Photocatalytic Degradation of Cefixime Trihydrate by Bismuth Ferrite Nanoparticles
Abstract
:1. Introduction
2. Experimental Methodology
2.1. Materials
2.2. Synthesis of Bismuth Ferrite (BFO) Nanoparticles
2.3. Degradation Studies
2.4. Characterization Techniques
3. Results and Discussion
3.1. X-ray Diffraction Analysis (XRD)
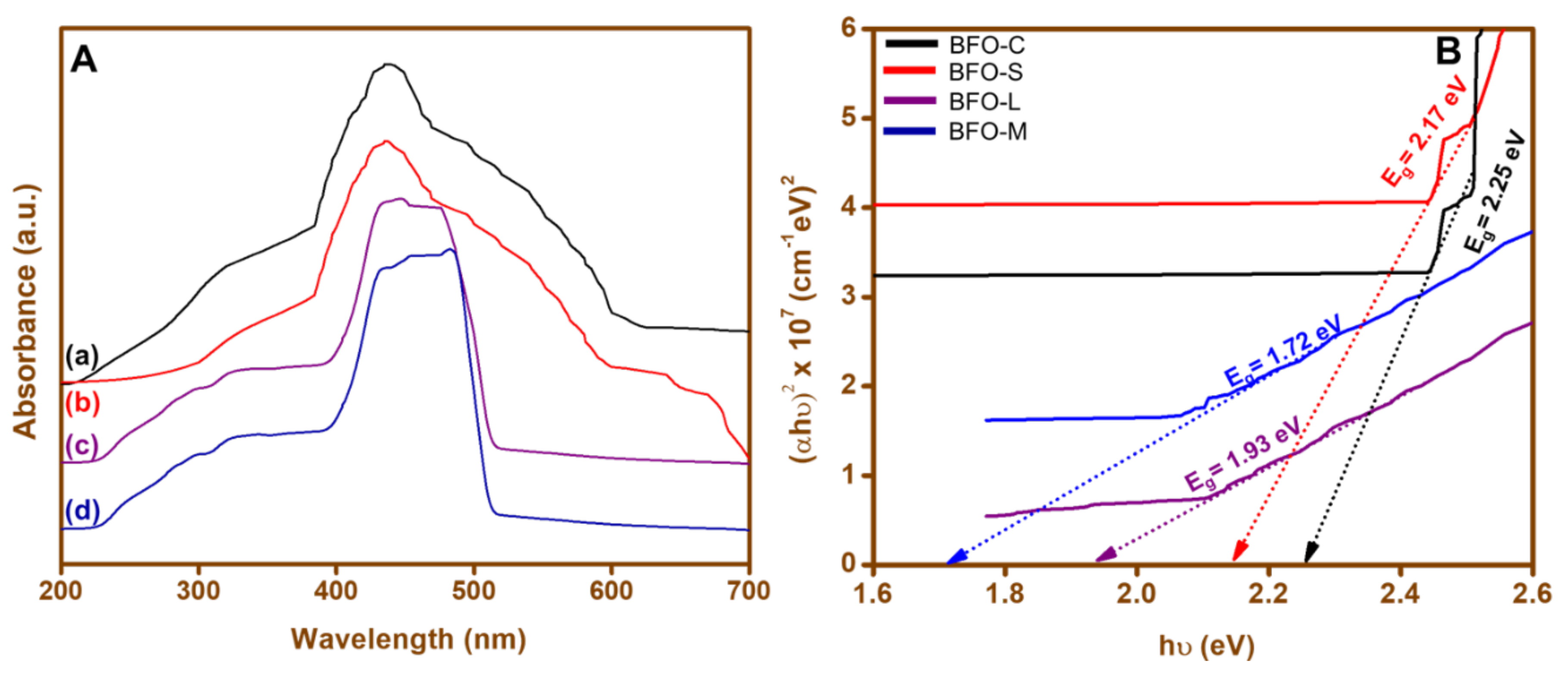
3.2. UV–Vis Absorbance Spectroscopy (UV-Vis)
3.3. Fourier-Transform Infrared Spectroscopy (FTIR)
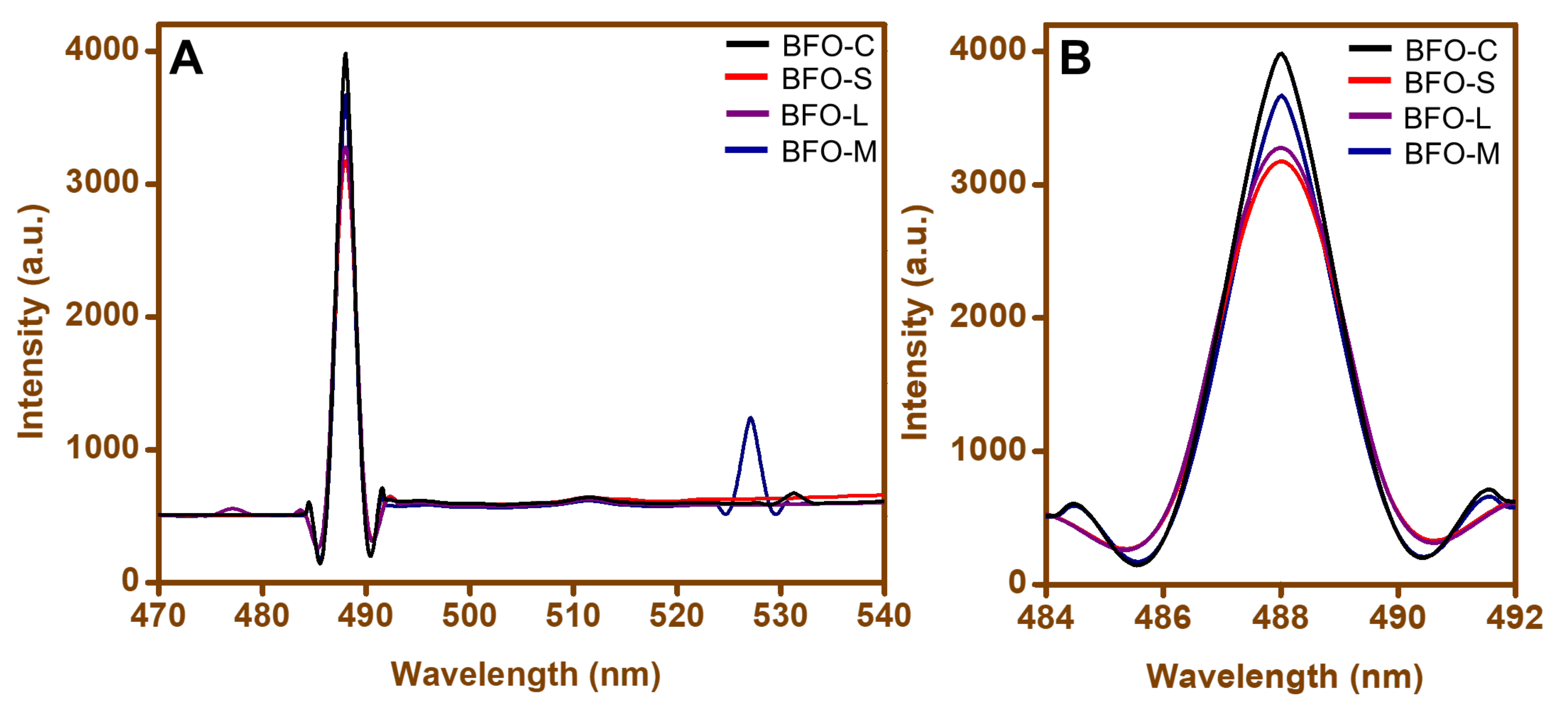
3.4. Photoluminescence (PL)
3.5. Scanning Electron Microscope (SEM)
3.6. Energy-Dispersive Spectroscopy (EDS)
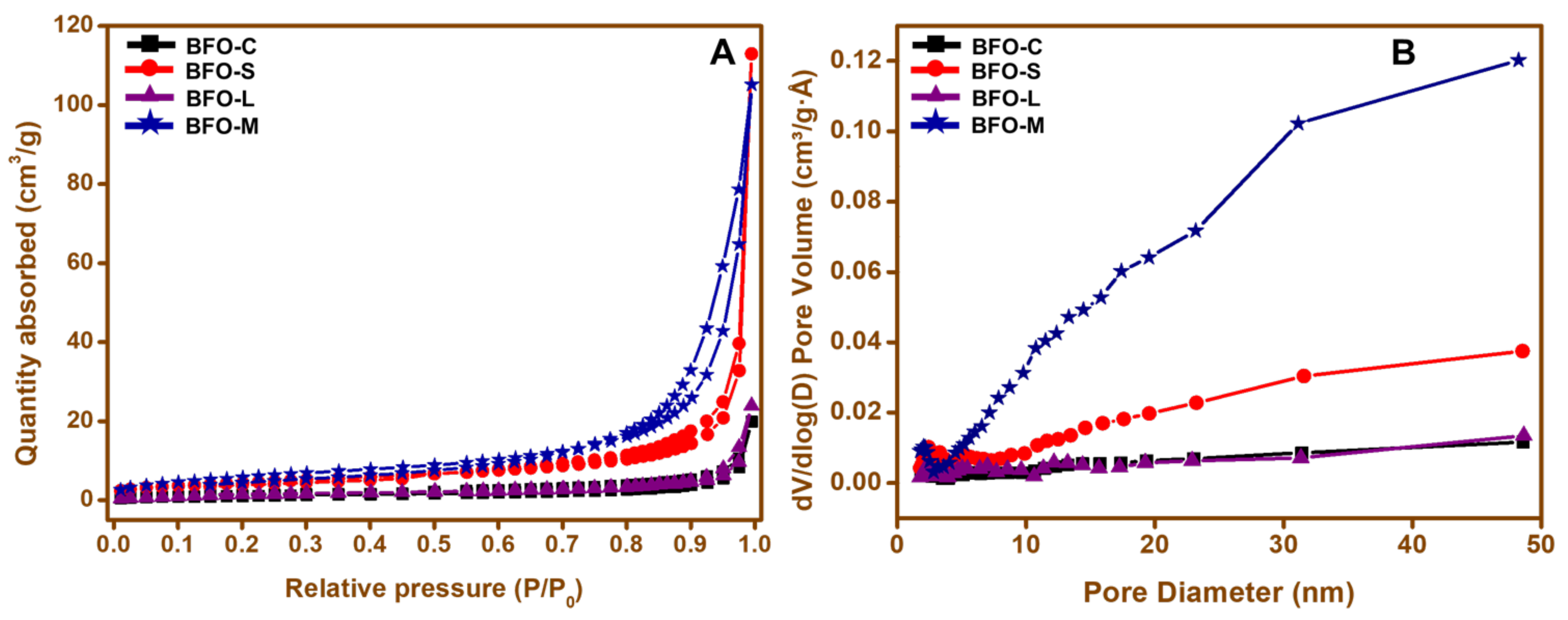
3.7. N2 Sorption Studies
