Improved Hydrogenation Kinetics of TiMn1.52 Alloy Coated with Palladium through Electroless Deposition
Abstract
:1. Introduction
2. Experimental Methods
2.1. Materials
2.2. Surface Modification of Alloy
2.3. Characterisation Techniques
3. Results and Discussion
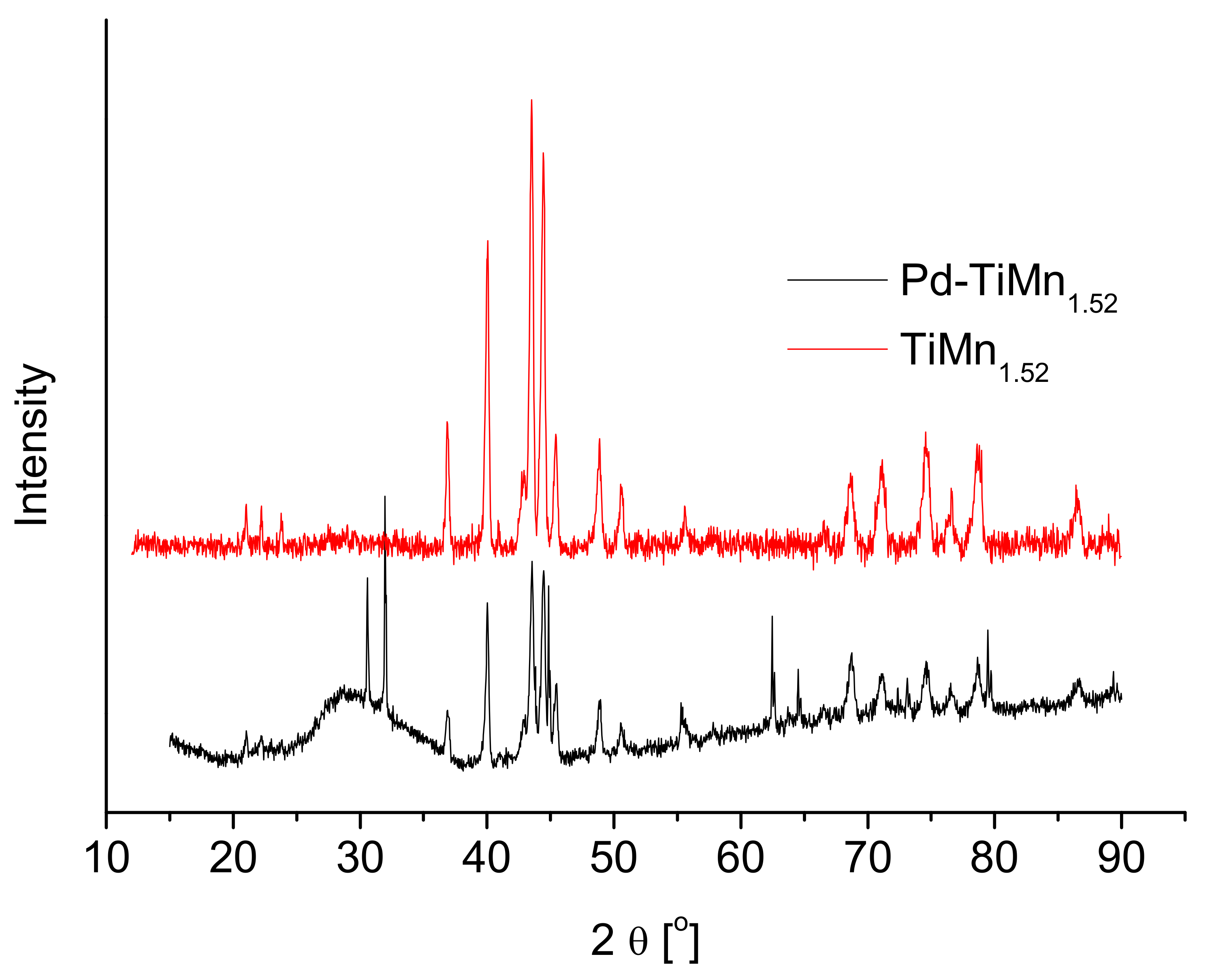
3.1. Structural Characterisation
3.2. Morphological and Elemental Characterisations
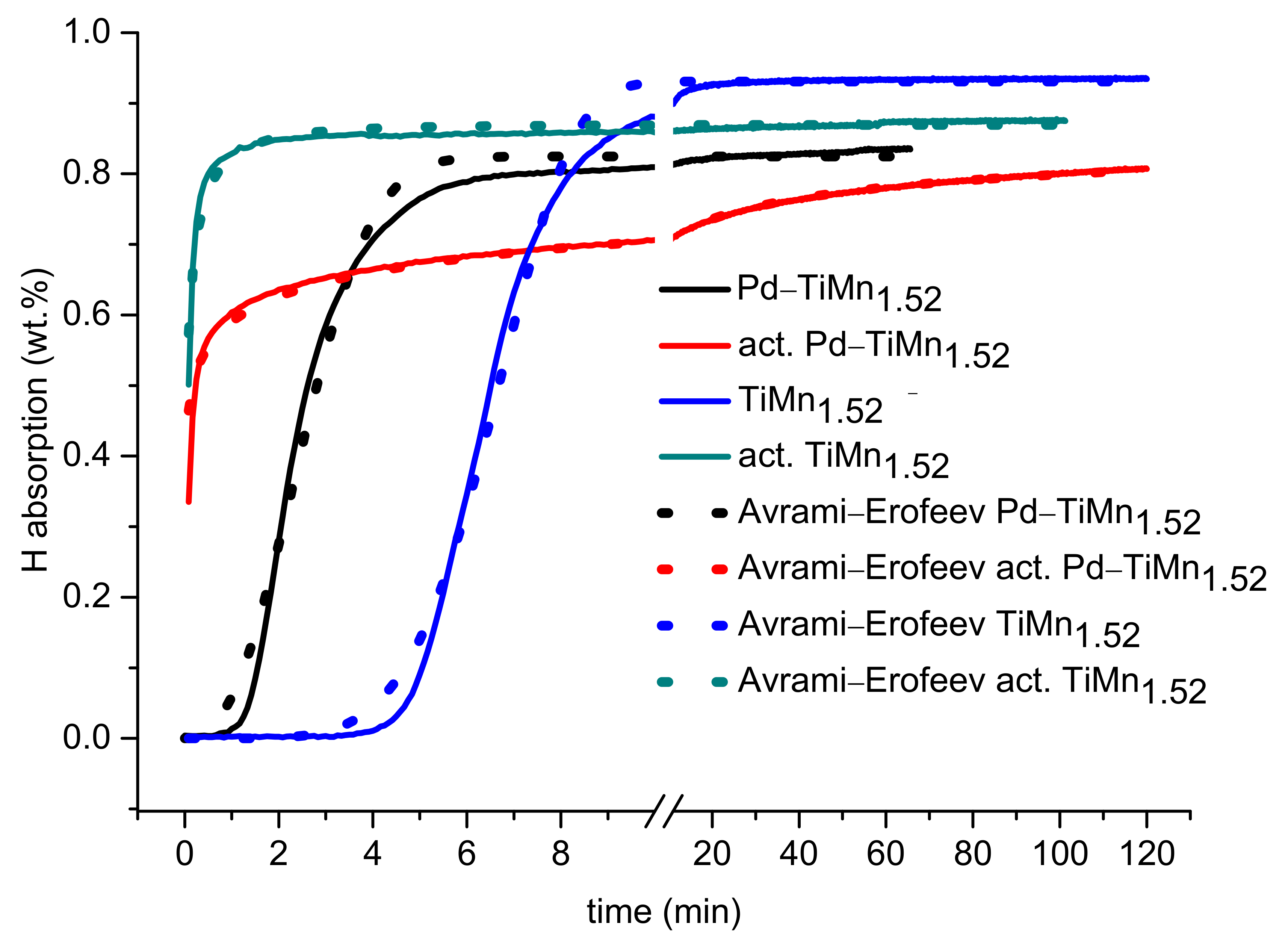
3.3. Hydrogen Absorption Kinetics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, P.; Xie, X.; Xu, L.; Li, X.; Liu, T. Hydrogen storage properties of (Ti 0.85 Zr 0.15) 1.05 Mn 1.2 Cr 0.6 V 0.1 M 0.1 (M=Ni, Fe, Cu) alloys easily activated at room temperature. Prog. Nat. Sci. Mater. Int. 2017, 27, 652–657. [Google Scholar] [CrossRef]
- Somo, T.R.; Maponya, T.C.; Davids, M.W.; Hato, M.J.; Lototskyy, M.V.; Modibane, K.D. A comprehensive review on hydrogen absorption behaviour of metal alloys prepared through mechanical alloying. Metals 2020, 10, 562. [Google Scholar] [CrossRef]
- El-eskandarany, M.S.; Al-Ajmi, F.; Banyan, M.; Al-duweesh, A. Synergic effect of reactive ball milling and cold pressing on enhancing the hydrogen storage behavior of nanocomposite. Int. J. Hydrog. Energy 2019, 44, 26428–26443. [Google Scholar] [CrossRef]
- Park, C. Volume expansion of TiMn2-type hydrogen storage alloy with hydrogenation. Trans. Korean Hydrog. New Energy Soc. 2017, 28, 459–464. [Google Scholar]
