Tacky-Free Polyurethanes Pressure-Sensitive Adhesives by Molecular-Weight and HDI Trimer Design
Abstract
:1. Introduction
2. Experimental
2.1. Materials
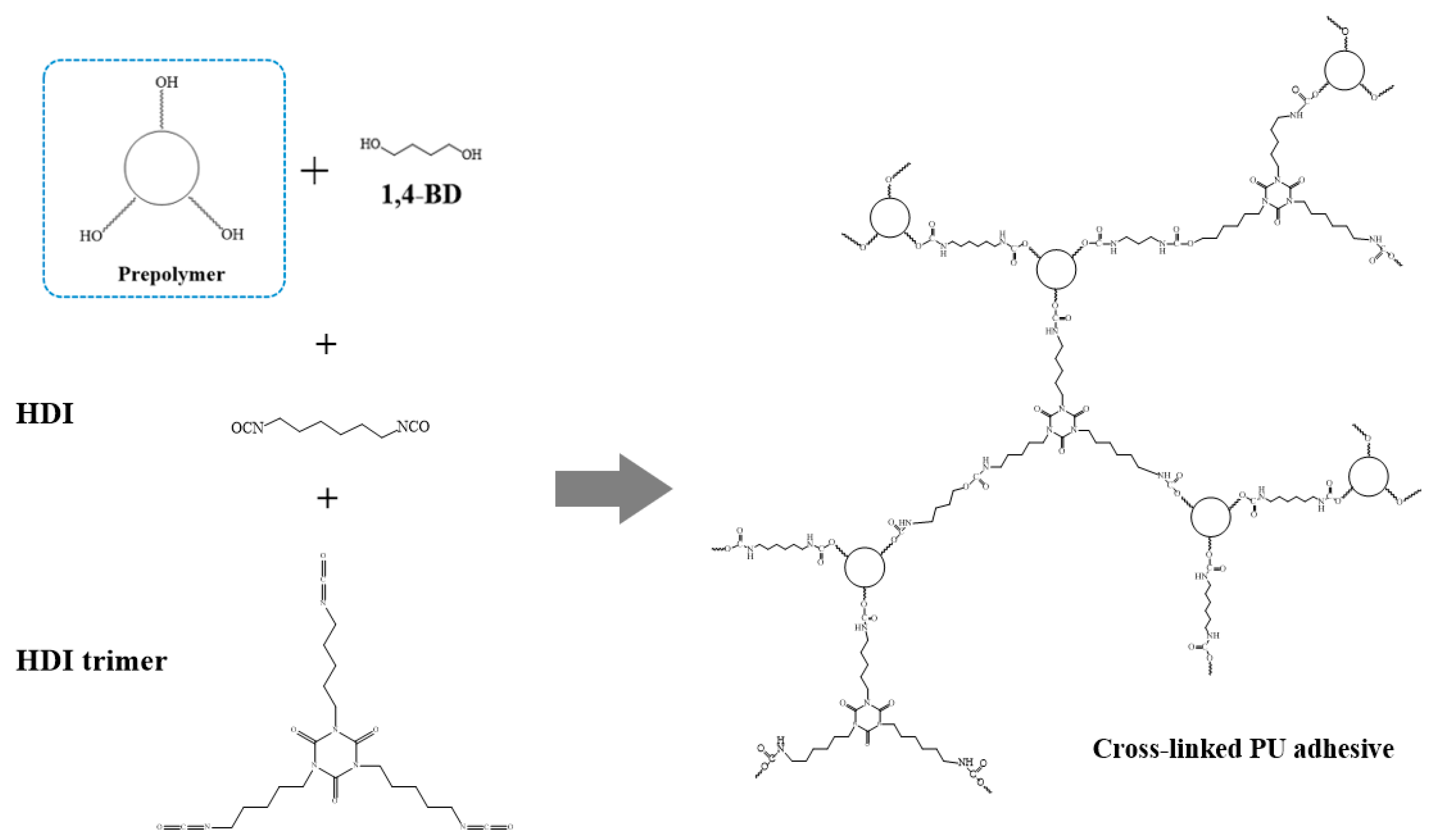
2.2. Synthesis of Polyurethane Pressure-Sensitive Adhesive (PU-PSA)
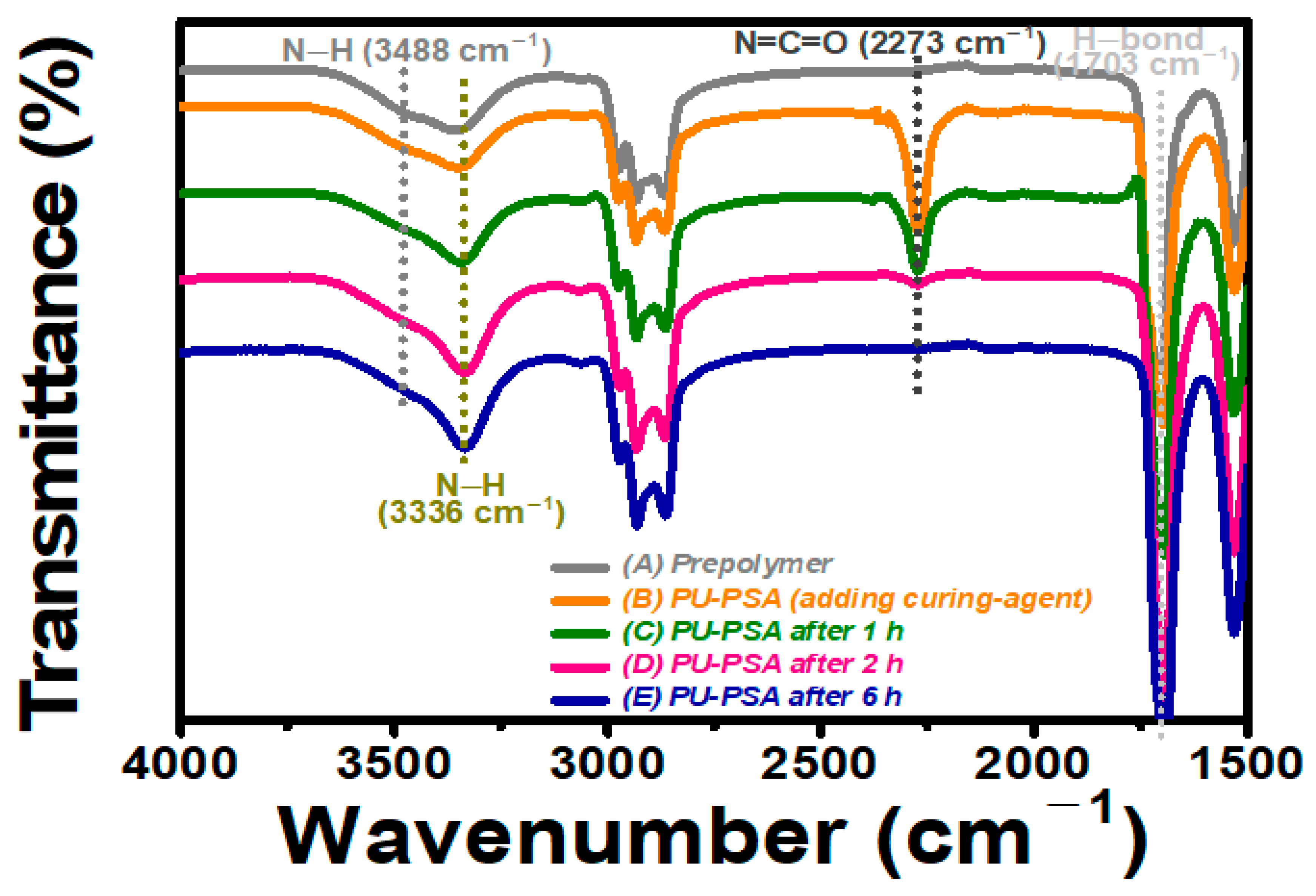
2.3. Characterization
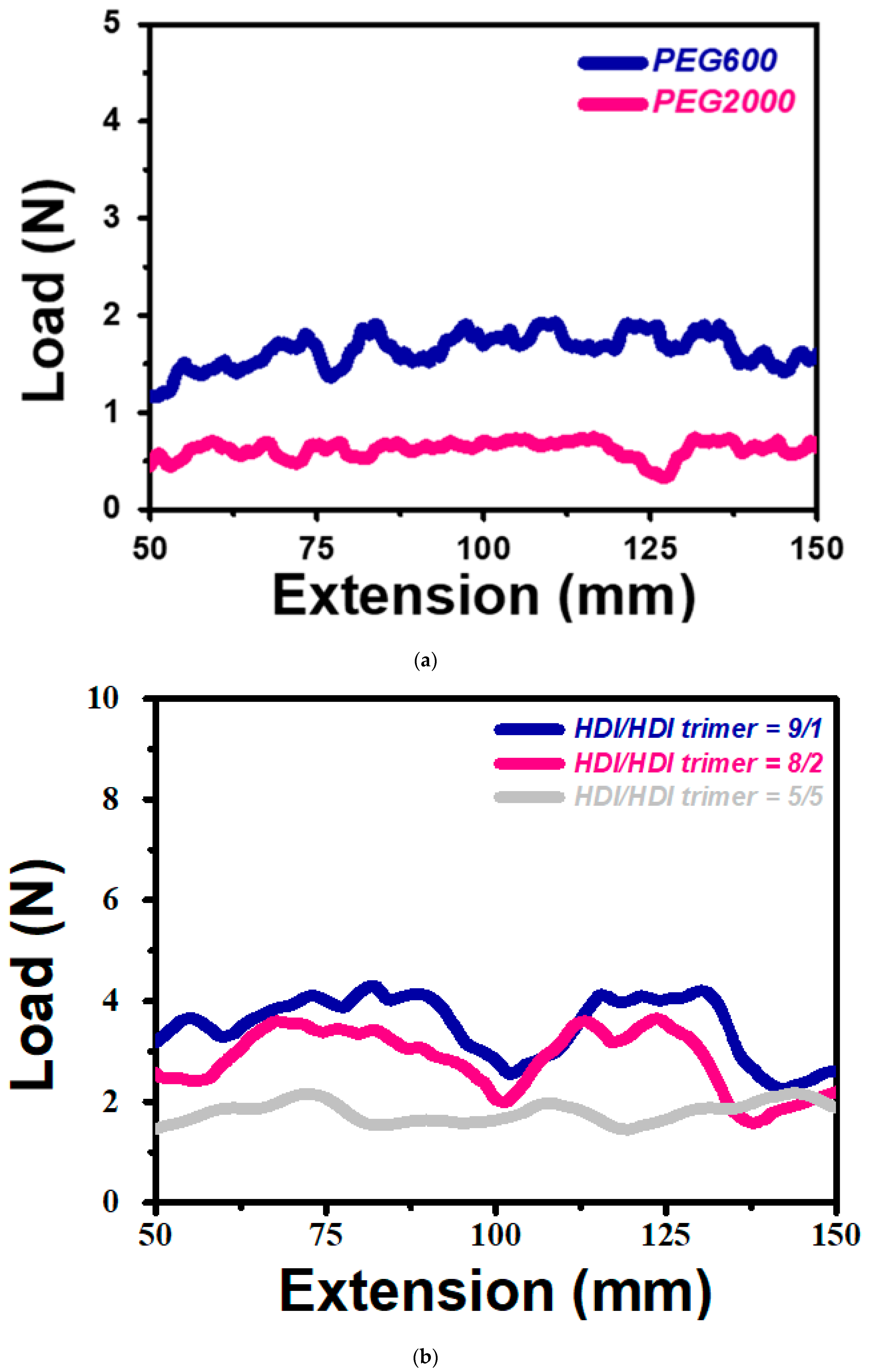
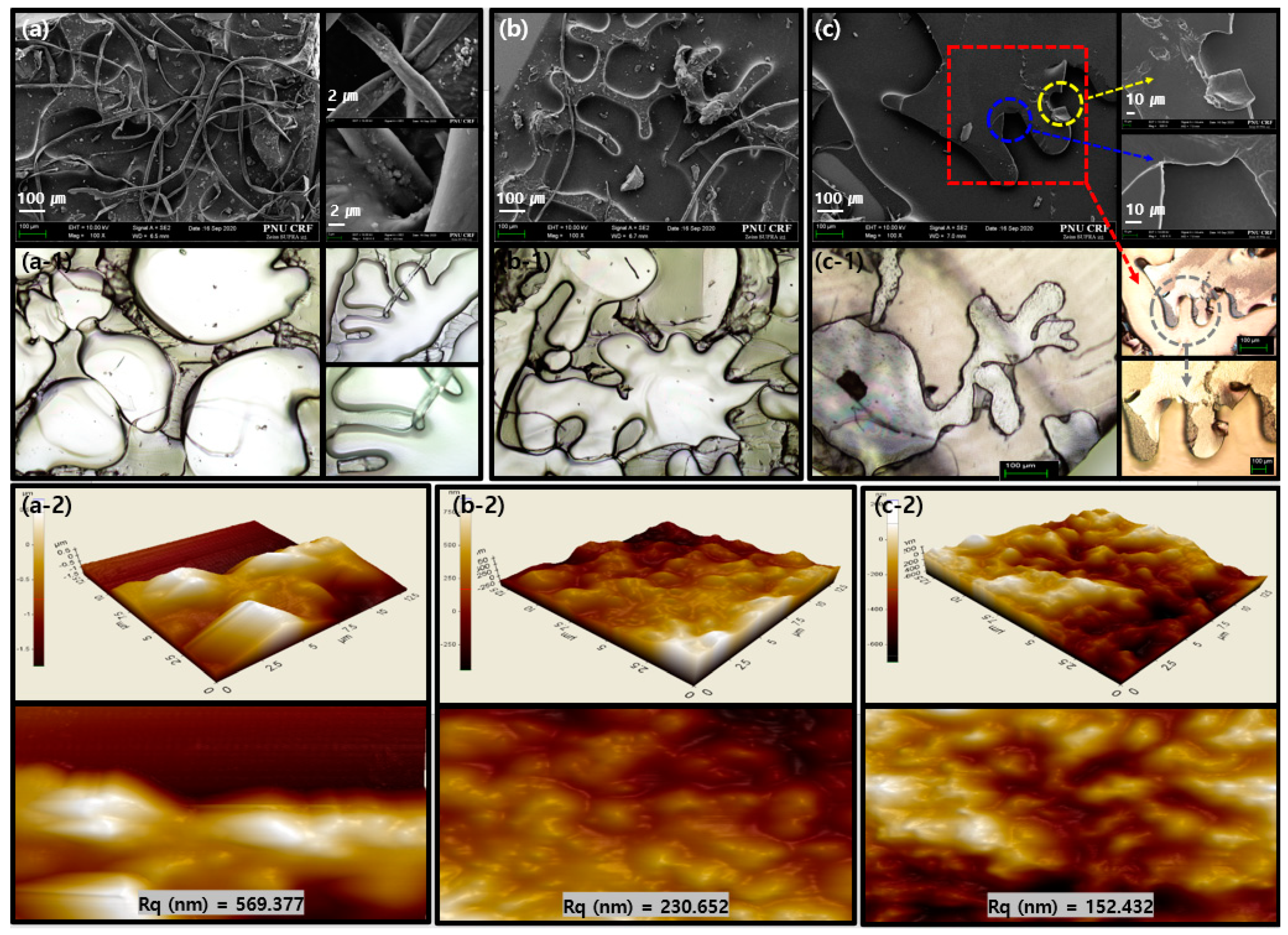
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feldstein, M.M.; Dormidontova, E.E.; Khokhlov, A.R. Pressure sensitive adhesives based on interpolymer complexes. Prog. Polym. Sci. 2015, 42, 79–153. [Google Scholar] [CrossRef]
- Sun, S.; Li, M.; Liu, A. A review on mechanical properties of pressure sensitive adhesives. Int. J. Adhes. Adhes. 2013, 41, 98–106. [Google Scholar] [CrossRef]
- Ho, K.Y.; Dodou, K. Rheological studies on pressure-sensitive silicone adhesives and drug-in-adhesive layers as a means to characterise adhesive performance. Int. J. Pharm. 