PROMETHEUS: A Copper-Based Polymetallic Catalyst for Automotive Applications. Part II: Catalytic Efficiency an Endurance as Compared with Original Catalysts
Abstract
1. Introduction
2. Materials and Methods
2.1. Tested Catalysts
2.2. X-ray Fluorescence Spectroscopy
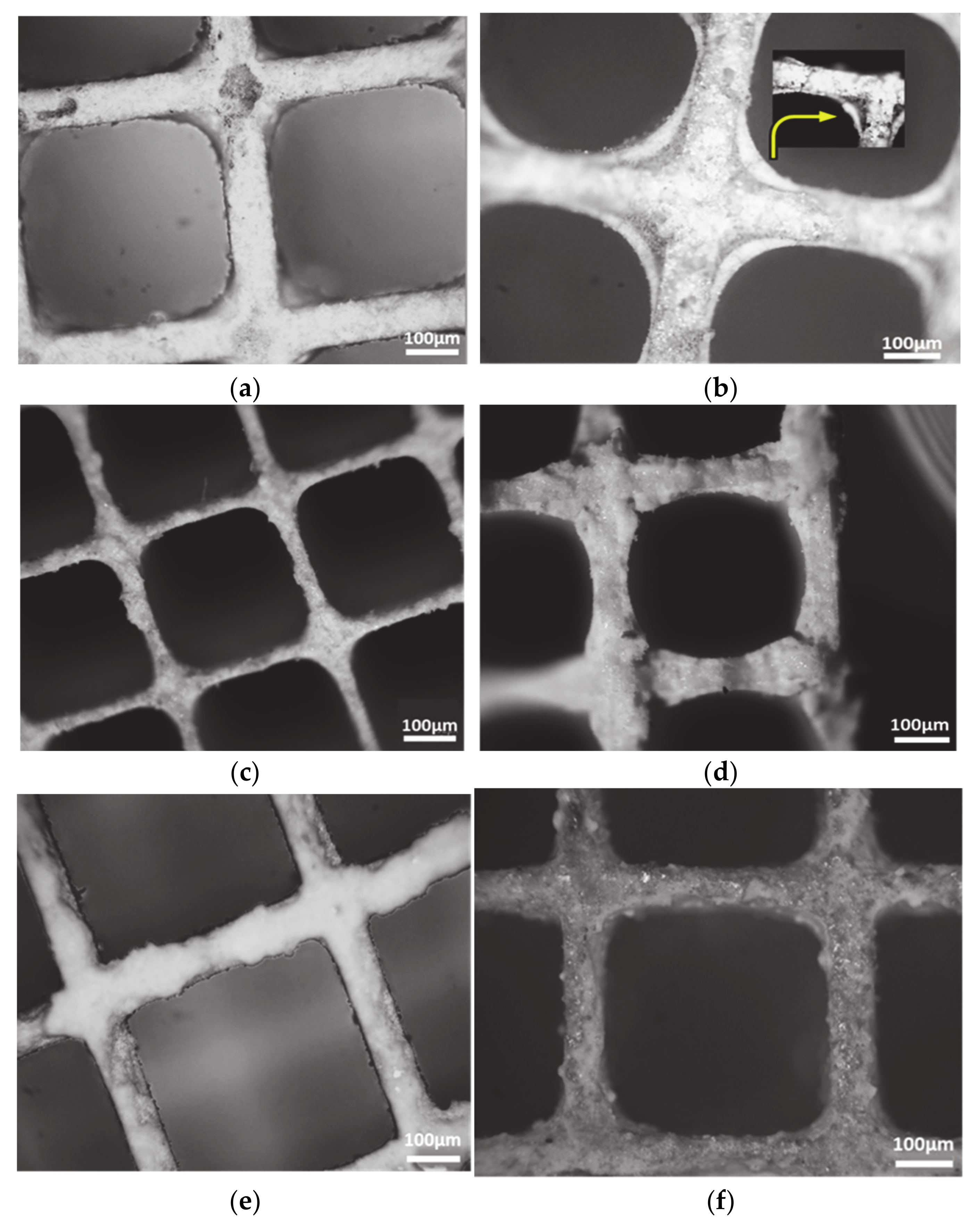
2.3. Optical Microscopy
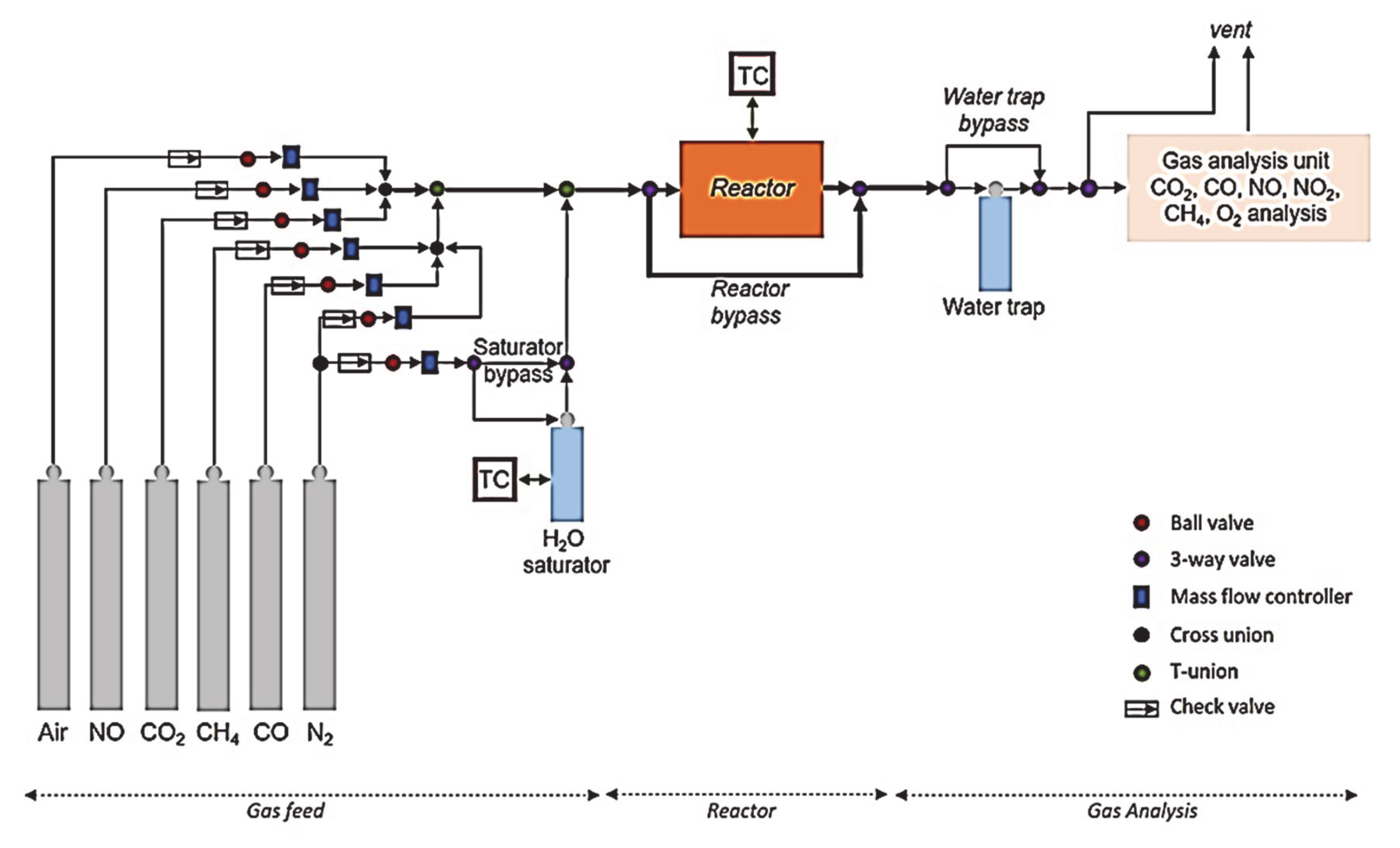
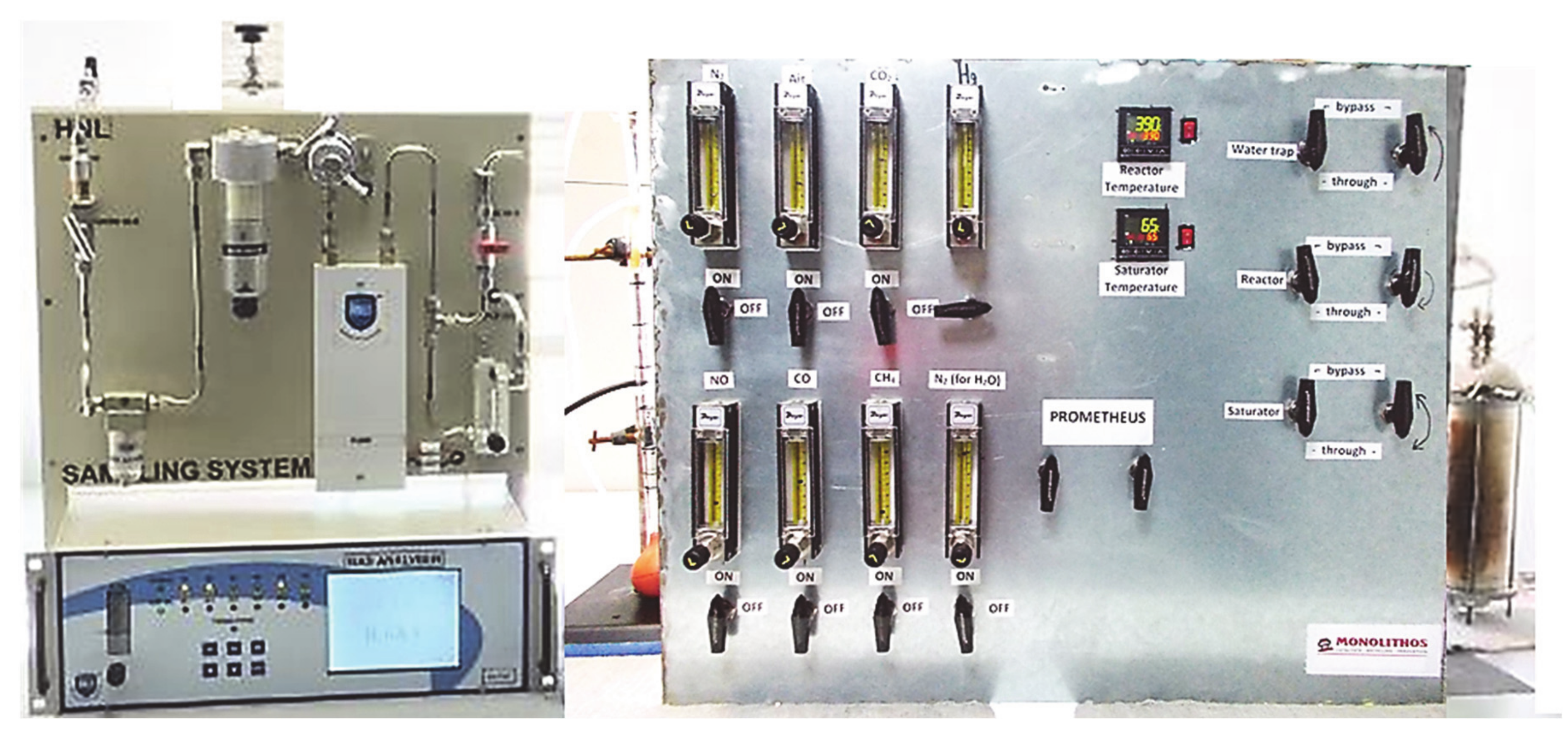
2.4. Synthetic Gas Bench (SGB)
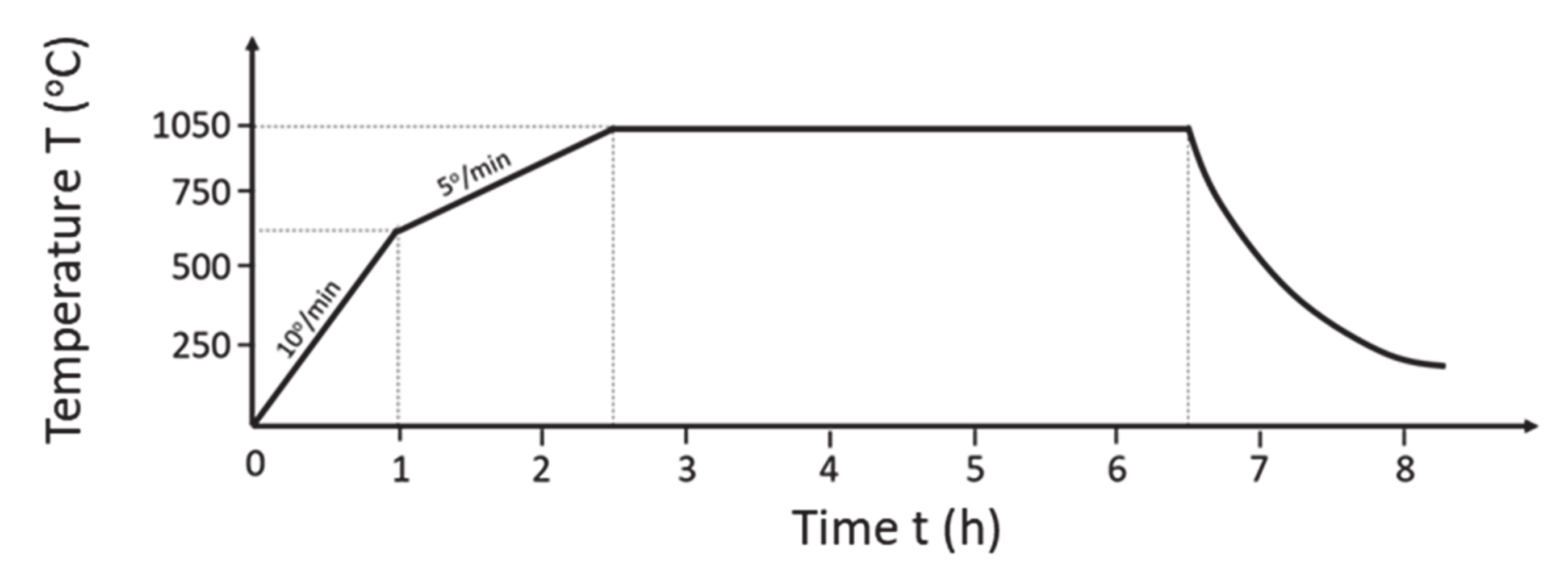
2.5. Catalyst Ageing Procedure
3. Results
3.1. Catalytic Activity of Fresh Catalysts
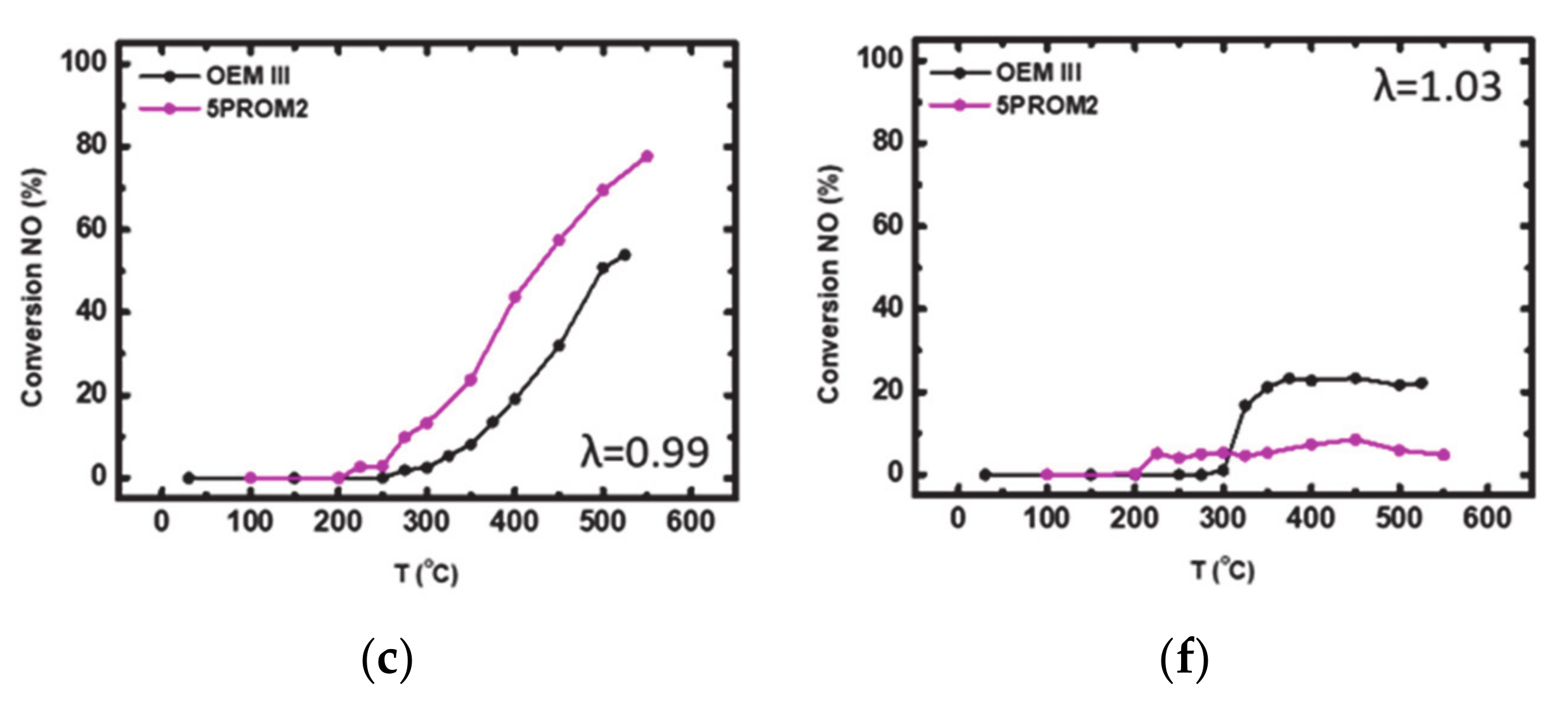
3.1.1. Catalytic Efficiency Comparison of OEM III with 5PROM2 Full Scale Catalyst (84.4% Substitution of PGMs with Copper NanoParticles)
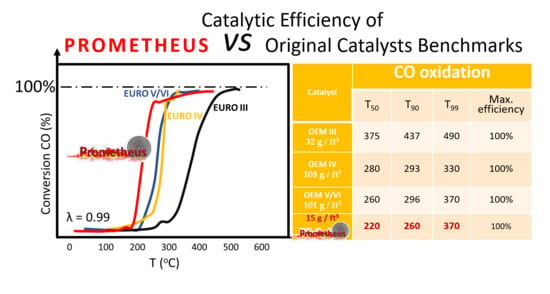
3.1.2. Catalytic Efficiency Comparison of OEM IV and OEM V/VI with 15PROM2 Full Scale Catalyst (85.1% Substitution of PGMs with Copper NanoParticles)
3.1.3. Comparison of the Tested Fresh Catalysts
3.2. The Effect of Ageing in Catalytic Activity
3.2.1. Catalytic Efficiency of AOEM III with A5PROM2 Full Scale Catalyst (84.4% Substitution of PGMs with Copper NanoParticles)
3.2.2. Catalytic Efficiency of AOEM IV and AOEM V/VI with A15PROMW Full Scale Catalyst (85.1% substitution of PGMs with Copper NanoParticles)
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kitco.com. Available online: https://www.kitco.com/ (accessed on 12 April 2021).
- Taylor, K.C. Automobile Catalytic Converters. In Catalysis and Automotive Pollution Control, 1st ed.; Crucq, A., Frennet, A., Eds.; Elsevier: Amsterdam, The Netherlands, 1987; Volume 30, p. 97. [Google Scholar]
- Lipshutz, B.H.; Pfeiffer, S.S. Copper: Organometallic Chemistry. In Encyclopedia of Inorganic and Bioinorganic Chemistry, 1st ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2011. [Google Scholar] [CrossRef]
