophthalmology 2006, 113, 1863–1868. [Google Scholar] [CrossRef] [PubMed]
- Kozakiewicz, M.; Elgalal, M.; Walkowiak, B.; Stefanczyk, L. Technical concept of patient-specific, ultrahigh molecular weight polyethylene, orbital wall implant. J. Cranio-Maxillofac. Surg. 2013, 41, 282–290. [Google Scholar] [CrossRef]
- Lieger, O.; Richards, R.; Liu, M.; Lloyd, T. Computer-assisted design and manufacture of implants in the late reconstruction of extensive orbital fractures. Arch. Facial Plast. Surg. 2010, 12, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Loba, P.; Kozakiewicz, M.; Elgalal, M.; Stefanczyk, L.; Broniarczyk-Loba, A.; Omulecki, W. The use of modern imaging techniques in the diagnosis and treatment planning of patients with orbital floor fractures. Med. Sci. Monit. 2011, 17, CS94–CS98. [Google Scholar] [CrossRef] [Green Version]
- Kozakiewicz, M.; Elgalal, M.; Loba, P.; Komunski, P.; Arkuszewski, P.; Broniarczyk-Loba, A.; Stefnczyk, L. Clinical application of 3D pre-bent titanium implants for orbital floor fractures. J. Cranio-Maxillofac. Surg. 2009, 37, 229–234. [Google Scholar] [CrossRef]
- Kozakiewicz, M.; Elgalal, M.; Piotr, L.; Broniarczyk-Loba, A.; Stefanczyk, L. Treatment with individual orbital wall implants in humans—1-Year ophthalmologic evaluation. J. Cranio-Maxillofac. Surg. 2011, 39, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Potter, J.K.; Ellis, E. Biomaterials for reconstruction of the internal orbit. J. Oral. Maxillofac. Surg. 2004, 62, 1280–1297. [Google Scholar] [CrossRef]
- Manicone, P.F.; Rossi Iommetti, P.; Raffaelli, L. An overview of zirconia ceramics: Basic properties and clinical applications. J. Dent. 2007, 35, 819–826. [Google Scholar] [CrossRef]
- Piconi, C.; Maccauro, G. Zirconia as a ceramic biomaterial. Biomaterials 1999, 20, 1–25. [Google Scholar] [CrossRef]
- Warashina, H.; Sakano, S.; Kitamura, S.; Yamauchi, K.I.; Yamaguchi, J.; Ishiguro, N.; Hasegawa, Y. Biological reaction to alumina, zirconia, titanium and polyethylene particles implanted onto murine calvaria. Biomaterials 2003, 24, 3655–3661. [Google Scholar] [CrossRef]
- Degidi, M.; Artese, L.; Scarano, A.; Perrotti, V.; Gehrke, P.; Piattelli, A. Inflammatory infiltrate, microvessel density, nitric oxide synthase expression, vascular endothelial growth factor expression, and proliferative activity in peri-implant soft tissues around titanium and zirconium oxide healing caps. J. Periodontol. 2006, 77, 73–80. [Google Scholar] [CrossRef]
- Borys, J.; Maciejczyk, M.; Antonowicz, B.; Kretowski, A.; Waszkiel, D.; Bortnik, P.; Czarniecka-Bargłowska, K.; Kocisz, M.; Szulimowska, J.; Czajkowski, M.; et al. Exposure to Ti4Al4V titanium alloy leads to redox abnormalities, oxidative stress, and oxidative damage in patients treated for mandible fractures. Oxidative Med. Cell. Longev. 2018, 2018, 1–10. [Google Scholar] [CrossRef]
- Kozakiewicz, M.; Wach, T.; Szymor, P.; Zieliński, R. Two different techniques of manufacturing TMJ replacements—A technical report. J. Cranio-Maxillofac. Surg. 2017, 45, 1432–1437. [Google Scholar] [CrossRef] [PubMed]
- ISO 5834-1:2005/COR 1:2007. In Implants for Surgery—Ultra-High-Molecular-Weight Polyethylene—Part 1: Powder form—Technical Corrigendum 1; International Organization for Standardization: Geneva, Switzerland, 2007.
- ISO 5834-2:2006. In Implants for Surgery—Ultra-High-Molecular-Weight Polyethylene—Part 2: Moulded Forms; International Organization for Standardization: Geneva, Switzerland, 2006.