2007, 333, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Feldstein, M.M.; Siegel, R.A. Molecular and nanoscale factors governing pressure-sensitive adhesion strength of viscoelastic polymers. J. Polym. Sci. Part B Polym. Phys. 2012, 50, 739–772. [Google Scholar] [CrossRef]
- Taghizadeh, S.M.; Ghasemi, D. Rheological and adhesion properties of acrylic pressure_sensitive adhesives. J. Appl. Polym. Sci. 2011, 120, 411–418. [Google Scholar] [CrossRef]
- Czech, Z. Solvent-based pressure-sensitive adhesives for removable products. Int. J. Adhes. Adhes. 2006, 26, 414–418. [Google Scholar] [CrossRef]
- Satas, D.; Satas, A.M. Hospital and first aid products. In Handbook of Pressure Sensitive Adhesive Technology; Satas, D., Ed.; Van Nostrand Reinhold: New York, NY, USA, 1989; pp. 627–642. [Google Scholar]
- Jang, S.J.; Baek, S.S.; Kim, J.Y.; Hwang, S.H. Preparation and adhesion performance of transparent acrylic pressure sensitive adhesives for touch screen panel. J. Adhes. Sci.Technol. 2014, 28, 1990–2000. [Google Scholar] [CrossRef]
- Fuensanta, M.; Martín-Martínez, J.M. Thermoplastic polyurethane coatings made with mixtures of polyethers of different molecular weights with pressure sensitive adhesion property. Prog. Org. Coat. 2018, 118, 148–156. [Google Scholar] [CrossRef] [Green Version]
- Fuensanta, M.; Martín-Martínez, J.M. Thermoplastic polyurethane pressure sensitive adhesives made with mixtures of polypropylene glycols of different molecular weights. Int. J. Adhes. Adhes. 2019, 88, 81–90. [Google Scholar] [CrossRef]
- Akram, N.; Zia, K.M.; Saeed, M.; Usman, M.; Saleem, S. Impact of macrodiols on the adhesion strength of polyurethane pressure-sensitive adhesives. J. Appl. Polym. Sci. 2018, 135, 46635. [Google Scholar] [CrossRef]
- Tan, C.; Tirri, T.; Wilen, C.E. Investigation on the Influence of Chain Extenders on the Performance of One-Component Moisture-Curable Polyurethane Adhesives. Polymers 2017, 9, 184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, M.; Cao, Z.; Zhou, H.; Xie, Y.; Li, G.; Wang, N.; Liu, Y.; He, L.; Qu, X. Preparation of environmentally friendly acrylic pressure-sensitive adhesives by bulk photopolymerization and their performance. RSC Adv. 2020, 10, 10277–10284. [Google Scholar] [CrossRef]