- Kanwal, I.; Mujahid, A.; Rasool, N.; Rizwan, K.; Malik, A.; Ahmad, G.; Ali Shah, S.A.; Rashid, U.; Nasir, N.M. Palladium and Copper Catalyzed Sonogashira cross Coupling an Excellent Methodology for C-C Bond Formation over 17 Years: A Review. Catalysts 2010, 10, 443. [Google Scholar] [CrossRef]
- Cao, Y.-X.; Dong, X.-Y.; Yang, J.; Jiang, S.-P.; Zhou, S.; Li, Z.-L.; Chen, G.-C.; Liu, X.-Y. A Copper-Catalyzed Sonogashira Coupling Reaction of Diverse Activated Alkyl Halides with Terminal Alkynes Under Ambient Conditions. Adv. Synth. Catal. 2020, 362, 2280–2284. [Google Scholar] [CrossRef]
- Lakshmi Kantam, M.; Gadipelly, C.; Deshmukh, G.; Rajender Reddy, K.; Bhargava, S. Copper Catalyzed C-H Activation. Chem. Rec. 2019, 19, 1302–1318. [Google Scholar] [CrossRef] [PubMed]
- Kirillova, M.V.; Fernandes, T.A.; André, V.; Kirillov, A.M. Mild C−H Functionalization of Alkanes Catalyzed by Bioinspired Copper (II) Cores. Org. Biomol. Chem. 2019, 17, 7706–7771. [Google Scholar] [CrossRef]
- Lu, C.-Y.; Chang, W.-C.; Wey, M.-Y. CuO/CeO2 catalysts prepared with different cerium supports for CO oxidation at low temperature. Mater. Chem. Phys. 2013, 141, 512–518. [Google Scholar] [CrossRef]
- Zeng, S.; Wang, Y.; Ding, S.; Sattler, J.J.H.B.; Borodina, E.; Zhang, L.; Weckhuysen, B.M.; Su, H. Active sites over CuO/CeO2 and inverse CeO2/CuO catalysts for preferential CO oxidation. J. Power Sources 2014, 256, 301–311. [Google Scholar] [CrossRef]
- Tanga, C.; Suna, J.; Yaoa, X.; Caoa, Y.; Liua, L.; Gea, C.; Gao, F.; Dong, L. Efficient fabrication of active CuO-CeO2/SBA-15 catalysts for preferential oxidation of CO by solid state impregnation. Appl. Catal. B 2014, 256, 301–311. [Google Scholar] [CrossRef]
- Yu-Yao, Y.-F. The Oxidation of CO and C2H4 over Metal Oxides. J. Catal. 1975, 39, 104–114. [Google Scholar] [CrossRef]
- Farrauto, R.J.; Wedding, B. Poisoning by SOx of some base metal oxide auto exhaust catalysts. J. Catal. 1974, 33, 249–255. [Google Scholar] [CrossRef]
- Fishel, N.A.; Lee, R.K.; Wilhelm, F.C. Poisoning of Vehicle Emission Control Catalysts by Sulfur Compounds. Environ. Sci. Technol. 1974, 8, 260–267. [Google Scholar] [CrossRef]
