Effects of Copper Surface Oxidation and Reduction on Shear-Bond Strength Using Functional Monomers
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
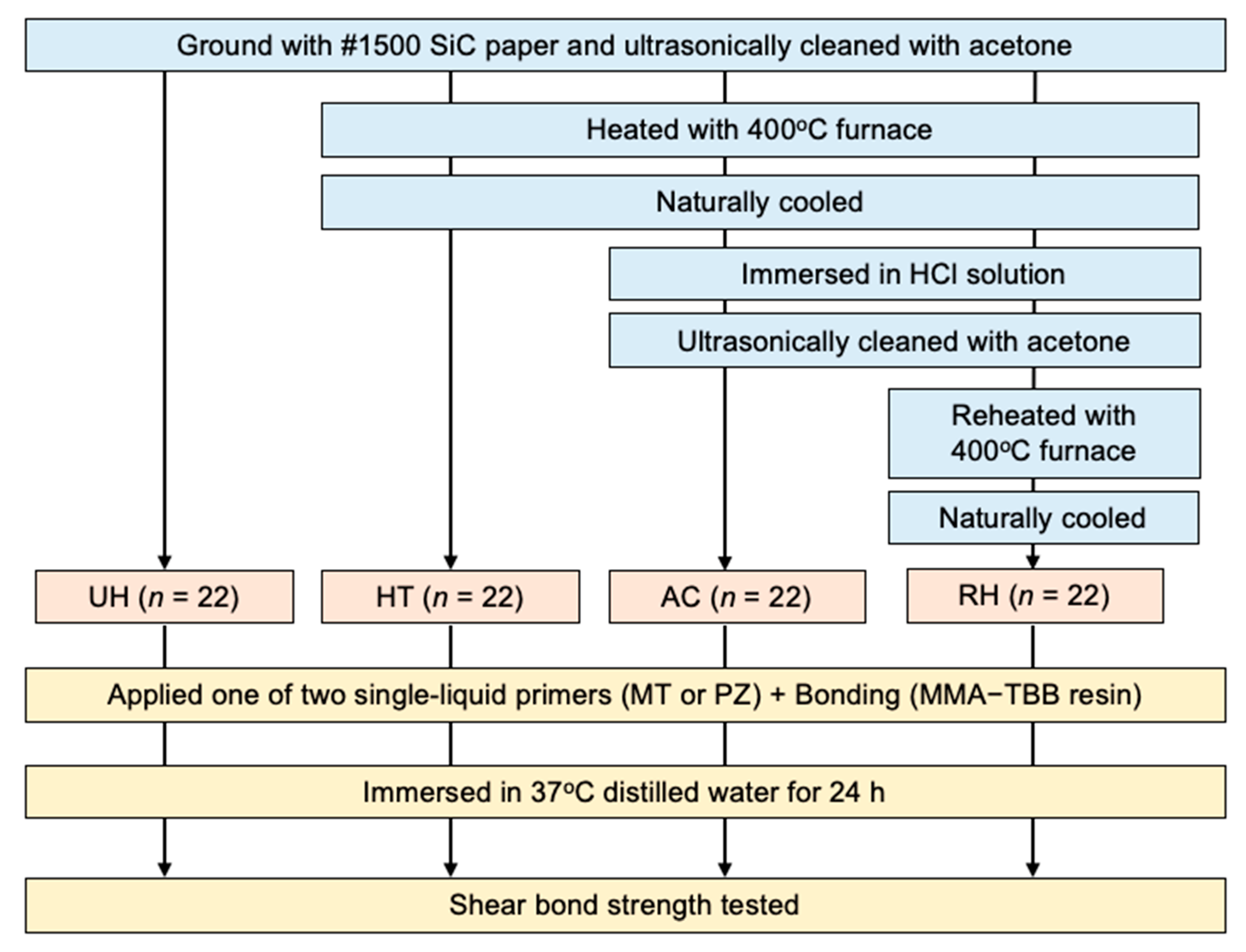
2.2. Preparation of Specimens
2.2.1. Heating
2.2.2. Immersion in HCl Solution
2.3. Surface Roughness Measurements
2.4. Specimen Preparation for Shear-Bond Strength Tests
2.5. Statistical Analysis
2.6. X-Ray Photoelectron Spectroscopy (XPS) Analysis
3. Results
3.1. Surface Roughness
3.2. Shear-Bond Strength Tests
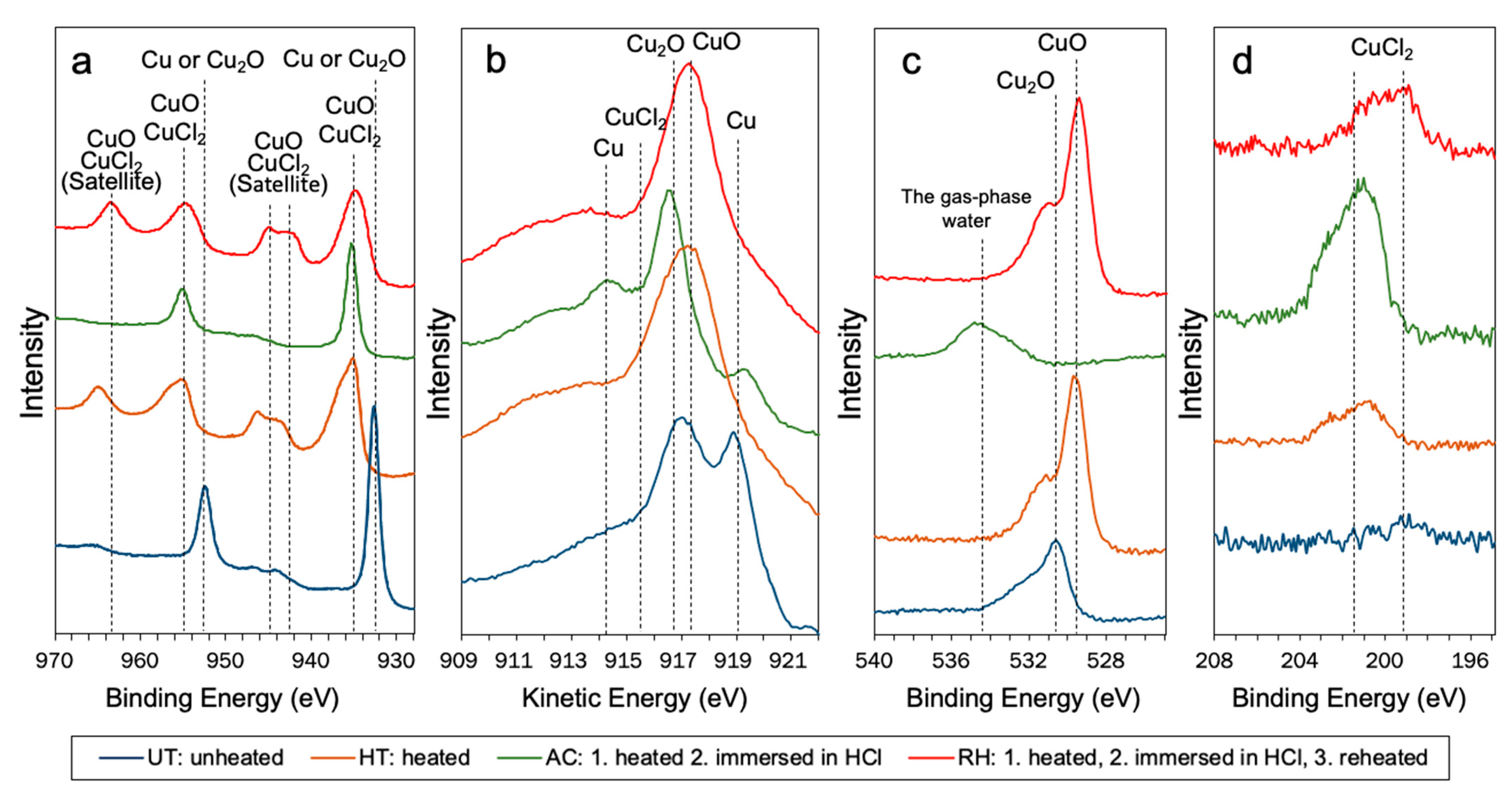
3.3. XPS Analysis
4. Discussion
5. Conclusions
- The unheated copper surface consisted of both Cu and Cu2O because of a gradual change from Cu to Cu2O over time. A copper oxide film (CuO) was formed on the copper surface upon heating and reheating.
- The copper oxide surface significantly increased the effectiveness of MDP to shear-bond strengths, while MTU-6 was significantly reduced. Cu, Cu2O, and CuCl2 was formed on the surface of the copper immersed in a concentrated HCl solution as the oxide film eliminated, remarkably increasing and decreasing the effectiveness of MTU-6 and MDP, respectively, to improve shear-bond strength.