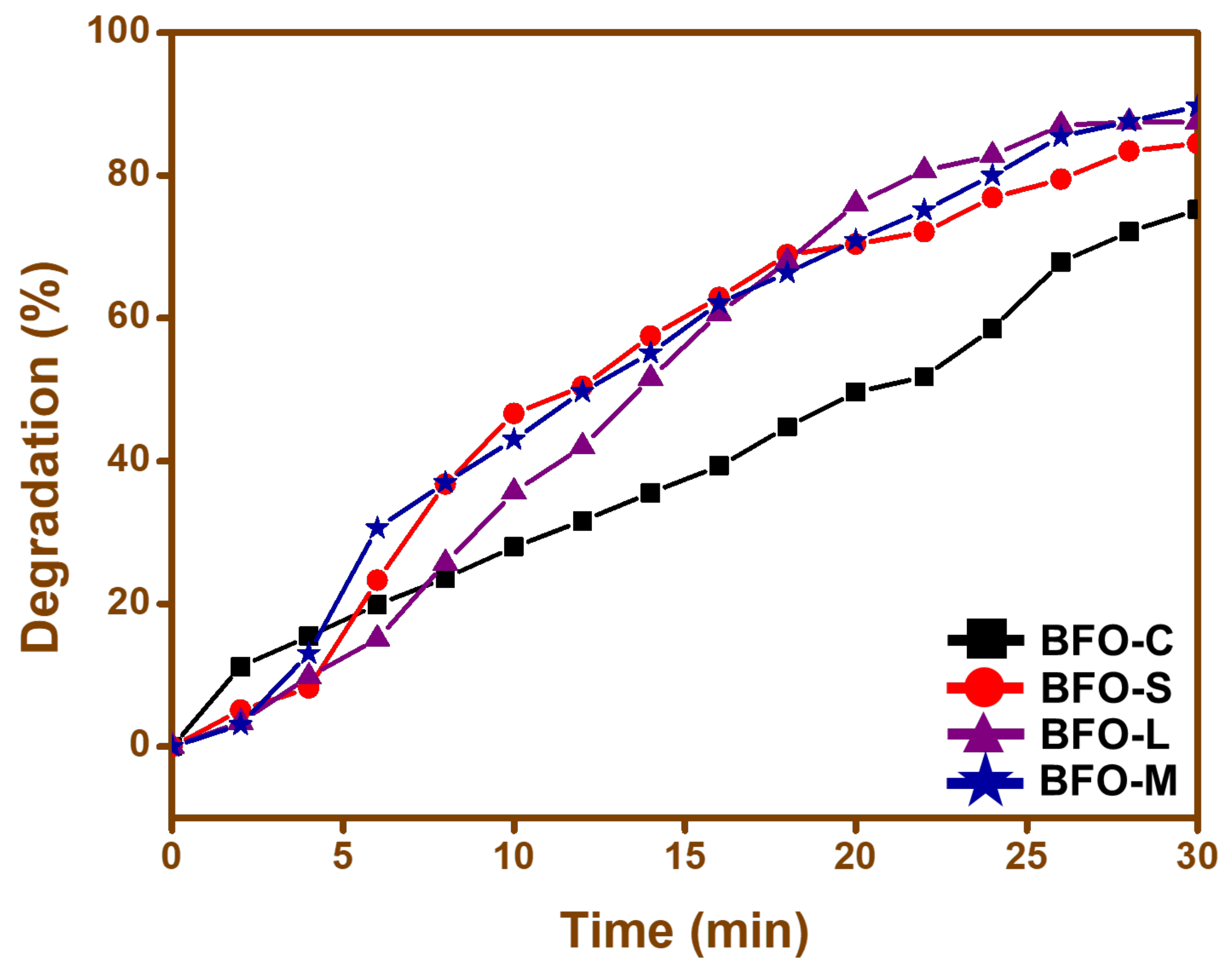
3.8. Degradation Studies
3.9. Optimization of Various Parameters
3.9.1. Effect of the Catalyst
3.9.2. Effect of Cefixime Trihydrate
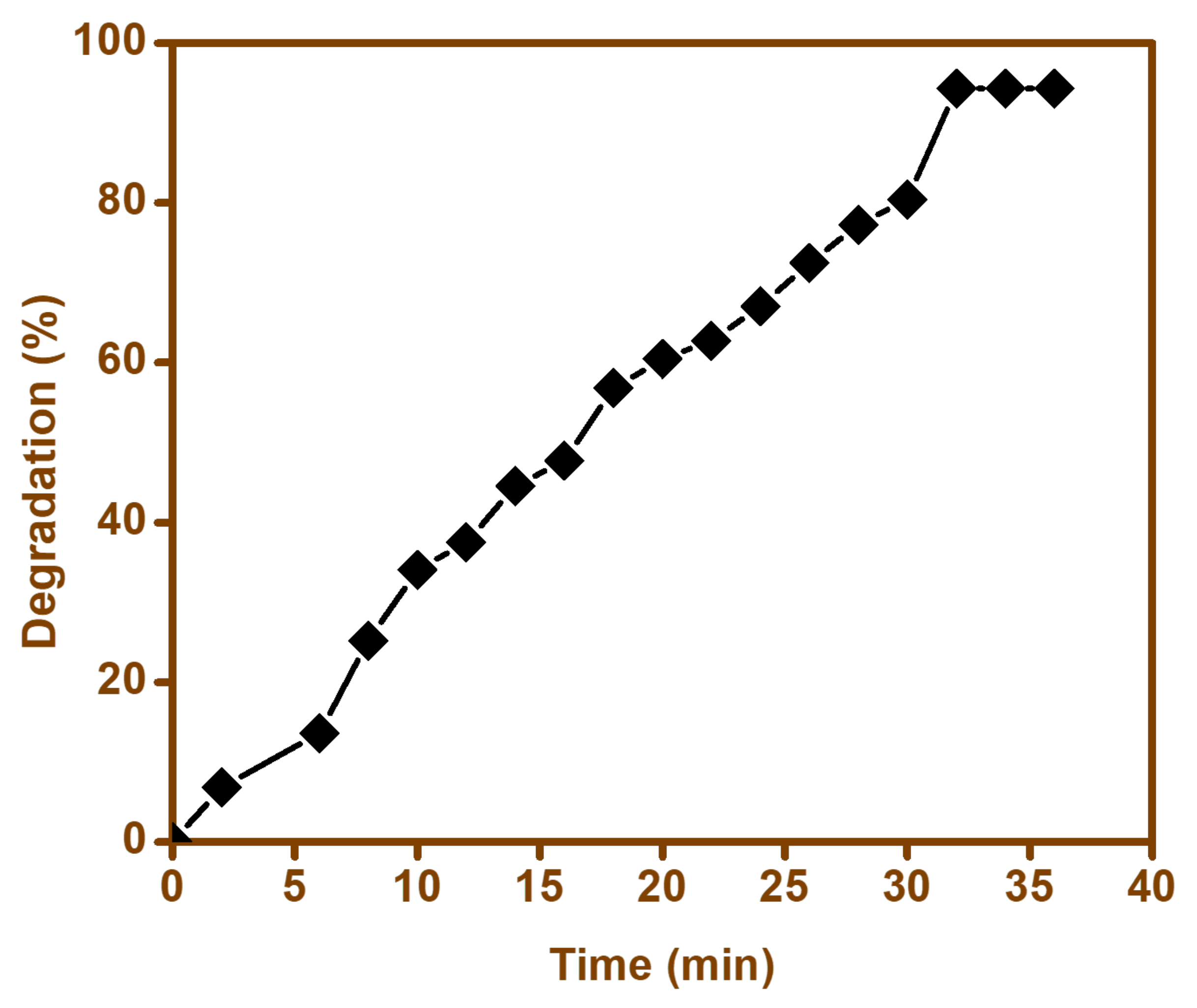
3.9.3. Effect of Time
3.9.4. Effect of pH
3.10. Kinectics Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mahmood, A.R.; Al-Haideri, H.H.; Hassan, F.M. Detection of Antibiotics in Drinking Water Treatment Plants in Baghdad City, Iraq. Adv. Public Health 2019, 2019, 7851354. [Google Scholar] [CrossRef] [Green Version]
- Manzetti, S.; Ghisi, R. The environmental release and fate of antibiotics. Mar. Pollut. Bull. 2014, 79, 7–15. [Google Scholar] [CrossRef]