- Baron, A.; Rodriguez-Hernandez, J.; Ibarboure, E.; Derail, C.; Papon, E. Adhesives based on polyurethane graft multiblock copolymers: Tack, rheology and first morphological analyses. Int. J. Adhes. Adhes. 2009, 29, 1–18. [Google Scholar] [CrossRef]
- Akram, N.; Gurney, R.S.; Zuber, M.; Ishaq, M.; Keddie, J.L. Influence of polyol molecular weight and type on the tack and peel properties of waterborne polyurethane pressure-sensitive adhesives. Macromol. React. Eng. 2013, 7, 493–503. [Google Scholar] [CrossRef]
- Yamamoto, T.; Shibayama, M.; Nomura, S. Structure and Properties of Fatigued Segmented Poly(urethaneurea)s III. Quantitative Analyses of Hydrogen Bond. Polym. J. 1989, 21, 895–903. [Google Scholar] [CrossRef] [Green Version]
- Asefnejad, A.; Khorasani, M.T.; Behnamghader, A.; Farsadzadeh, B.; Bonakdar, S. Manufacturing of biodegradable polyurethane sca_olds based on polycaprolactone using a phase separation method: Physical properties and in vitro assay. Int. J. Nanomed. 2011, 6, 2375–2384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Datta, J.; Kosiorek, P.; Włoch, M. Synthesis, structure and properties of poly(ether urethane)s synthesized using a tri-functional oxypropylated glycerol as a polyol. J. Therm. Anal. Calorim. 2017, 128, 155–167. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bae, J.-H.; Won, J.C.; Lim, W.B.; Kim, B.J.; Lee, J.H.; Min, J.G.; Seo, M.J.; Mo, Y.H.; Huh, P. Tacky-Free Polyurethanes Pressure-Sensitive Adhesives by Molecular-Weight and HDI Trimer Design. Materials 2021, 14, 2164. https://doi.org/10.3390/ma14092164
Bae J-H, Won JC, Lim WB, Kim BJ, Lee JH, Min JG, Seo MJ, Mo YH, Huh P. Tacky-Free Polyurethanes Pressure-Sensitive Adhesives by Molecular-Weight and HDI Trimer Design. Materials. 2021; 14(9):2164. https://doi.org/10.3390/ma14092164
Chicago/Turabian StyleBae, Ji-Hong, Jong Chan Won, Won Bin Lim, Byeong Joo Kim, Ju Hong Lee, Jin Gyu Min, Min Ji Seo, Young Hyun Mo, and PilHo Huh. 2021. "Tacky-Free Polyurethanes Pressure-Sensitive Adhesives by Molecular-Weight and HDI Trimer Design" Materials 14, no. 9: 2164. https://doi.org/10.3390/ma14092164