- Xu, S.-D.; Fang, L.; Ding, X.-L. Effect of structural factors on the hydrogen storage capacity of nonstoichiometric TiMnx alloys. Acta Physicochim. Sin. 2016, 32, 780–786. [Google Scholar] [CrossRef]
- Zhu, J.; Dai, L.; Yu, Y.; Cao, J.; Wang, L. A direct electrochemical route from oxides to TiMn2 hydrogen storage alloy. Chin. J. Chem. Eng. 2015, 23, 1865–1870. [Google Scholar] [CrossRef]
- Davids, M.; Martin, T.; Lototskyy, M.; Denys, R.; Yartys, V. Study of hydrogen storage properties of oxygen modified Ti- based AB2 type metal hydride alloy. Int. J. Hydrog. Energy 2020, 5, 215. [Google Scholar] [CrossRef]
- Hu, T.; Huang, X.; Li, J.; Zhang, J.; Cai, G.; Jin, Z. Experimental investigation and thermodynamic calculation of Ti-Hf-Mn system. Calphad Comput. Coupling Phase Diagr. Thermochem. 2020, 70, 101776. [Google Scholar] [CrossRef]
- Cui, R.; Yang, C.; Li, M.; Jin, B.; Ding, X.; Jiang, Q. Enhanced high-rate performance of ball-milled MmNi3.55Co0.75Mn0.4Al0.3 hydrogen storage alloys with graphene nanoplatelets. J. Alloys Compd. 2017, 693, 126–131. [Google Scholar] [CrossRef]
- Dymek, M.; Nowak, M.; Jurczyk, M.; Bala, H. Encapsulation of La1.5Mg0.5Ni7 nanocrystalline hydrogen storage alloy with Ni coatings and its electrochemical characterization. J. Alloys Compd. 2018, 749, 534–542. [Google Scholar] [CrossRef]
- Somo, T.R.; Mabokela, T.E.; Teffu, D.M.; Sekgobela, T.K.; Hato, M.J.; Modibane, K.D. Review on the effect of metal oxides as surface coatings on hydrogen storage properties of porous and non-porous materials. Chem. Pap. 2021, 10, 1–15. [Google Scholar] [CrossRef]