- Storz, G.; Hengge, R. Bacterial Stress Responses; American Society for Microbiology Press: Washington, DC, USA, 2010. [Google Scholar]
- Glassmeyer, S.T.; Hinchey, E.K.; Boehme, S.E.; Daughton, C.G.; Ruhoy, I.S.; Conerly, O.; Daniels, R.L.; Lauer, L.; McCarthy, M.; Nettesheim, T.G.; et al. Disposal practices for unwanted residential medications in the United States. Environ. Int. 2009, 35, 566–572. [Google Scholar] [CrossRef]
- Hussain, S.; Naeem, M.; Chaudhry, M.N. Estimation of Residual Antibiotics in Pharmaceutical Effluents and their Fate in Affected Areas. Pol. J. Environ. Stud. 2016, 25, 607–614. [Google Scholar] [CrossRef]
- Bound, J.P.; Kitsou, K.; Voulvoulis, N. Household disposal of pharmaceuticals and perception of risk to the environment. Environ. Toxicol. Pharmacol. 2006, 21, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Abuin, S.; Codony, R.; Compañó, R.; Granados, M.; Prat, M.D. Analysis of macrolide antibiotics in river water by solid-phase extraction and liquid chromatography–mass spectrometry. J. Chromatogr. A 2006, 1114, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Seifrtová, M.; Nováková, L.; Lino, C.; Pena, A.; Solich, P. An overview of analytical methodologies for the determination of antibiotics in environmental waters. Anal. Chim. Acta 2009, 649, 158–179. [Google Scholar] [CrossRef]