- The results of this study clearly showed that the presence or absence of the oxide film on the metal surface has a strong effect on shear-bond strength using functional monomers.
- A combination with sulfur-containing and acidic functional monomers is assumed to be effective for the noble metal alloys that contain both non-oxidizable noble metals, e.g., Au and Pt, and a copper oxide.
- The present results could complement the previous studies on the adhesive bonding between metals and acrylic resin and suggest that sulfur-containing and acidic functional monomers were better to combine after alumina-based airborne-particle abrasion when bonding copper-containing noble-metal alloys with an acrylic resin.
- The improvement of the bonding strength of noble-metal alloys by a simple method is important in the clinical setting because dentists can make the choice to apply a resin-bonded fixed partial denture to make miniaturization of the cut amount of abutment teeth of patients.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wataha, J.C.; Malcolm, C.T.; Hanks, C.T. Correlation between cytotoxicity and the elements released by dental casting alloys. Int. J. Prosthodont. 1995, 8, 9–14. [Google Scholar]
- Elshahawy, W.; Watanabe, I. Biocompatibility of dental alloys used in dental fixed prosthodontics. Tanta Dent. J. 2014, 11, 150–159. [Google Scholar] [CrossRef]
- Hanawa, T. Overview of metals and applications. In Metals for Biomedical Devices, 2nd ed.; Niinomi, M., Ed.; Woodhead Publishing: Cambridge, UK, 2019; pp. 3–29. [Google Scholar] [CrossRef]
- Lupu, A. Thermogravimetry of copper and copper oxides (Cu2O-CuO). J. Therm. Anal. Calorim. 1970, 2, 445–458. [Google Scholar] [CrossRef]
- Wagner, C. Oxidation of Alloys Involving Noble Metals. J. Electrochem. Soc. 1956, 103, 571–580. [Google Scholar] [CrossRef]
- Meijering, J.L. Internal Oxidation and Related Phenomena in Alloys. Angew. Chem. Int. Ed. 1969, 8, 775. [Google Scholar] [CrossRef]
- Taira, Y.; Matsumura, H.; Yoshida, K.; Tanaka, T.; Atsuta, M. Adhesive bonding of titanium with a methacrylate?phosphate primer and self-curing adhesive resins. J. Oral Rehabil. 1995, 22, 409–412. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Atsuta, M.; Nakabayashi, N.; Masuhara, E. Surface treatment of gold alloys for adhesion. J. Prosthet. Dent. 1988, 60, 271–279. [Google Scholar] [CrossRef]
- Matsumura, H.; Kawahara, M.; Tanaka, T.; Atsuta, M. Surface preparations for metal frameworks of composite resin veneered prostheses made with an adhesive opaque resin. J. Prosthet. Dent. 1991, 66, 10–15. [Google Scholar] [CrossRef]
- Miyahara, H.; Ikeda, H.; Anggraini, S.A.; Fujio, Y.; Yoshii, S.; Nagamatsu, Y.; Kitamura, C.; Shimizu, H. Adhesive bonding of alumina air-abraded Ag-Pd-Cu-Au alloy with 10-methacryloyloxydecyl dihydrogen phosphate. Dent. Mater. J. 2020, 39, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Yamamoto, M.; Fujishima, A.; Miyazaki, T.; Hisamitsu, H.; Kojima, K.; Kadoma, Y. Raman and IR studies on adsorption behavior of adhesive monomers in a metal primer for Au, Ag, Cu, and Cr surfaces. J. Biomed. Mater. Res. 2002, 62, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Nakamura, Y. Study on triazine thiols. V. Polymerization of 6-(4-vinylbenzyl propyl) amino-1,3,5-triazine-2,4-dithiol on copper plates and their corrosion resistance. J. Polym. Sci. Polym. Lett. 1983, 21, 889–895. [Google Scholar] [CrossRef]
