Experimental Study on the Influence of Tool Electrode Material on Electrochemical Micromachining of 304 Stainless Steel
Abstract
:1. Introduction
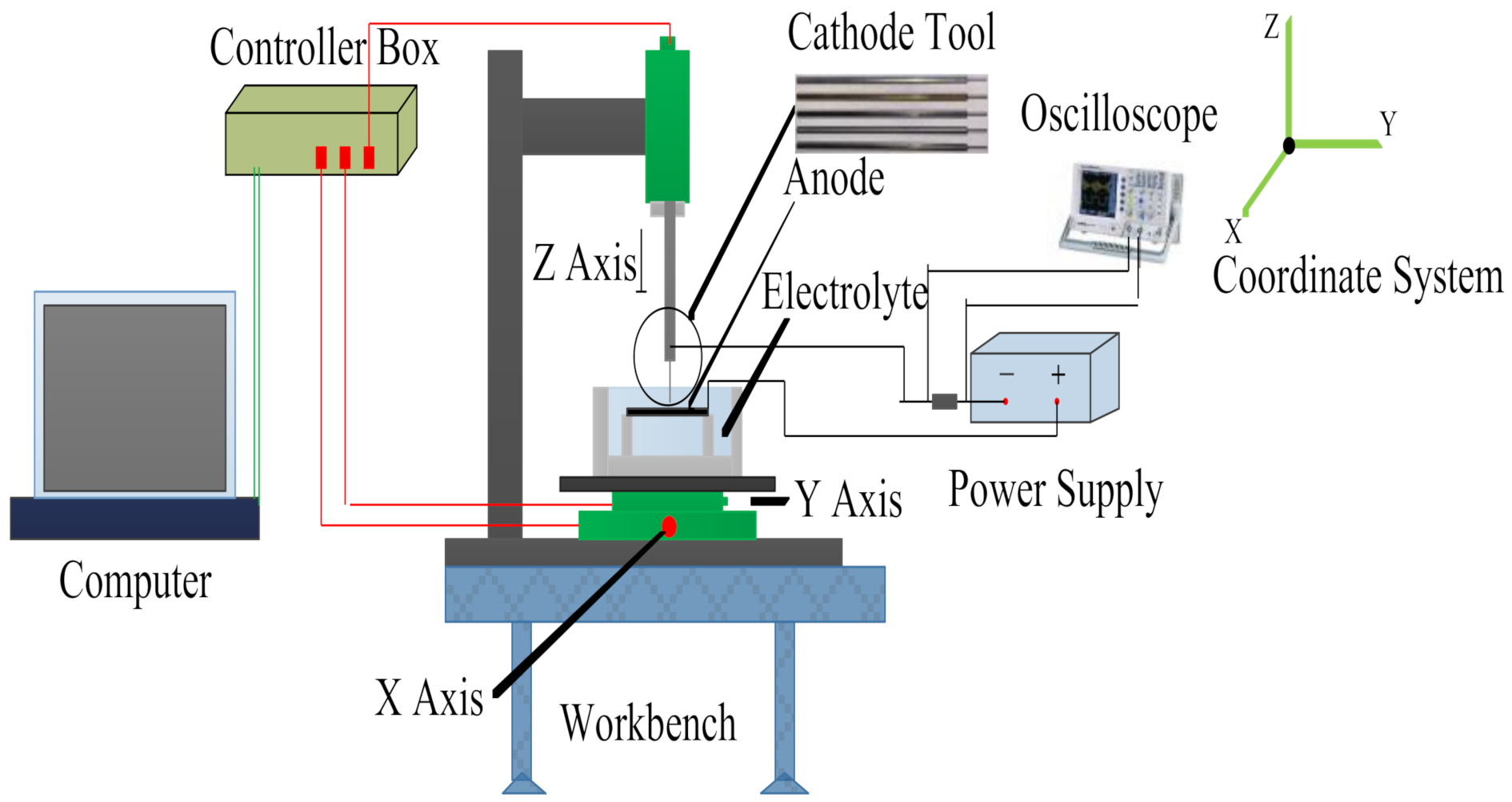
2. Experimental Equipment
3. Simulation of Electric Field
3.1. Physical Simulation Model
3.2. Physical Simulation Model
- (a)
- The distribution of surface current density on the anode workpiece is decided by the Ohm effect.