- Luo, M.-F.; Fang, P.; He, M.; Xie, Y.-L. In situ XRD, Raman, and TPR studies of CuO/Al2O3 catalysts for CO oxidation. J. Mol. Catal. A Chem. 2005, 239, 243–248. [Google Scholar] [CrossRef]
- Martinez-Arias, A.; Fernadez-Garcia, M.; Galvez, O.; Coronado, J.M.; Anderson, J.A.; Conesa, J.C.; Soria, J.; Munuera, G. Comparative Study on Redox Properties and Catalytic Behavior for CO Oxidation of CuO/CeO2 and CuO/ZrCeO4 Catalysts. J. Catal. 2000, 195, 207–216. [Google Scholar] [CrossRef]
- Yang, Z.; He, B.; Lu, Z.; Hermansson, K. Physisorbed, Chemisorbed, and Oxidized CO on Highly Active Cu-CeO2 (111). J. Phys. Chem. C 2010, 114, 4486–4494. [Google Scholar] [CrossRef]
- Jia, A.; Jiang, S.; Lu, J.; Luo, M. Study of Catalytic Activity at the CuO-CeO2 Interface for CO Oxidation. J. Phys. Chem. C 2010, 114, 21605–21610. [Google Scholar] [CrossRef]
- Mrabet, D.; Abassi, A.; Cherizol, R.; Do, T.-O. One-pot Solvothermal Synthesis of Mixed Cu-Ce-Ox Nanocatalysts and Their Catalytic Activity for Low Temperature CO Oxidation. Appl. Catal. A 2012, 447–448, 60–66. [Google Scholar] [CrossRef]
- Aneggi, E.; Llorca, J.; Boaro, M.; Trovarelli, A. Surface-structure Sensitivity of CO Oxidation over Polycrystalline Ceria Powders. J. Catal. 2005, 234, 88–95. [Google Scholar] [CrossRef]
- Pacella, M.; Carbujo, A.; Fabro, J.; Gulotto, M.; Xin, Q.; Natile, M.M.; Canu, P.; Cool, P.; Glisenti, A. PGM-free CuO/LaCoO3 nanocomposites: New opportunities for TWC application. Appl. Catal. B 2018, 227, 446–458. [Google Scholar] [CrossRef]
- Moretti, E.; Lenardaa, M.; Rielloa, P.; Storaro, L.; Talona, A.; Frattini, R.; Reyes-Carmona, A.; Jiménez-López, A.; Rodríguez-Castelló, E. Influence of synthesis parameters on the performance of CeO2–CuO and CeO2–ZrO2–CuO systems in the catalytic oxidation of CO in excess of hydrogen. Appl. Catal. B 2013, 129, 556–565. [Google Scholar] [CrossRef]