- Eftekhari, A.; Fang, B. Electrochemical hydrogen storage: Opportunities for fuel storage, batteries, fuel cells, and supercapacitors. Int. J. Hydrog. Energy 2017, 42, 25143–25165. [Google Scholar] [CrossRef]
- Williams, M.; Lototsky, M.; Davids, M.; Linkov, V.; Yartys, V.; Solberg, J. Chemical surface modification for the improvement of the hydrogenation kinetics and poisoning resistance of TiFe. J. Alloys Compd. 2011, 509, S770–S774. [Google Scholar] [CrossRef]
- Bratanich, T.I.; Bulanov, V.N.; Skorokhod, V.V.; Klimenko, V.P. Reversable hydriding of LaNi5−xAlx−Pd composite in the presence of carbon monoxide. Powder Metall. Met. Ceram. 2001, 39, 575–583. [Google Scholar] [CrossRef]
- Zaluski, L.; Zaluska, A.; Tessier, P.Y.; Ström-Olsen, J.; Schulz, R. Catalytic effect of Pd on hydrogen absorption in mechanically alloyed Mg2Ni, LaNi5 and FeTi. J. Alloys Compd. 1995, 217, 295–300. [Google Scholar] [CrossRef]
- Uchida, H.; Wulz, H.-G.; Fromm, E. Catalytic effect of nickel, iron and palladium on hydriding titanium and storage materials. J. Less Common Met. 1991, 172–174, 1076–1083. [Google Scholar] [CrossRef]
- Williams, M.; Lototsky, M.; Nechaev, A.; Yartys, V.; Solberg, J.K.; Denys, R.V.; Linkov, V.M. Palladium mixed-metal surface-modified AB5-type intermetallides enhance hydrogen sorption kinetics. South Afr. J. Sci. 2010, 106, 6. [Google Scholar] [CrossRef] [Green Version]