- ASTM F 648-07. In Standard Specification for Ultra-High-Molecular-Weight Polyethylene Powder and Fabricated Form for Surgical Implants; ASTM International: West Conshohocken, PA, USA, 2007.
- Kozakiewicz, M. Computer-aided orbital wall defects treatment by individual design ultrahigh molecular weight polyethylene implants. J. Cranio-Maxillofac. Surg. 2014, 42, 283–289. [Google Scholar] [CrossRef]
- Tessier, P. The conjunctival approach to the orbital floor and maxilla in congenital malformation and trauma. J. Maxillofac. Surg. 1973, 1, 3–8. [Google Scholar] [CrossRef]
- Markowska-Szczupak, A.; Endo-Kimura, M.; Paszkiewicz, O.; Kowalska, E. Are Titania Photocatalysts and Titanium Implants Safe? Review on the Toxicity of Titanium Compounds. Nanomaterials (Basel) 2020, 10, 2065. [Google Scholar] [CrossRef] [PubMed]
- López-Jornet, P.; Perrez, F.P.; Calvo-Guirado, J.L.; Ros-Llor, I.; Ramírez-Fernández, P. Metallic ion content and damage to the DNA in oral mucosa cells patients treated dental implants. J. Mater. Sci. Mater. Med. 2014, 25, 1819–1824. [Google Scholar] [CrossRef]
- Liao, H.; Wurtz, T.; Li, J. Influence of titanium ion on mineral formation and properties of osteoid nodules in rat calvaria cultures. J. Biomed. Mater. Res. 1999, 47, 220–227. [Google Scholar] [CrossRef]
- Borys, J.; Maciejczyk, M.; Antonowicz, B.; Kretowski, A.; Sidun, J.; Domel, E.; Dąbrowski, J.; Ładny, J.R.; Morawska, K.; Zalewska, A. Glutathione metabolism, mitochondria activity, and nitrosative stress in patients treated for mandible fractures. J. Clin. Med. 2019, 8, 127. [Google Scholar] [CrossRef] [Green Version]
- Obando-Pereda, G.A.; Fischer, L.; Stach-Machado, D.R. Titanium and zirconia particle-induced pro-inflammatory gene expression in cultured macrophages and osteolysis, inflammatory hyperalgesia and edema in vivo. Life Sci. 2014, 97, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Attarilar, S.; Yang, J.; Ebrahimi, M.; Wang, Q.; Liu, J.; Tang, Y.; Yang, J. The Toxicity Phenomenon and the Related Occurrence in Metal and Metal Oxide Nanoparticles: A Brief Review from the Biomedical Perspective. Front. Bioeng. Biotechnol. 2020, 8, 822. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Q.; Yin, L.H.; Tang, M.; Pu, Y.P. ZnO, TiO(2), SiO(2,) and Al(2)O(3) nanoparticles-induced toxic effects on human fetal lung fibroblasts. Biomed. Environ. Sci. 2011, 24, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.A.; Khan, H.M.; Khan, A.A.; Cameotra, S.S.; Saquib, Q.; Musarrat, J. Interaction of Al2O3 nanoparticles with Escherichia coli and their cell envelope biomolecules. J. Appl. Microbiol. 2013, 116, 772–783. [Google Scholar] [CrossRef]