- Sun, S.; Mao, D.; Yu, J.; Yang, Z.; Lua, G.; Mab, Z. Low-temperature CO oxidation on CuO/CeO2 catalysts: The significant effect of copper precursor and calcination temperature. Catal. Sci. Technol. 2015, 5, 3166–3181. [Google Scholar] [CrossRef]
- Papadopoulos, C.; Kappisa, K.; Papavasiliou, J.; Vakros, J.; Kuśmierz, M.; Gac, W.; Georgiou, Y.; Deligiannakis, Y.; Avgouropoulos, G. Copper-promoted ceria catalysts for CO oxidation reaction. Catal. Today 2020, 355, 647–653. [Google Scholar] [CrossRef]
- Yakoumis, I. PROMETHEUS: A Copper Based Polymetallic Catalyst for Automotive Applications. Part I: Synthesis and Characterization. Materials 2021, 14, 622. [Google Scholar] [CrossRef]
- Bedford, R.E.; LaBarge, W.J. Base Metal Automotive Exhaust Catalyst with Improved Activity and Stability and Method of Making the Catalysts. U.S. Patent 5,063,193, 5 November 1991. [Google Scholar]
- Shore, L.; Ruettinger, W.F.; Farrauto, R.J. Platinum Group Metal Promoted Copper Oxidation Catalysts and Methods for Carbon Monoxide Remediation. U.S. Patent 2002/0131915 A1, 19 September 2002. [Google Scholar]
- Park, S.E.; Kim, G.-M.; Lee, Y.-J.; Chang, J.-S.; Han, S.-H. Method for Removing Nitrogen Oxides in Exhaust Gas by Selective Catalytic Reduction and Catalyst for Reduction of Nitrogen Oxides. U.S. Patent 5,879,645, 9 March 1999. [Google Scholar]
- Hu, Y.; Dong, L.; Shen, M.; Liu, D.; Wang, J.; Ding, W.; Chen, Y. Influence of supports on the activities of copper oxide species in the low-temperature NO + CO reaction. Appl. Catal. B 2001, 31, 61–69. [Google Scholar] [CrossRef]
- Yakoumis, I.; Moschovi, A.; Panou, M.; Panias, D. Single-Step Hydrometallurgical Method for the Platinum Group Metals Leaching from Commercial Spent Automotive Catalysts. J. Sustain. Metall. 2020, 6, 259–268. [Google Scholar] [CrossRef]
- Dalianis, G.; Nanaki, E.; Xydis, G.; Zervas, E. Aspects to Greenhouse New Gas Mitigation Policies for Low Carbon Cities. Energies 2016, 9, 128. [Google Scholar] [CrossRef]
