Catalytic Reduction of Organic Dyes by Multilayered Graphene Platelets and Silver Nanoparticles in Polyacrylic Acid Hydrogel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation Process of MLGPs
2.3. Preparation Process of MLGP/PAA Hydrogel and MLGP/Ag0/PAA Hydrogel
2.4. Characterization Techniques
2.5. Catalytic Experiment
3. Results and Discussion
3.1. Preparation of MLGPs by Liquid-Based Direct Exfoliation
3.2. Preparation of MLGP/PAA Hydrogels and MLGP/Ag0/PAA Hydrogels
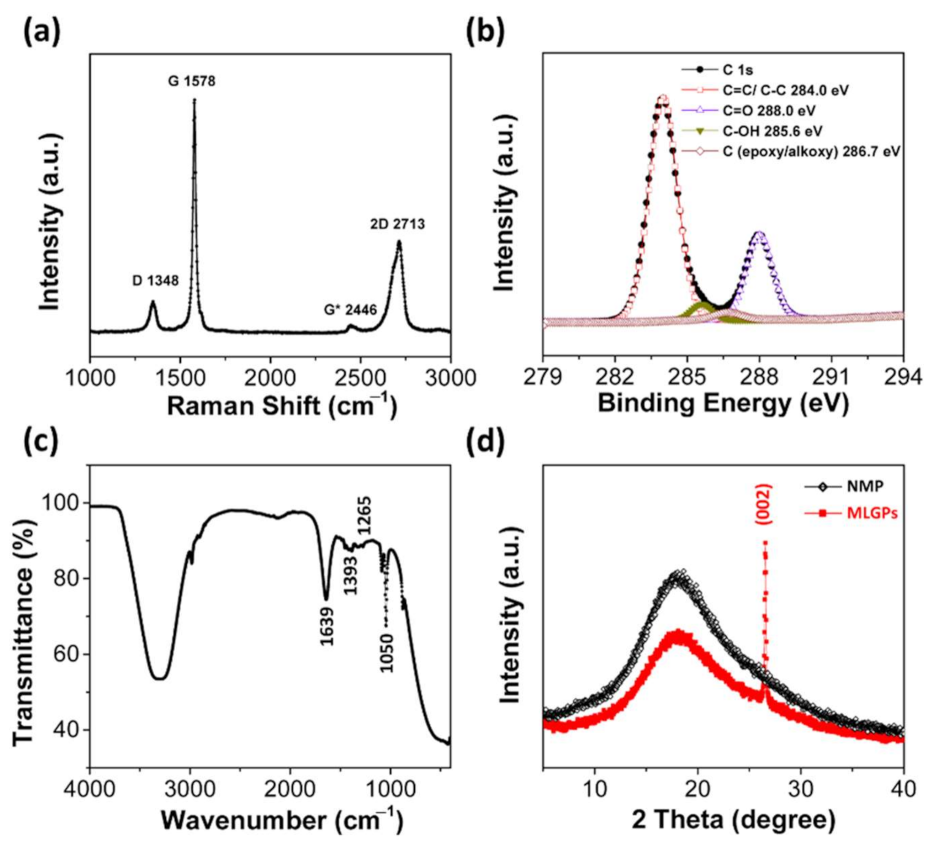
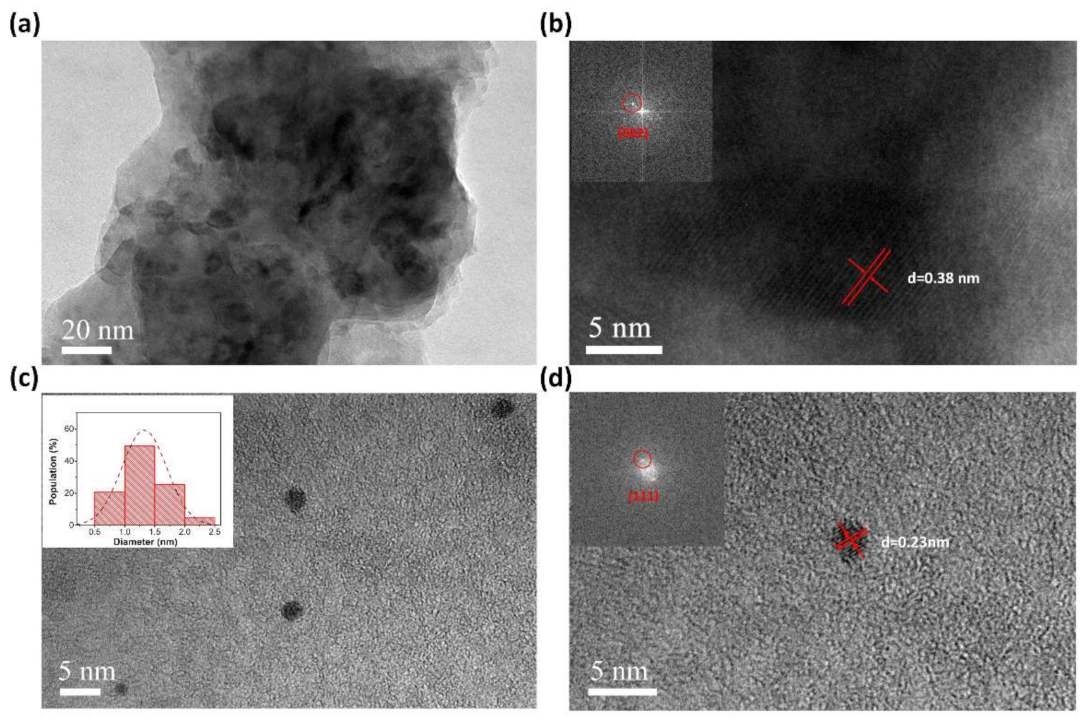
3.3. Characterization of MLGPs Prepared from Liquid-Based Direct Exfoliation
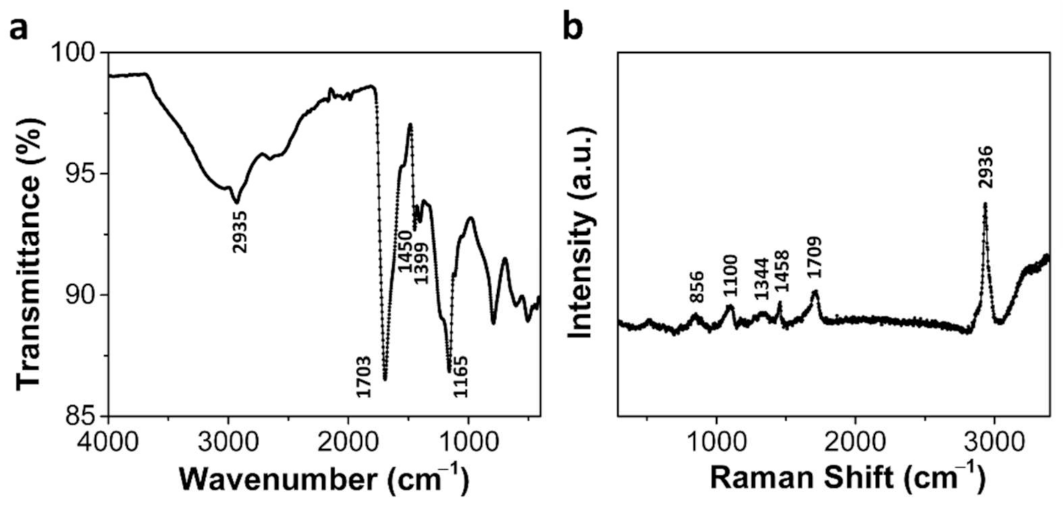
3.4. Characterization of MLGP/PAA and MLGP/Ag0/PAA Hydrogels
3.5. Catalytic Performance of MLGP/PAA and MLGP/Ag0/PAA Hydrogels
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MLGPs | Multilayered Graphene Platelets |
| PAA | polyacrylic acid |
| MLGP/PAA hydrogel | multilayered graphene platelets/polyacrylic acid hydrogel composite |
| Ag0 | silver nanoparticles |
| MLGP/Ag0/PAA hydrogel | multilayered graphene platelets/silver nanoparticles/polyacrylic acid hydrogel composite |
| NMP | N-methyl pyrrolidone |
| IPA | isopropanol |
| MB | methylene blue |
| 4-NP | 4-nitrophenol |
References
- Yang, F.; Deng, D.H.; Pan, X.L.; Fu, Q.; Bao, X.H. Understanding nano effects in catalysis. Natl. Sci. Rev. 2015, 2, 183–201. [Google Scholar] [CrossRef] [Green Version]
- Kong, X.K.; Chen, C.L.; Chen, Q.W. Doped graphene for metal-free catalysis. Chem. Soc. Rev. 2014, 43, 2841–2857. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Pumera, M. Electrochemical catalysis at low dimensional carbons: Graphene, carbon nanotubes and beyond—A review. Appl. Mater. Today 2016, 5, 134–141. [Google Scholar] [CrossRef]
- Qiu, B.C.; Xing, M.Y.; Zhang, J.L. Recent advances in three-dimensional graphene based materials for catalysis applications. Chem. Soc. Rev. 2018, 47, 2165–2216. [Google Scholar] [CrossRef]