- (b)
- The electric conductivity k of the electrolyte is fixed.
- (c)
- The tool electrode is defined as the equipotential surface.
3.3. Simulation and Experimental Parameters
3.4. Simulation Results
4. Experimental Results and Discussion
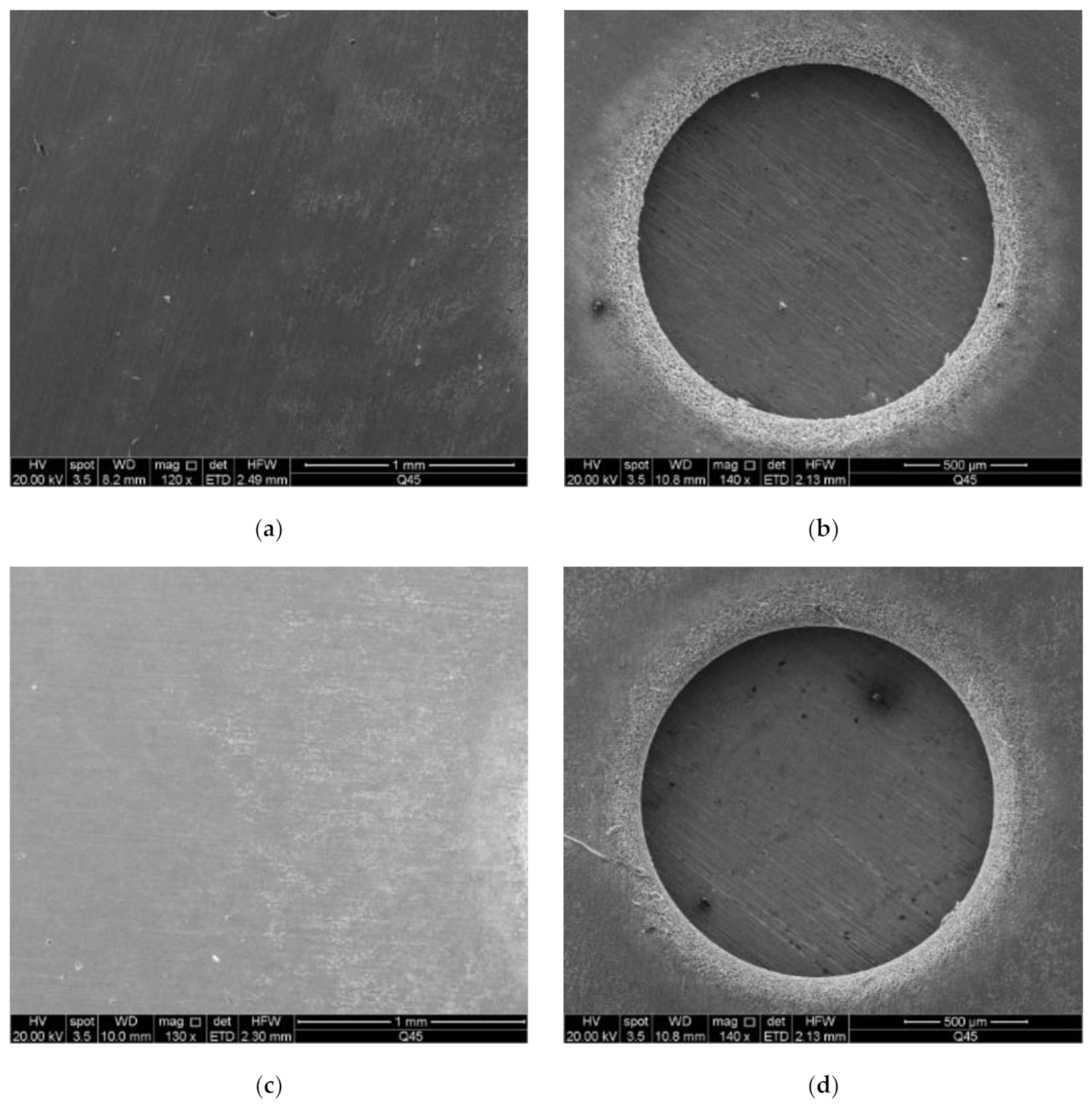
4.1. The Stray Current Corrosion of Hole
4.2. Taper of Hole
4.3. Material Removal Rate
5. Conclusions
- Simulation was conducted to the current density and electric field distribution on the anode surface, and the results show that the variation of the cathode tool could not change the current density mode and electric field mode generated on the anode surface.