- Palka, L.; Mazurek-Popczyk, J.; Arkusz, K.; Baldy-Chudzik, K. Susceptibility to biofilm formation on 3D-printed titanium fixation plates used in the mandible: A preliminary study. J. Oral. Microbiol. 2020, 12, 1838164. [Google Scholar] [CrossRef]
- Skinner, H.B. Review Ceramic bearing surfaces. Clin. Orthop. Relat. Res. 1999, 83–91. [Google Scholar] [CrossRef]
- Bierbaum, B.E.; Nairus, J.; Kuesis, D.; Morrison, J.C.; Ward, D. Ceramic-on-ceramic bearings in total hip arthroplasty. Clin. Orthop. Relat. Res. 2002, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Min, B.W.; Song, K.S.; Kang, C.H.; Bae, K.C.; Won, Y.Y.; Lee, K.Y. Delayed fracture of a ceramic insert with modern ceramic total hip replacement. J. Arthroplast. 2007, 22, 136–139. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.; Glatzer, C. Individual CAD/CAM fabricated glass-bioceramic implants in reconstructive surgery of the bony orbital floor. Plast. Reconstr. Surg. 2006, 117, 565–570. [Google Scholar] [CrossRef]
- Converse, J.M.; Smith, B.; Obear, M.F.; Wood-Smith, D. Orbital blowout fractures: A ten-year survey. Plast. Reconstr. Surg. 1967, 39, 20–36. [Google Scholar] [CrossRef]
- Koornneef, L. Details of the orbital connective tissue system in the adult. Acta Morphol. Neerl. Scand. 1977, 15, 1–34. [Google Scholar] [PubMed]
- Bartkowski, S.B.; Kurek, M.; Stypulkowska, J.; Krzystkowa, K.M.; Zapala, J. Foreign bodies in the orbit. Review of 20 cases. J. Maxillofac. Surg. 1984, 12, 97–102. [Google Scholar] [CrossRef]
- Galiè, M.; Consorti, G.; Clauser, L.C.; Kawamoto, H.K. Craniofacial surgical strategies for the correction of pneumosinus dilatans frontalis. J. Cranio-Maxillofac. Surg. 2013, 41, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Tieghi, R.; Consorti, G.; Banchini, S.; Elia, G.; Illiano, F.; Clauser, L.C. Cranial bone grafts in forehead reconstruction after resection for benign tumors. J. Craniofac. Surg. 2013, 24, 505–507. [Google Scholar] [CrossRef] [PubMed]
- Ellis, E., 3rd; Tan, Y. Assessment of internal orbital reconstructions for pure blowout fractures: Cranial bone grafts versus titanium mesh. J. Oral Maxillofac. Surg. 2003, 61, 442–453. [Google Scholar] [CrossRef]
- Dougherty, W.R.; Wellisz, T. The natural history of alloplastic implants in orbital floor reconstruction: An animal model. J. Craniofac. Surg. 1994, 5, 26–33. [Google Scholar] [CrossRef]
- Hemprich, A.; Breier, T. Secondary correction of traumatogenic enophthalmos with auto- and alloplastic implants. Rev. Stomatol. Chir. Maxillofac. 1993, 94, 37–39. [Google Scholar]