- Neyestanaki, A.K.; Klingstedt, F.; Salmi, T.; Murzin, D.Y. Deactivation of Postcombustion Catalysts, a Review. Fuel 2014, 83, 395–408. [Google Scholar] [CrossRef]
- More, K.L.; Kenik, E.A.; Coffey, D.W.; Geer, T.S.; LaBarge, W.J.; Beckmeyer, R.F.; Theis, J. Thermally-Induced Microstructural Changes in a Three-Way Automotive Catalyst. SAE Tech. Paper Ser. 1997, 1255–1263. [Google Scholar] [CrossRef]
- Winkler, A.; Ferri, D.; Hauert, R. Influence of Aging Effects on the Conversion Efficiency of Automotive Exhaust Gas Catalysts. Catal. Today 2010, 155, 140–146. [Google Scholar] [CrossRef]
- Sheng, S.; Lai, Y.; Hao, C.; Hou, P.; Lv, T.; Ge, Y. Review of Rapid Ageing Testing Methods of Three-Way Catalyst for Gasoline Engine. Int. J. Veh. Perform. 2020, 6, 277–293. [Google Scholar] [CrossRef]
- Kang, S.B.; Kwon, H.J.; Nam, I.-S.; Song, Y.I.; Oh, S.H. Activity Function for Describing Alteration of Three-Way Catalyst Performance over Palladium-Only Three-Way Catalysts by Catalyst Mileage. Int. Eng. Chem. Res. 2011, 50, 5499–5509. [Google Scholar] [CrossRef]
- Giuliano, M.; Valsania, M.C.; Ticali, P.; Sartoretti, E.; Morandi, S.; Bensaid, S.; Ricchiardi, G.; Sgoi, M. Characterization of the Evolution of Noble Metal Particles in a Commercial Three-Way Catalyst: Correlation Between Real and Simulated Ageing. Catalysts 2021, 11, 247. [Google Scholar] [CrossRef]
- Nicole, J.; Tsiplakides, D.; Pliangos, C.; Verykios, X.E.; Comninellis, C.; Vayenas, C.G. Electrochemical Promotion and Metal–Support Interactions. J. Catal. 2001, 204, 23–34. [Google Scholar] [CrossRef]
- Vernoux, P.; Lizarraga, L.; Tsampas, M.N.; Sapountzi, F.M.; De Lucas-Consuegra, A.; Valverde, J.-L.; Souentie, S.; Vayenas, C.G.; Tsiplakides, D.; Balomenou, S.; et al. Ionically Conducting Ceramics as Active Catalyst Supports. Chem. Rev. 2013, 113, 8192–8260. [Google Scholar] [CrossRef]
- Zafiris, G.S.; Gorte, R.J. Evidence for Low-Temperature Oxygen Migration from Ceria to Rh. J. Catal. 1993, 139, 561–567. [Google Scholar] [CrossRef]