- According to the simulation and experimental results, the oxide film generated on the aluminum electrode surface could provide side insulation. In the machining of the 304 stainless steel workpiece, the tool cathode of aluminum alloy 6061 could provide better machining quality than other common tool electrodes. In addition, the oxide film generated on the aluminum electrode surface could alleviate secondary corrosion of the anode workpiece, which could help reduce the taper of the hole structure, and the stray current corrosion could also be reduced. Combining the aspects of machining quality and material removal rate, aluminum alloy 6061 is a better choice of cathode used in the electrochemical machining of 304 stainless steel.
- The insulation characteristics of its oxide film can facilitate the promotion and application of the aluminum alloy 6061 tool cathode in the field of electrochemical machining.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Razali, A.R.; Qin, Y. A Review on Micro-manufacturing, Micro-forming and Their Key Issues. Procedia Eng. 2013, 53, 665–672. [Google Scholar] [CrossRef] [Green Version]
- Bian, J.X.; Ma, B.J.; Liu, X.F. Experimental Study of Tool Wear in Electrochemical Discharge Machining. Appl. Sci. 2020, 10, 5039. [Google Scholar] [CrossRef]
- Spieser, A.; Ivanov, A. Design of a pulse power supply unit for micro-ECM. Int. J. Adv. Manuf. Technol. 2015, 78, 537–547. [Google Scholar] [CrossRef] [Green Version]
- Giandomenico, N.; Meylan, O. Development of a New Generator for Electrochemical Micro-machining. Procedia CIRP 2016, 42, 804–808. [Google Scholar] [CrossRef]
- Xu, Z.Y.; Sun, L.Y.; Hu, Y. Flow Field Design and Experimental Investigation of Electrochemical Machining on Blisk Cascade Passage. Int. J. Adv. Manuf. Technol. 2014, 71, 459–469. [Google Scholar] [CrossRef]
- Qu, N.S.; Hu, Y.; Zhu, D. Electrochemical Machining of Blisk Channels with Progressive-Pressure Electrolyte Flow. Mater. Manuf. Process. 2014, 29, 572–578. [Google Scholar] [CrossRef]
- Dabrowski, L.; Paczkowski, T. Computer Simulation of Two-Dimensional Electrolyte Flow in Electrochemical Machining. J. Electrochem. 2005, 41, 91–98. [Google Scholar] [CrossRef]
- Kozak, J.; Dabrowski, L.; Lubkowski, K. CAE-ECM System for Electrochemical Technology of Parts and Tools. J. Mater. Process. Technol. 2000, 107, 293–299. [Google Scholar] [CrossRef]
- Fujisawa, T.; Inaba, K.; Yamamoto, M. Multiphysics Simulation of Electrochemical Machining Process for Three-Dimensional Compressor Blade. J. Fluid. Eng. 2008, 130, 081602. [Google Scholar] [CrossRef]
- Zhang, C.; Ai, H.; Yan, Z.; Jiang, X.; Cheng, P.; Hu, Y.; Tian, H. Cathode optimization and multi-physics simulation of pulse electrochemical machining for small inner-walled ring grooves. Int. J. Adv. Manuf. Technol. 2020, 106, 401–416. [Google Scholar] [CrossRef]