- Silikas, N.; Wincott, P.; Vaughan, D.; Watts, D.; Eliades, G. Surface characterization of precious alloys treated with thione metal primers. Dent. Mater. 2007, 23, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Hiraba, H.; Koizumi, H.; Nogawa, H.; Kodaira, A.; Okamura, K.; Matsumura, H. Trace of organic sulfur compounds detected from debonded interface between transparent acrylic resin and gold alloy. J. Oral Sci. 2017, 59, 511–517. [Google Scholar] [CrossRef][Green Version]
- Yamashita, M.; Koizumi, H.; Ishii, T.; Nakayama, D.; Oba, Y.; Matsumura, H. Adhesive performance of silver-palladium-copper-gold alloy and component metals bonded with organic sulfur-based priming agents and a tri-n-butylborane initiated luting material. Acta Odontol. Scand. 2012, 71, 196–204. [Google Scholar] [CrossRef]
- Imai, H.; Koizumi, H.; Shimoe, S.; Hirata, I.; Matsumura, H.; Nikawa, H. Effect of thione primers on adhesive bonding between an indirect composite material and Ag-Pd-Cu-Au alloy. Dent. Mater. J. 2014, 33, 681–688. [Google Scholar] [CrossRef]
- Hiraba, H.; Nogawa, H.; Koizumi, H.; Kodaira, A.; Akahane, S. Effect of multi-purpose primers on the bond durability between tri-n-butylborane initiated resin and gold alloy. J. Prosthodont. Res. 2019, 63, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Hiraba, H.; Koizumi, H.; Kodaira, A.; Nogawa, H.; Yoneyama, T.; Matsumura, H. Influence of Oxidation of Copper on Shear Bond Strength to an Acrylic Resin Using an Organic Sulfur Compound. Materials 2020, 13, 2092. [Google Scholar] [CrossRef]
- Taira, Y.; Kamada, K.; Atsuta, M. Effects of primers containing thiouracil and phosphate monomers on bonding of resin to Ag-Pd-Au alloy. Dent. Mater. J. 2008, 27, 69–74. [Google Scholar] [CrossRef][Green Version]
- Miyahara, H.; Ikeda, H.; Fujio, Y.; Yoshii, S.; Nagamatsu, Y.; Kitamura, C.; Shimizu, H. Chemical alteration of Ag-Pd-Cu-Au alloy surface by alumina air-abrasion and its effect on bonding to resin cement. Dent. Mater. J. 2019, 38, 630–637. [Google Scholar] [CrossRef]
- Ghijsen, J.; Tjeng, L.H.; Van Elp, J.; Eskes, H.; Westerink, J.; Sawatzky, G.A.; Czyzyk, M.T. Electronic structure ofCu2O and CuO. Phys. Rev. B Condens. Matter. 1988, 38, 11322–11330. [Google Scholar] [CrossRef]
- Poulston, S.; Parlett, P.M.; Stone, P.; Bowker, M. Surface oxidation and reduction of CuO and Cu2O studied using XPS and XAES. Surf. Interface Anal. 1996, 24, 811–820. [Google Scholar] [CrossRef]
- Biesinger, M.C. Advanced analysis of copper X-ray photoelectron spectra. Surf. Interface Anal. 2017, 49, 1325–1334. [Google Scholar] [CrossRef]
- Kaushik, V.K. Identification of oxidation states of copper in mixed oxides and chlorides using ESCA. Spectrochim. Acta Part B At. Spectrosc. 1989, 44, 581–587. [Google Scholar] [CrossRef]
- Yamamoto, S.; Bluhm, H.; Andersson, K.; Ketteler, G.; Ogasawara, H.; Salmeron, M.; Nilsson, A. In situx-ray photoelectron spectroscopy studies of water on metals and oxides at ambient conditions. J. Phys. Condens. Matter 2008, 20, 184025. [Google Scholar] [CrossRef]
- Srivastava, S.; Badrinarayanan, S.; Mukhedkar, A. X-ray photoelectron spectra of metal complexes of substituted 2,4-pentanediones. Polyhedron 1985, 4, 409–414. [Google Scholar] [CrossRef]
- Wataha, J.C. Biocompatibility of dental casting alloys: A review. J. Prosthet. Dent. 2000, 83, 223–234. [Google Scholar] [CrossRef]