- Zhang, C.; Guo, N.; Yam, K.M. Graphene based solid-state catalysis: From computational understanding to applications. J. Mater. Sci. Nanotechnol. 2019, 3, 17. [Google Scholar]
- Komeily-Nia, Z.; Qu, L.-T.; Li, J.-L. Progress in the Understanding and Applications of the Intrinsic Reactivity of Graphene-Based Materials. Small Sci. 2021, 1, 2000026. [Google Scholar] [CrossRef]
- Presolski, S.; Pumera, M. Graphene Oxide: Carbocatalyst or Reagent? Angew. Chem. Int. Ed. 2018, 57, 16713–16715. [Google Scholar] [CrossRef]
- Ahn, Y.; Oh, H.; Yoon, Y.; Park, W.K.; Yang, W.S.; Kang, J.W. Effect of graphene oxidation degree on the catalytic activity of graphene for ozone catalysis. J. Environ. Chem. Eng. 2017, 5, 3882–3894. [Google Scholar] [CrossRef]
- Zhu, S.H.; Wang, J.G.; Fan, W.B. Graphene-based catalysis for biomass conversion. Catal. Sci. Technol. 2015, 5, 3845–3858. [Google Scholar] [CrossRef]
- Niu, L.; Coleman, J.N.; Zhang, H.; Shin, H.; Chhowalla, M.; Zheng, Z. Production of Two-Dimensional Nanomaterials via Liquid-Based Direct Exfoliation. Small 2016, 12, 272–293. [Google Scholar] [CrossRef]
- Nicolosi, V.; Chhowalla, M.; Kanatzidis, M.G.; Strano, M.S.; Coleman, J.N. Liquid exfoliation of layered materials. Science 2013, 340, 1226419. [Google Scholar] [CrossRef] [Green Version]
- Cui, X.; Zhang, C.; Hao, R.; Hou, Y. Liquid-phase exfoliation, functionalization and applications of graphene. Nanoscale 2011, 3, 2118. [Google Scholar] [CrossRef]
- Backes, C.; Abdelkader, A.M.; Alonso, C.; Andrieux-Ledier, A.; Arenal, R.; Azpeitia, J.; Balakrishnan, N.; Banszerus, L.; Barjon, J.; Bartali, R.; et al. Production and processing of graphene and related materials. 2D Mater. 2020, 7, 022001. [Google Scholar] [CrossRef]
- Yang, N.; Qi, P.; Ren, J.; Yu, H.; Liu, S.; Li, J.; Chen, W.; Kaplan, D.L.; Ling, S. Polyvinyl Alcohol/Silk Fibroin/Borax Hydrogel Ionotronics: A Highly Stretchable, Self-Healable, and Biocompatible Sensing Platform. ACS Appl. Mater. Interfaces 2019, 11, 23632–23638. [Google Scholar] [CrossRef]