- Zhan, S.D.; Zhao, Y.H. Plasma-assisted electrochemical machining of microtools and microstructures. Int. J. Mach Tool. Manuf. 2020, 156, 103596. [Google Scholar] [CrossRef]
- Hung, J.C.; Liu, Y.R.; Tsui, H.P. Electrode insulation layer for electrochemical machining fabricated through hot-dip aluminizing and microarc oxidation on a stainless-steel substrate. Surf. Coat. Technol. 2019, 378, 124995. [Google Scholar] [CrossRef]
- Wang, F.; Yao, J.; Kang, M. Electrochemical machining of a rhombus hole with synchronization of pulse current and low-frequency oscillations. J. Manuf. Process. 2020, 57, 91–104. [Google Scholar] [CrossRef]
- Lian, H.; Guo, Z.; Huang, Z. Experimental Research of Al6061 on Ultrasonic Vibration Assisted Micro-Milling. Procedia CIRP 2013, 6, 561–564. [Google Scholar] [CrossRef] [Green Version]
- Natsu, W.; Nakayama, H.; Yu, Z. Improvement of ECM characteristics by applying ultrasonic vibration. Int. J. Precis. Eng. Manuf. 2012, 13, 1131–1136. [Google Scholar] [CrossRef]
- Yang, I.; Min, S.P.; Chu, C.N. Micro ECM with ultrasonic vibrations using a semi-cylindrical tool. Int. J. Precis. Eng. Manuf. 2009, 10, 5–10. [Google Scholar] [CrossRef]
- Pei, W.; Yu, Z.; Li, J. Influence of Abrasive Particle Movement in Micro USM. Procedia CIRP 2013, 6, 551–555. [Google Scholar] [CrossRef] [Green Version]
- Yao, Z.; Guo, Z.N.; Zhang, Y.J. Research on the Frequency Tracking in Rotary Ultrasonic Machining. Procedia CIRP 2013, 6, 556–560. [Google Scholar] [CrossRef] [Green Version]
- Shibuya, N.; Ito, Y.; Natsu, W. Electrochemical machining of tungsten carbide alloy micro-pin with NaNO3 solution. Int. J. Precis. Eng. Manuf. 2012, 13, 2075–2078. [Google Scholar] [CrossRef]
- Cole, K.M.; Kirk, D.W.; Singh, C.V. Optimizing electrochemical micromachining parameters for Zr-based bulk metallic glass. J. Manuf. Process. 2017, 25, 227–234. [Google Scholar] [CrossRef]
- Rathod, V.; Doloi, B.; Bhattacharyya, B. Experimental Investigations into Machining Accuracy and Surface Roughness of Microgrooves Fabricated by Electrochemical Micromachining. Proc. Inst. Mech. Eng. Part B J. Eng. Manuf. 2015, 229, 1781–1802. [Google Scholar] [CrossRef]
- Abuzied, H.H.; Awad, M.A.; Senbel, H.A. Prediction of Electrochemical Machining Process Parameters Using Artificial Neural Networks. Int. J. Comput. Sci. Eng. 2012, 4, 129–137. [Google Scholar]
- ChenthilJegan, T.M.; Ravindran, D. Electrochemical Machining Process Parameter Optimization Using Particle Swarm Optimization. Comput. Intell. 2017, 33, 1–19. [Google Scholar]
- Senthilkumar, C.; Ganesan, G.; Karthikeyan, R. Parametric Optimization of Electrochemical Machining of Al/15%SiCp Composites Using NSGA-II. Trans. Nonferrous Met. Soc. China 2011, 21, 2294–2300. [Google Scholar] [CrossRef]
- Chakradhar, D.; Gopal, A.V. Multi-objective Optimization of Electrochemical Machining of EN31 Steel by Grey Relational Analysis. Int. J. Model. Optim. 2011, 1, 113–117. [Google Scholar] [CrossRef]