- Kozakiewicz, M.; Elgalal, M.; Piotr, L.; Broniarczyk-Loba, A.; Stefanczyk, L. Patient specific implants, designed using Rapied Prototyping and diagnostic imaging, for the repair of orbital fractures. Med. Sci. Monit. 2010, 16, 75–79. [Google Scholar] [CrossRef]
- Cavalcanti, A.N.; Foxton, R.M.; Watson, T.F.; Oliveira, M.T.; Giannini, M.; Marchi, G.M. Y-TZP ceramics: Key concepts for clinical application. Oper. Dent. 2009, 34, 344–351. [Google Scholar] [CrossRef]
- Wenz, H.J.; Bartsch, J.; Wolfart, S.; Kern, M. Osseointegration and clinical success of zirconia dental implants: A systematic review. Int. J. Prosthodont. 2008, 21, 27–36. [Google Scholar]
- Andreiotelli, M.; Wenz, H.J.; Kohal, R.J. Are ceramic implants a viable alternative to titanium implants? A systematic literature review. Clin. Oral Implants Res. 2009, 20 (Suppl. 4), 32–47. [Google Scholar] [CrossRef]
- Candido, L.; Miotto, L.; Fais, L.; Cesar, P.; Pinelli, L. Mechanical and Surface Properties of Monolithic Zirconia. Operat. Dent. 2018, 43, E119–E128. [Google Scholar] [CrossRef]
- Stawarczyk, B.; Emslander, A.; Roos, M.; Sener, B.; Noack, F.; Keul, C. Zirconia ceramics, their contrast ratio and grain size depending on sintering parameters. Dent. Mat. J. 2014, 33, 591–598. [Google Scholar] [CrossRef] [Green Version]
- Keen, M. Complications of harvesting cranial bone grafts. Plast. Reconstr. Surg. 1995, 96, 1753. [Google Scholar] [CrossRef] [PubMed]
- Glassman, R.D.; Manson, P.N.; Vanderkolk, C.A.; Iliff, N.T.; Yaremchuk, M.J.; Petty, P.; Defresne, C.R.; Markowitz, B.L. Rigid fixation of internal orbital fractures. Plast. Reconstr. Surg. 1990, 86, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Ghayor, C.; Weber, F.E. Osteoconductive Microarchitecture of Bone Substitutes for Bone Regeneration Revisited. Front. Physiol. 2018, 9, 960. [Google Scholar] [CrossRef] [PubMed]
- Clauser, L.C.; Tieghi, R.; Galiè, M.; Carinci, F. Structural fat grafting: Facial volumetric restoration in complex reconstructive surgery. J. Craniofac. Surg. 2011, 22, 1695–1701. [Google Scholar] [CrossRef] [PubMed]
- Coleman, S.R. Facial augmentation with structural fat grafting. Clin. Plast. Surg. 2006, 33, 567–577. [Google Scholar] [CrossRef]
- Consorti, G.; Tieghi, R.; Clauser, L.C. Frontal linear scleroderma: Long-term result in volumetric restoration of the fronto-orbital area by structural fat grafting. J. Craniofac. Surg. 2012, 23, 263–265. [Google Scholar] [CrossRef]
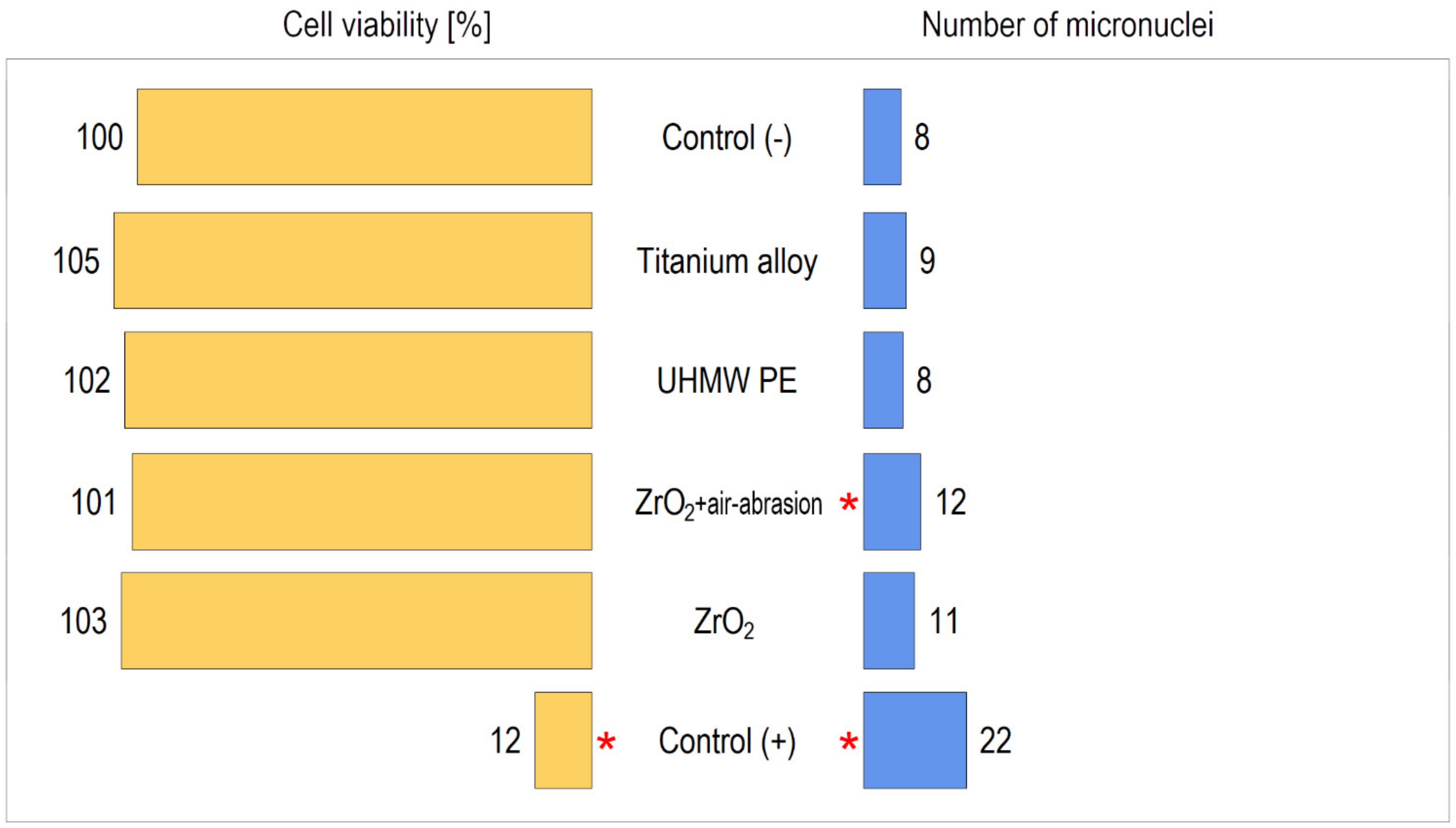