- Simon, D.T.; Gabrielsson, E.O.; Tybrandt, K.; Berggren, M. Organic bioelectronics: Bridging the signaling gap between biology and technology. Chem. Rev. 2016, 116, 13009–13041. [Google Scholar] [CrossRef] [Green Version]
- Yuk, H.; Lu, B.; Zhao, X. Hydrogel bioelectronics. Chem. Soc. Rev. 2019, 48, 1642–1667. [Google Scholar] [CrossRef] [Green Version]
- Rivnay, J.; Owens, R.M.; Malliaras, G.G. The Rise of Organic Bioelectronics. Chem. Mater. 2014, 26, 679–685. [Google Scholar] [CrossRef]
- De France, K.J.; Xu, F.; Hoare, T. Structured Macroporous Hydrogels: Progress, Challenges, and Opportunities. Adv. Healthc. Mater. 2018, 7, 1700927. [Google Scholar] [CrossRef]
- Mei, X.; Liu, J.; Guo, Z.; Li, P.; Bi, S.; Wang, Y.; Yang, Y.; Shen, W.; Wang, Y.; Xiao, Y.; et al. Simultaneous p-nitrophenol and nitrogen removal in PNP wastewater treatment: Comparison of two integrated membrane-aerated bioreactor systems. J. Hazard. Mater. 2019, 363, 99–108. [Google Scholar] [CrossRef]
- Rashed, M.N.; El Taher, M.A.E.D.; Fadlalla, S.M.M. Adsorption of methylene blue using modified adsorbents from drinking water treatment sludge. Water Sci. Technol. 2016, 74, 1885–1898. [Google Scholar] [CrossRef]
- Dod, R.; Banerjee, G.; Saini, D.R. Removal of methylene blue (MB) dye from water environment by processed Jowar Stalk [Sorghum bicolor (L.) Moench] adsorbent. Clean Technol. Environ. Policy 2015, 17, 2349–2359. [Google Scholar] [CrossRef]
- Huo, C.; Yan, Z.; Song, X.; Zeng, H. 2D materials via liquid exfoliation: A review on fabrication and applications. Sci. Bull. 2015, 60, 1994–2008. [Google Scholar] [CrossRef]
- Skaltsas, T.; Ke, X.; Bittencourt, C.; Tagmatarchis, N. Ultrasonication Induces Oxygenated Species and Defects onto Exfoliated Graphene. J. Phys. Chem. C 2013, 117, 23272–23278. [Google Scholar] [CrossRef]