- Schön, G. ESCA studies of Cu, Cu2O and CuO. Surf. Sci. 1973, 35, 96–108. [Google Scholar] [CrossRef]
- Tran, M.-P.; Gonzalez-Aguirre, P.; Beitia, C.; Lundgren, J.; Moon, S.-I.; Fontaine, H. Deposition of hydrogen chloride gas on copper wafer depending on humidity and HCl concentration. Microelectron. Eng. 2019, 207, 1–6. [Google Scholar] [CrossRef]


| Material/Trade Name | Manufacturer | Lot | Composition |
|---|---|---|---|
| Element metal | - | - | - |
| Copper metal | Nilaco Corp., Tokyo, Japan | 44225602 | Cu 99.9, mass% |
| Primer | - | - | - |
| Metaltite | Tokuyama Dental Corp., Tokyo, Japan | 0382 | MTU-6, Ethanol |
| Super-Bond PZ Primer (Liquid A) | Sun Medical Co., Ltd., Moriyama, Japan | SM1 | MDP, MMA |
| Luting material | - | - | - |
| Super-Bond C&B Catalyst V | Sun Medical Co., Ltd. | SX11 | TBB, TBB-O, hydrocarbon |
| Super-Bond C&B Opaque Ivory Powder | Sun Medical Co., Ltd. | RM1 | PMMA, titanium oxide |
| Methyl methacrylate | Tokyo Chemical Industry Co., Ltd., Tokyo, Japan | ZJ3WJIJ | MMA, 99.8% |
| Treatment | Median | IQR |
|---|---|---|
| UH | 0.62 a | 0.006 |
| HT | 0.55 b | 0.009 |
| AC | 0.52 c | 0.005 |
| RH | 0.54 b | 0.006 |
| Treatment | MTU-6 (MT Group) | MDP (PZ Group) | M-U | p | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Median | IQR | A | CA | Median | IQR | A | CA | |||
| UH | 27.6 a | 2.0 | 0 | 11 | 25.8 c | 1.4 | 0 | 11 | S | 0.038 |
| HT | 10.1 b | 4.3 | 11 | 0 | 26.1 c | 3.1 | 0 | 11 | S | <0.0001 |
| AC | 27.3 a | 2.6 | 0 | 11 | 3.5 d | 0.7 | 11 | 0 | S | <0.0001 |
| RH | 7.0 b | 2.0 | 11 | 0 | 23.5 e | 3.1 | 0 | 11 | S | <0.0001 |
| Element | Peak Energy (eV) | Peak Assignment (Compound) | Reference |
|---|---|---|---|
| Cu 2p3/2 | 932.6 | Cu2O or Cu | [21,22,23] |
| Cu 2p1/2 | 952.2 | Cu2O or Cu | [21,22,23] |
| Cu 2p3/2 | 935.1 | CuO | [21,22,23] |
| Cu 2p3/2 | 934.9 | CuCl2 | [21,22,23] |
| Cu 2p3/2 | 943.4, 943.6 | CuO, CuCl2 satellite peaks | [21,22,23] |
| Cu 2p1/2 | 955.3, 954.7 | CuO | [21,22,23] |
| Cu 2p1/2 | 964.9, 963.6 | CuO, CuCl2 satellite peaks | [21,22,23] |
| Cu LMM | 915.4 | CuCl2 | [21,22,23] |
| Cu LMM | 916.6 | Cu2O | [23,24] |
| Cu LMM | 917.5 | CuO | [23,24] |
| Cu LMM | 914.3, 918.5 | Cu | [23,24] |
| O 1s | 529.7 | CuO | [23,24] |
| O 1s | 530.6 | Cu2O | [23,24] |
| O 1s | 534.7 | The gas-phase water | [25] |
| Cl 2p3/2 | 199.2, 201.6 | CuCl2 | [23,24,26] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hiraba, H.; Koizumi, H.; Kodaira, A.; Takehana, K.; Yoneyama, T.; Matsumura, H. Effects of Copper Surface Oxidation and Reduction on Shear-Bond Strength Using Functional Monomers. Materials 2021, 14, 1753. https://doi.org/10.3390/ma14071753
Hiraba H, Koizumi H, Kodaira A, Takehana K, Yoneyama T, Matsumura H. Effects of Copper Surface Oxidation and Reduction on Shear-Bond Strength Using Functional Monomers. Materials. 2021; 14(7):1753. https://doi.org/10.3390/ma14071753
Chicago/Turabian StyleHiraba, Haruto, Hiroyasu Koizumi, Akihisa Kodaira, Kosuke Takehana, Takayuki Yoneyama, and Hideo Matsumura. 2021. "Effects of Copper Surface Oxidation and Reduction on Shear-Bond Strength Using Functional Monomers" Materials 14, no. 7: 1753. https://doi.org/10.3390/ma14071753
APA StyleHiraba, H., Koizumi, H., Kodaira, A., Takehana, K., Yoneyama, T., & Matsumura, H. (2021). Effects of Copper Surface Oxidation and Reduction on Shear-Bond Strength Using Functional Monomers. Materials, 14(7), 1753. https://doi.org/10.3390/ma14071753