- Modibane, K.; Lototskyy, M.; Davids, M.; Williams, M.; Hato, M.; Molapo, K. Influence of co-milling with palladium black on hydrogen sorption performance and poisoning tolerance of surface modified AB5-type hydrogen storage alloy. J. Alloys Compd. 2018, 750, 523–529. [Google Scholar] [CrossRef]
- Dekhtyarenko, V.; Pryadko, T.; Savvakin, D.; Bondarchuk, V.; Mogylnyy, G. Hydrogenation process in multiphase alloys of Ti-Zr-Mn-V system on the example of Ti42.75Zr27Mn20.25V10 alloy. Int. J. Hydrog. Energy 2021, 46, 8040–8047. [Google Scholar] [CrossRef]
- Ha, T.; Lee, S.-I.; Hong, J.; Lee, Y.-S.; Kim, D.-I.; Suh, J.-Y.; Cho, Y.W.; Hwang, B.; Lee, J.; Shim, J.-H. Hydrogen storage behavior and microstructural feature of a TiFe–ZrCr2 alloy. J. Alloys Compd. 2021, 853, 157099. [Google Scholar] [CrossRef]
- Sarswat, P.K.; Sarkar, S.; Cho, J.; Bhattacharyya, D.; Free, M.L. Structural and electrical irregularities caused by selected dopants in black-phosphorus. ECS J. Solid State Sci. Technol. 2016, 5, Q3026–Q3032. [Google Scholar] [CrossRef]
- Sheikh, A.M.; Silva, E.L.; Abellah, M.Y. Pd-based catalysts for ethanol oxidation in alkaline electrolyte. Am. J. Min. Metall. 2014, 2, 64–69. [Google Scholar] [CrossRef]
- Davids, M.W.; Lototskyy, M.; Nechaev, A.; Naidoo, Q.; Williams, M.; Klochko, Y. Surface modification of TiFe hydrogen storage alloy by metal-organic chemical vapour deposition of palladium. Int. J. Hydrog. Energy 2011, 36, 9743–9750. [Google Scholar] [CrossRef]