- Mukherjee, R.; Chakraborty, S. Selection of the Optimal Electrochemical Machining Process Parameters Using Biogeography-based Optimization Algorithm. Int. J. Adv. Manuf. Technol. 2013, 64, 781–791. [Google Scholar] [CrossRef]
- Thangamani, G.; Kalaichelvan, K.; Thangaraj, M. Influence of Coated Tool Electrode on Drilling Inconel Alloy 718 in Electrochemical Micro Machining. Procedia CIRP 2016, 46, 127–130. [Google Scholar]
- Meng, L.C.; Zeng, Y.B.; Zhu, D. Helical Carbon Nanotube Fiber Tool Cathode for Wire Electrochemical Micromachining. J. Electrochem. Soc. 2018, 165, 665–673. [Google Scholar] [CrossRef]
- Niu, S.; Qu, N.S.; Fu, S.X.; Fang, X.L.; Li, H.S. Investigation of inner-jet electrochemical milling of nickel-based alloy GH4169/Inconel 718. Int. J. Adv. Manuf. Technol. 2017, 93, 2123–2132. [Google Scholar] [CrossRef]
- Liu, J.; Wang, H.; Zhu, D. Electrochemical Machining of γ-TiAl Intermetallic Blades by Using the Stainless Steel Anti-copied Tool Electrodes. Procedia CIRP 2018, 68, 757–761. [Google Scholar] [CrossRef]
- Rahman, Z.; Das, A.K.; Chattopadhyaya, S. Microhole drilling through electrochemical processes: A review. Adv. Manuf. Process. 2018, 33, 1379–1405. [Google Scholar] [CrossRef]






| Parameters | Values |
|---|---|
| Cathode material | H62 brass|6061 aluminum|304 stainless steel 45 steel|YK15 tungsten steel |
| Anode material | 304 stainless steel |
| Cathode size (mm) | 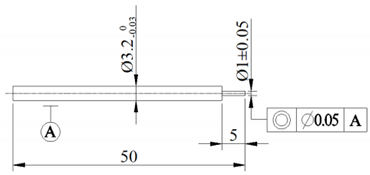 |
| Anode size (mm) | 25 × 25 × 0.3 |
| Electrolyte | 1 mol/L NaNO3 + 0.1 mol/L C6H8O7 |
| Cathode potential (V) | 10 |
| Anode potential (V) | 0 |
| Initial interelectrode gap (IEG) (mm) | 0.2 |
| Electrolyte conductivity (S/m) | 1.542 |
| Temperature (°C) | 20 |
| Spindle speed (rpm) | 3000 |
| Feed speed (μm/s) | 1.0 |
| Workpiece thickness(mm) | 0.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bian, J.; Ma, B.; Ai, H.; Qi, L. Experimental Study on the Influence of Tool Electrode Material on Electrochemical Micromachining of 304 Stainless Steel. Materials 2021, 14, 2311. https://doi.org/10.3390/ma14092311
Bian J, Ma B, Ai H, Qi L. Experimental Study on the Influence of Tool Electrode Material on Electrochemical Micromachining of 304 Stainless Steel. Materials. 2021; 14(9):2311. https://doi.org/10.3390/ma14092311
Chicago/Turabian StyleBian, Jianxiao, Baoji Ma, Haihong Ai, and Lijun Qi. 2021. "Experimental Study on the Influence of Tool Electrode Material on Electrochemical Micromachining of 304 Stainless Steel" Materials 14, no. 9: 2311. https://doi.org/10.3390/ma14092311
APA StyleBian, J., Ma, B., Ai, H., & Qi, L. (2021). Experimental Study on the Influence of Tool Electrode Material on Electrochemical Micromachining of 304 Stainless Steel. Materials, 14(9), 2311. https://doi.org/10.3390/ma14092311