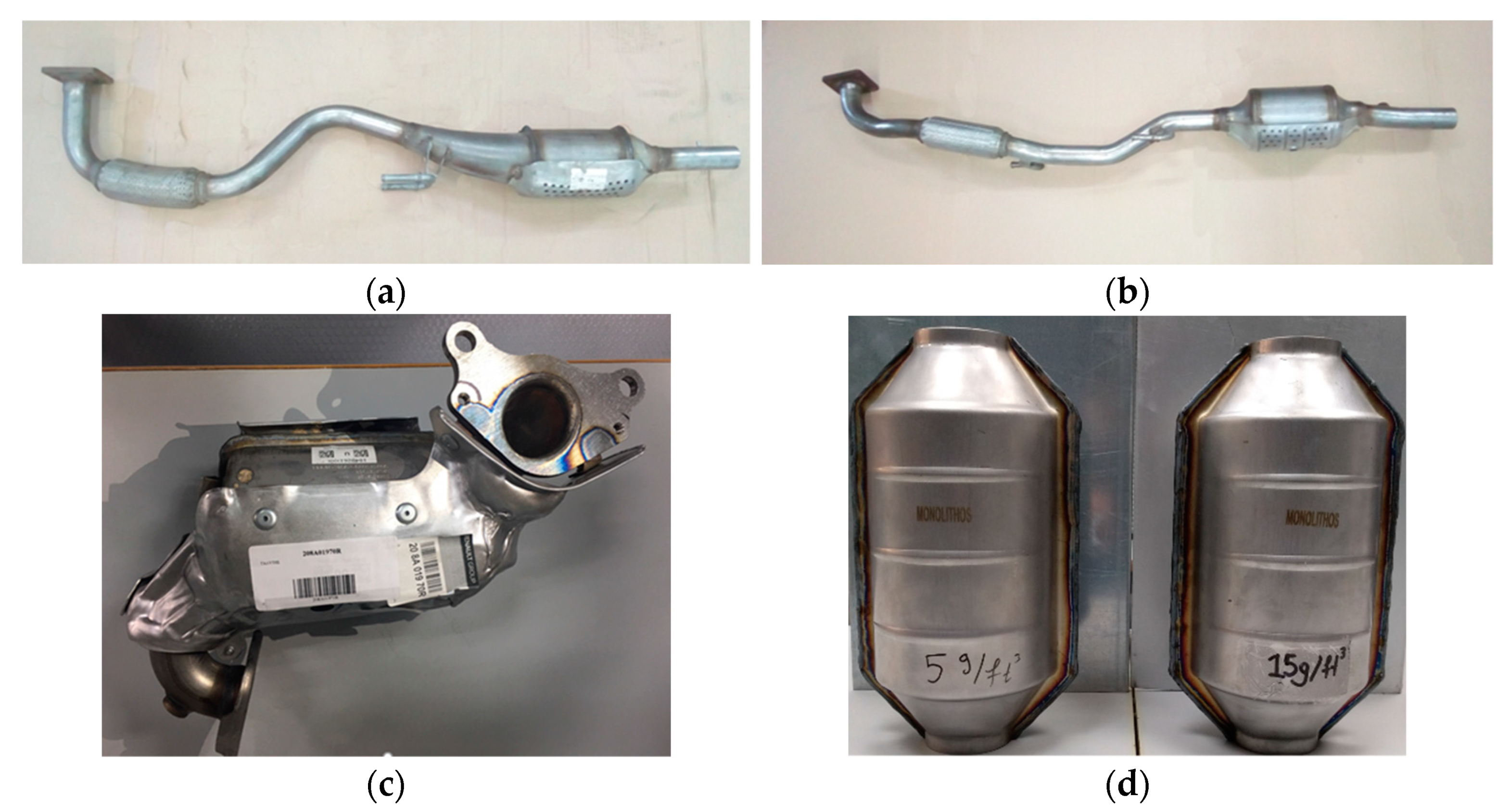






| Abbreviation | Company | Model | Motor | Euro | Cu (ppm/%wt.) | Pd (ppm/%wt.) | Rh (ppm/%wt.) | PGM Loading (g/ft3) 1 |
|---|---|---|---|---|---|---|---|---|
| 5PROM2 | MONOLITHOS | Universal | Up to 1.6 Lt | III | 820 0.082 | 280 0.028 | 40 0.004 | 5 |
| OEM III | VW Group | Polo | 1.4 Lt | III | — | 1392 0.1392 | 295 0.0295 | 32 |
| OEM IV | VW Group | Polo | 1.4 Lt | IV | — | 4872 0.4872 | 306 0.0306 | 108 |
| OEM V/VI | Renault | Meganne | 1.2 Lt turbo | V/VI | — | 5642 0.5642 | 471 0.0471 | 101 |
| 15PROM2 | MONOLITHOS | Universal | Up to 1.6 Lt | VI | 2320 0.232 | 780 0.078 | 110 0.011 | 15 |
| λ Factor Values | Gas Component | |||||
|---|---|---|---|---|---|---|
| CO (%) | CO2 (%) | O2 (%) | NO (ppm) | CH4 1 (ppm) | H2O (%) | |
| Rich-burn conditions λ = 0.99 | 1 | 12 | 0.91 | 800 | 2500 | 10 |
| Lean-burn conditions λ = 1.03 | 1 | 12 | 0.95 | 800 | 2500 | 10 |
| Catalyst | CO Oxidation | CH4 Oxidation | NO Reduction | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T50 (°C) | T90 (°C) | T99 (°C) | Max. Efficiency (%) | T50 (°C) | T90 (°C) | T99 (°C) | Max. Efficiency (%) | T50 (°C) | T90 (°C) | T99 (°C) | Max. Efficiency (%) | |
| OEM III | 375 | 437 | 490 | 100% | 375 | — | — | 87% | 500 | — | — | 54% |
| 5PROM2 | 215 | 306 | 388 | 100% | 212 | 375 | — | 95% | 425 | — | — | 78% |
| Catalyst | CO Oxidation | CH4 Oxidation | NO Reduction | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T50 (°C) | T90 (°C) | T99 (°C) | Max. Efficiency (%) | T50 (°C) | T90 (°C) | T99 (°C) | Max. Efficiency (%) | T50 (°C) | T90 (°C) | T99 (°C) | Max. Efficiency (%) | |
| OEM III | 345 | 390 | 440 | 100% | 350 | 420 | — | 93% | — | — | — | 23% |
| 5PROM2 | 240 | 350 | 430 | 100% | 240 | 375 | — | 92% | — | — | — | 8% |