- Elliott, J.E.; Macdonald, M.; Nie, J.; Bowman, C.N. Structure and swelling of poly(acrylic acid) hydrogels: Effect of pH, ionic strength, and dilution on the crosslinked polymer structure. Polymer 2004, 45, 1503–1510. [Google Scholar] [CrossRef]
- Yonezawa, Y.; Sato, T.; Kuroda, S. Photochemical Formation of Colloidal Silver: Peptizing Action of Acetone Ketyl Radical. J. Chem. Soc. Faraday Trans. 1991, 87, 1905–1910. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Basko, D.M. Raman spectroscopy as a versatile tool for studying the properties of graphene. Nat. Nanotechnol. 2013, 8, 235–246. [Google Scholar] [CrossRef] [Green Version]
- Cançado, L.G.; Jorio, A.; Ferreira, E.H.M.; Stavale, F.; Achete, C.A.; Capaz, R.B.; Moutinho, M.V.O.; Lombardo, A.; Kulmala, T.S.; Ferrari, A.C. Quantifying Defects in Graphene via Raman Spectroscopy at Different Excitation Energies. Nano Lett. 2011, 11, 3190–3196. [Google Scholar] [CrossRef] [Green Version]
- Zhu, C.; Guo, S.; Fang, Y.; Dong, S. Reducing Sugar: New Functional Molecules for the Green Synthesis of Graphene Nanosheets. ACS Nano 2010, 4, 2429–2437. [Google Scholar] [CrossRef]
- Rattana, T.; Chaiyakun, S.; Witit-anun, N.; Nuntawong, N.; Chindaudom, P.; Oaew, S.; Kedkeaw, C.; Limsuwan, P. Preparation and characterization of graphene oxide nanosheets. Procedia Eng. 2012, 32, 759–764. [Google Scholar] [CrossRef] [Green Version]
- Krishnamoorthy, K.; Kim, G.-S.; Kim, S.J. Graphene nanosheets: Ultrasound assisted synthesis and characterization. Ultrason. Sonochem. 2013, 20, 644–649. [Google Scholar] [CrossRef]
- Dong, J.; Ozaki, Y.; Nakashima, K. FTIR Studies of Conformational Energies of Poly(acrylic Acid) in Cast Films. J. Polym. Sci. Part B: Polym. Phys. 1997, 35, 507–515. [Google Scholar] [CrossRef]
- De la Fuente, J.L.; Wilhelm, M.; Spiess, H.W.; Madruga, E.L.; Fernández-Garcia, M.; Cerrada, M.L. Thermal, morphological and rheological characterization of poly(acrylic acid-g-styrene) amphiphilic graft copolymers. Polymer 2005, 46, 4544–4553. [Google Scholar] [CrossRef]