- Li, B.; Kado, S.; Mukainakano, Y.; Miyazawa, T.; Miyao, T.; Naito, S.; Okumura, K.; Kunimori, K.; Tomishige, K. Surface modification of Ni catalysts with trace Pt for oxidative steam reforming of methane. J. Catal. 2007, 245, 144–155. [Google Scholar] [CrossRef]

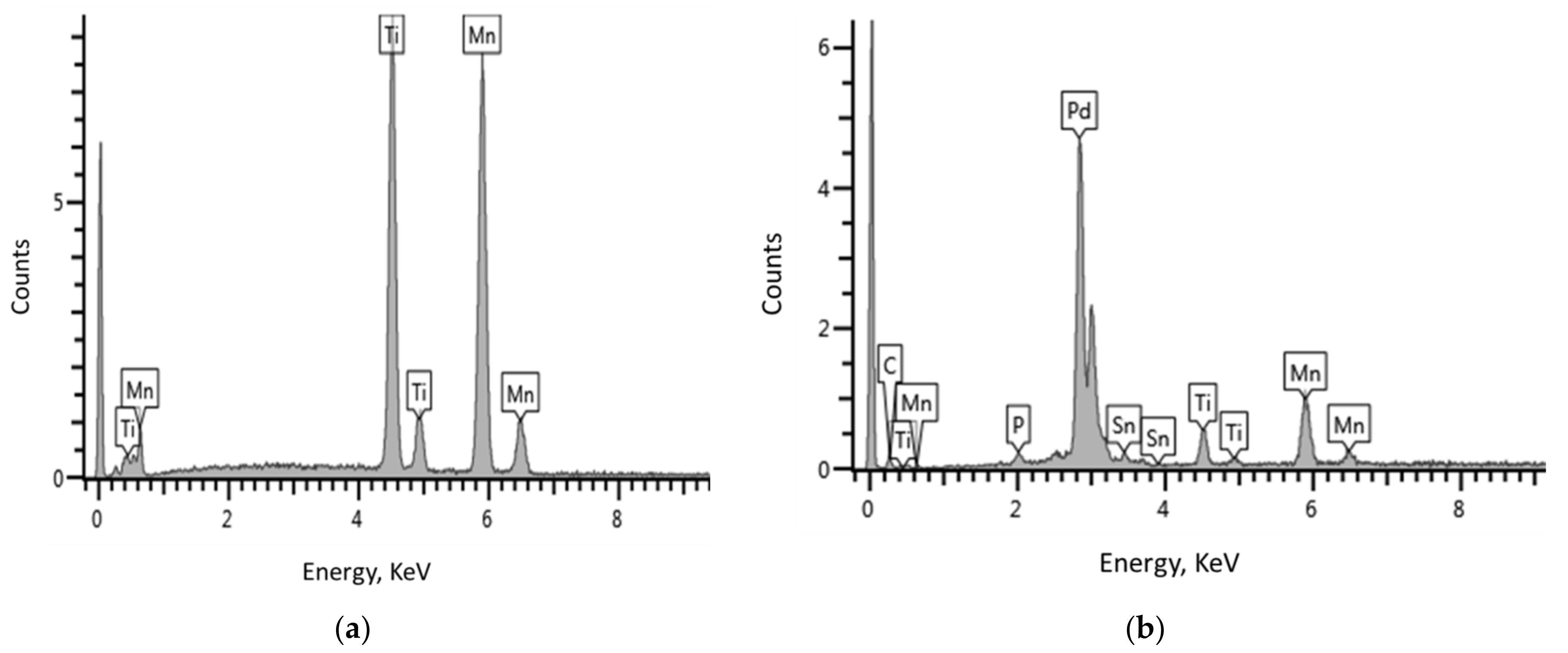
| Material | Element | EDS Data | Target Composition | |
|---|---|---|---|---|
| Net Counts | Wt.% | Wt.% | ||
| TiMn1.52 | Ti | 10.25 | 37.00 | 36.44 |
| Mn | 9.7 | 63.00 | 63.56 | |
| Total | - | 100 | 100 | |
| Material | Rate Constant, k (min−1) | Index of Power, n | ||||
|---|---|---|---|---|---|---|
| State | Non-activated | Activated | Non-activated | Activated | Non-activated | Activated |
| TiMn1.52 | 0.933 | 0.869 | 0.438 | 1.05 | 1.37 | 0.50 |
| Pd–TiMn1.52 | 0.827 | 0.779 | 0.771 | 13.2 | 0.925 | 0.50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Somo, T.R.; Davids, M.W.; Lototskyy, M.V.; Hato, M.J.; Modibane, K.D. Improved Hydrogenation Kinetics of TiMn1.52 Alloy Coated with Palladium through Electroless Deposition. Materials 2021, 14, 1833. https://doi.org/10.3390/ma14081833
Somo TR, Davids MW, Lototskyy MV, Hato MJ, Modibane KD. Improved Hydrogenation Kinetics of TiMn1.52 Alloy Coated with Palladium through Electroless Deposition. Materials. 2021; 14(8):1833. https://doi.org/10.3390/ma14081833
Chicago/Turabian StyleSomo, Thabang R., Moegamat W. Davids, Mykhaylo V. Lototskyy, Mpitloane J. Hato, and Kwena D. Modibane. 2021. "Improved Hydrogenation Kinetics of TiMn1.52 Alloy Coated with Palladium through Electroless Deposition" Materials 14, no. 8: 1833. https://doi.org/10.3390/ma14081833