| Material | Cell Viability ± SD [%] | Note |
|---|---|---|
| Control (−) | 100 ± 5.6 | NS |
| Titanium alloy | 105.3 ± 7.2 | NS |
| UHMW PE | 102.9 ± 8.5 | NS |
| ZrO2+ air-abrasion | 101.2 ± 6.7 | NS |
| ZrO2 | 103.6 ± 5.9 | NS |
| Control (+) | 12.5 ± 1.1 | p < 0.05 vs. other materials |
| Material | Number of Micronuclei ± SD | Note |
|---|---|---|
| Control (−) | 8.09 ± 0.91 | |
| Titanium alloy | 9.36 ± 3.10 | NS |
| UHMW PE | 8.56 ± 1.52 | NS |
| ZrO2+ air-abrasion | 12.51 ± 2.12 | p < 0.01 |
| ZrO2 | 11.18 ± 1.92 | NS |
| Control (+) | 22.53 ± 2.86 | p < 0.001 |
| Case | Sex | Age | ODI | Area of Reconstruction | Approach | Fixing Screws | Follow-Up [Months] | Soft Tissue Shift [mm] | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 19 | 1 | Orbital wall defect |  | Tc | 2 | 99 | 2.01 |
| 2 | F | 22 | 3 | Orbital wall defect |  | Tc | 2 | 98 | 2.58 |
| 3 | M | 23 | 1 | Orbital wall defect |  | Tc | 2 | 96 | 1.98 |
| 4 | M | 32 | 1 | Orbital wall defect |  | Tc | 2 | 94 | 2.13 |
| 5 | F | 20 | 3 | Orbital wall defect |  | Tc | 2 | 90 | 2.96 |
| 6 | M | 21 | 1 | Orbital wall defect |  | Tc | 2 | 89 | 1.27 |
| 7 | M | 24 | 5 | Orbital wall defect |  | Tc | 1 | 73 | 4.00 |
| 8 | M | 27 | 2 | Zygomatic deformation |  | Tm | 1 | 91 | 1.78 |
| 9 | M | 31 | 2 | Zygomatic deformation |  | Tm | 1 | 89 | 6.63 |
| 10 | F | 29 | 2 | Zygomatic deformation |  | Tm | 1 | 88 | 5.42 |
| 11 | M | 46 | 2 | Zygomatic deformation |  | Tm | 1 | 86 | 5.21 |
| 12 | M | 37 | 2 | Zygomatic deformation |  | Tm | 1 | 82 | 5.32 |
| 13 | M | 28 | 7 | Orbital rim and forehead |  | Se | 2 | 82 | 3.89 |
| 14 | M | 24 | 7 | Orbital rim and forehead |  | Sc | 4 | 81 | 7.84 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozakiewicz, M.; Gmyrek, T.; Zajdel, R.; Konieczny, B. Custom-Made Zirconium Dioxide Implants for Craniofacial Bone Reconstruction. Materials 2021, 14, 840. https://doi.org/10.3390/ma14040840
Kozakiewicz M, Gmyrek T, Zajdel R, Konieczny B. Custom-Made Zirconium Dioxide Implants for Craniofacial Bone Reconstruction. Materials. 2021; 14(4):840. https://doi.org/10.3390/ma14040840
Chicago/Turabian StyleKozakiewicz, Marcin, Tomasz Gmyrek, Radosław Zajdel, and Bartłomiej Konieczny. 2021. "Custom-Made Zirconium Dioxide Implants for Craniofacial Bone Reconstruction" Materials 14, no. 4: 840. https://doi.org/10.3390/ma14040840
APA StyleKozakiewicz, M., Gmyrek, T., Zajdel, R., & Konieczny, B. (2021). Custom-Made Zirconium Dioxide Implants for Craniofacial Bone Reconstruction. Materials, 14(4), 840. https://doi.org/10.3390/ma14040840