| Catalyst | CO Oxidation | CH4 Oxidation | NO Reduction | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T50 (°C) | T90 (°C) | T99 (°C) | Max. Efficiency (%) | T50 (°C) | T90 (°C) | T99 (°C) | Max. Efficiency (%) | T50 (°C) | T90 (°C) | T99 (°C) | Max. Efficiency (%) | |
| OEM IV | 280 | 293 | 330 | 100% | 280 | — | — | 87% | 286 | 300 | — | 96% |
| OEM V/VI | 260 | 296 | 370 | 100% | 265 | — | — | 88% | 270 | 325 | — | 96% |
| 15PROM2 | 220 | 260 | 370 | 100% | 220 | — | — | 87% | 290 | 390 | — | 96% |
| Catalyst | CO Oxidation | CH4 Oxidation | NO Reduction | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T50 (°C) | T90 (°C) | T99 (°C) | Max. Efficiency (%) | T50 (°C) | T90 (°C) | T99 (°C) | Max. Efficiency (%) | T50 (°C) | T90 (°C) | T99 (°C) | Max. Efficiency (%) | |
| OEM IV | 240 | 258 | 260 | 100% | 244 | 268 | — | 93% | — | — | — | 15% |
| OEM V/VI | 233 | 285 | 320 | 100% | 237 | 305 | 400 | 99% | — | — | — | 22% |
| 15PROM2 | 160 | 190 | 210 | 100% | 160 | 190 | 215 | 100% | — | — | — | 6% |
| Catalyst | CO Oxidation | CH4 Oxidation | NO Reduction | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T50 (°C) | T90 (°C) | T99 (°C) | Max. Efficiency (%) | T50 (°C) | T90 (°C) | T99 (°C) | Max. Efficiency (%) | T50 (°C) | T90 (°C) | T99 (°C) | Max. Efficiency (%) | |
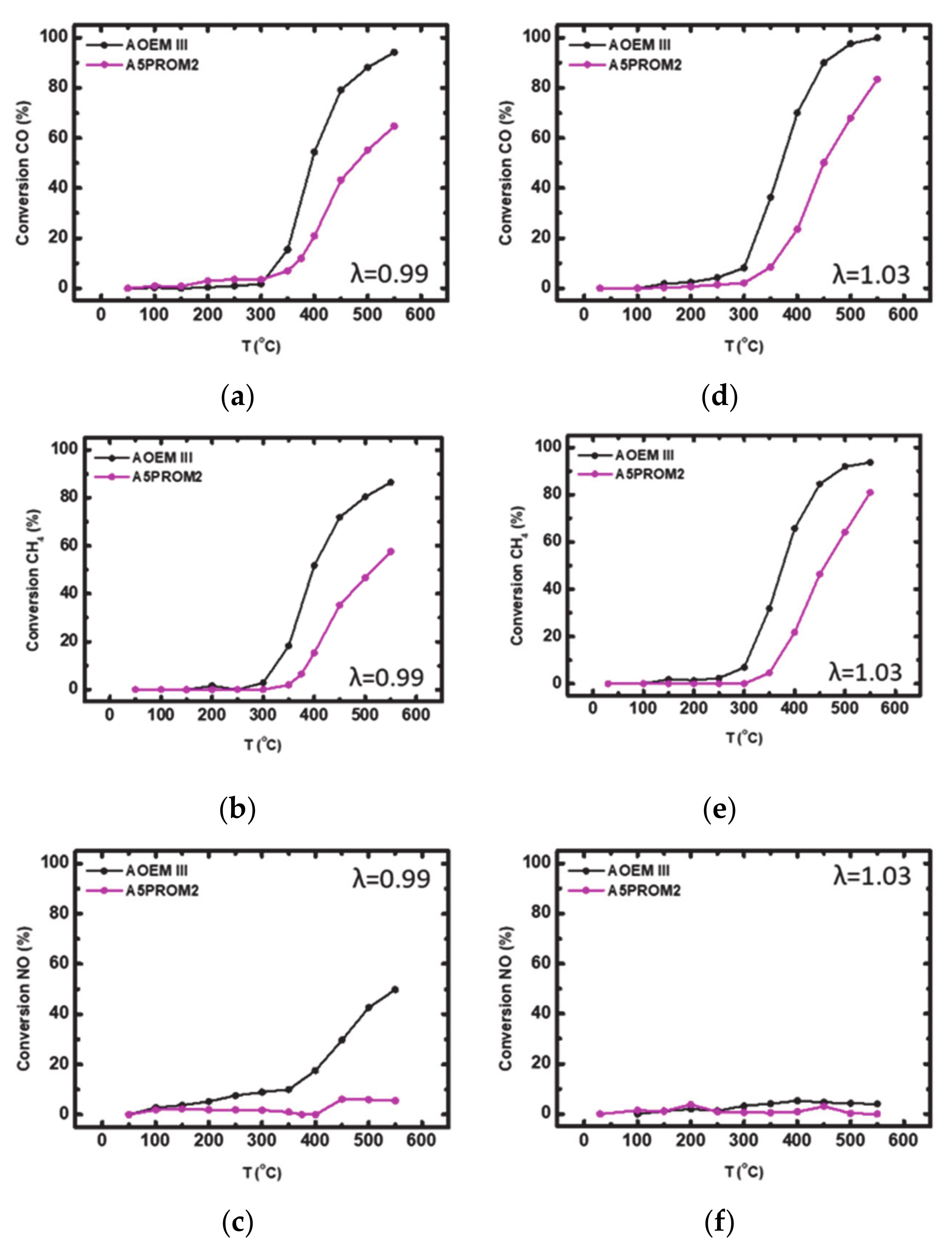
| AOEM III | 400 | 515 | — | 94 | 400 | — | — | 87 | 550 | — | — | 50 |
| A5PROM2 | 482 | — | — | 65 | 514 | — | — | 58 | — | — | — | 6 |
| Catalyst | CO Oxidation | CH4 Oxidation | NO Reduction | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T50 (°C) | T90 (°C) | T99 (°C) | Max. Efficiency (%) | T50 (°C) | T90 (°C) | T99 (°C) | Max. Efficiency (%) | T50 (°C) | T90 (°C) | T99 (°C) | Max. Efficiency (%) | |
| AOEM III | 370 | 450 | 525 | 100 | 370 | 490 | — | 94 | — | — | — | 5 |
| A5PROM2 | 449 | — | — | 83 | 460 | — | — | 81 | — | — | — | 0 |
| Catalyst | CO Oxidation | CH4 Oxidation | NO Reduction | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T50 (°C) | T90 (°C) | T99 (°C) | Max. Efficiency (%) | T50 (°C) | T90 (°C) | T99 (°C) | Max. Efficiency (%) | T50 (°C) | T90 (°C) | T99 (°C) | Max. Efficiency (%) | |