- Krivorotova, T.; Jonikaite-Svegzdiene, J.; Radzevicius, P.; Makuska, R. Synthesis by RAFT polymerization and properties of anionic cylindrical molecular brushes bearing poly(acrylic acid) side chains. React. Funct. Polym. 2014, 76, 32–40. [Google Scholar] [CrossRef]
- Abdollahi, R.; Taghizadeh, M.T.; Savani, S. Thermal and mechanical properties of graphene oxide nanocomposite hydrogel based on poly(acrylic acid) grafted onto amylose. Polym. Degrad. Stab. 2018, 147, 151–158. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Chen, D.-H. Catalytic reduction of 4-nitrophenol by magnetically recoverable Au nanocatalyst. J. Hazard. Mater. 2009, 165, 664–669. [Google Scholar] [CrossRef]
- Pradhan, N.; Pal, A.; Pal, T. Silver nanoparticle catalyzed reduction of aromatic nitro compounds. Colloids Surf. A 2002, 196, 247–257. [Google Scholar] [CrossRef]
- Naik, B.; Hazra, S.; Prasad, V.S.; Ghosh, N.N. Synthesis of Ag nanoparticles within the pores of SBA15: An efficient catalyst for reduction of 4-nitrophenol. Catal. Commun. 2011, 12, 1104–1108. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, H.; Nie, D.; Cui, N.; Li, K.; Zhang, X.; Zhang, L. Catalytic Reduction of Organic Dyes by Multilayered Graphene Platelets and Silver Nanoparticles in Polyacrylic Acid Hydrogel. Materials 2021, 14, 2274. https://doi.org/10.3390/ma14092274
Ding H, Nie D, Cui N, Li K, Zhang X, Zhang L. Catalytic Reduction of Organic Dyes by Multilayered Graphene Platelets and Silver Nanoparticles in Polyacrylic Acid Hydrogel. Materials. 2021; 14(9):2274. https://doi.org/10.3390/ma14092274
Chicago/Turabian StyleDing, Hanyuan, Dexi Nie, Naiyuan Cui, Kaili Li, Xiaojing Zhang, and Lei Zhang. 2021. "Catalytic Reduction of Organic Dyes by Multilayered Graphene Platelets and Silver Nanoparticles in Polyacrylic Acid Hydrogel" Materials 14, no. 9: 2274. https://doi.org/10.3390/ma14092274