| AOEM IV | 390 | — | — | 82 | 390 | — | — | 79 | — | — | — | 42 |
| AOEM V/VI | 315 | — | — | 83 | 325 | — | — | 78 | 340 | — | — | 80 |
| A15PROM2 | 330 | 390 | — | 94 | 330 | 395 | — | 97 | 390 | — | — | 70 |
| Catalyst | CO Oxidation | CH4 Oxidation | NO Reduction | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T50 (°C) | T90 (°C) | T99 (°C) | Max. Efficiency (%) | T50 (°C) | T90 (°C) | T99 (°C) | Max. Efficiency (%) | T50 (°C) | T90 (°C) | T99 (°C) | Max. Efficiency (%) | |
| AOEM IV | 345 | 450 | 500 | 100 | 358 | 495 | - | 94 | — | — | — | 0 |
| AOEM V/VI | 260 | 289 | 345 | 100 | 260 | — | - | 81 | — | — | — | 9 |
| A15PROM2 | 330 | 366 | 404 | 100 | 325 | 410 | - | 90 | — | — | — | 9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yakoumis, I.; Polyzou, Ε.; Moschovi, A.M. PROMETHEUS: A Copper-Based Polymetallic Catalyst for Automotive Applications. Part II: Catalytic Efficiency an Endurance as Compared with Original Catalysts. Materials 2021, 14, 2226. https://doi.org/10.3390/ma14092226
Yakoumis I, Polyzou Ε, Moschovi AM. PROMETHEUS: A Copper-Based Polymetallic Catalyst for Automotive Applications. Part II: Catalytic Efficiency an Endurance as Compared with Original Catalysts. Materials. 2021; 14(9):2226. https://doi.org/10.3390/ma14092226
Chicago/Turabian StyleYakoumis, Iakovos, Εkaterini Polyzou, and Anastasia Maria Moschovi. 2021. "PROMETHEUS: A Copper-Based Polymetallic Catalyst for Automotive Applications. Part II: Catalytic Efficiency an Endurance as Compared with Original Catalysts" Materials 14, no. 9: 2226. https://doi.org/10.3390/ma14092226
APA StyleYakoumis, I., Polyzou, Ε., & Moschovi, A. M. (2021). PROMETHEUS: A Copper-Based Polymetallic Catalyst for Automotive Applications. Part II: Catalytic Efficiency an Endurance as Compared with Original Catalysts. Materials, 14(9), 2226. https://doi.org/10.3390/ma14092226