- Yousef Tizhoosh, N.; Khataee, A.; Hassandoost, R.; Darvishi Cheshmeh Soltani, R.; Doustkhah, E. Ultrasound-engineered synthesis of WS2@CeO2 heterostructure for sonocatalytic degradation of tylosin. Ultrason. Sonochem. 2020, 67, 105114. [Google Scholar] [CrossRef]
- Hassandoost, R.; Pouran, S.R.; Khataee, A.; Orooji, Y.; Joo, S.W. Hierarchically structured ternary heterojunctions based on Ce3+/Ce4+ modified Fe3O4 nanoparticles anchored onto graphene oxide sheets as magnetic visible-light-active photocatalysts for decontamination of oxytetracycline. J. Hazard. Mater. 2019, 376, 200–211. [Google Scholar] [CrossRef]
- Arandiyan, H.; Mofarah, S.S.; Sorrell, C.C.; Doustkhah, E.; Sajjadi, B.; Hao, D.; Wang, Y.; Sun, H.; Ni, B.-J.; Rezaei, M. Defect engineering of oxide perovskites for catalysis and energy storage: Synthesis of chemistry and materials science. Chem. Soc. Rev. 2021, 50, 10116–10211. [Google Scholar] [CrossRef]
- Alibeigi, A.N.; Javid, N.; Amiri Gharaghani, M.; Honarmandrad, Z.; Parsaie, F. Synthesis, characteristics, and photocatalytic activity of zinc oxide nanoparticles stabilized on the stone surface for degradation of metronidazole from aqueous solution. Environ. Health Eng. Manag. J. 2021, 8, 55–63. [Google Scholar] [CrossRef]
- Song, M.; Qi, K.; Wen, Y.; Zhang, X.; Yuan, Y.; Xie, X.; Wang, Z. Rational design of novel three-dimensional reticulated Ag2O/ZnO Z-scheme heterojunction on Ni foam for promising practical photocatalysis. Sci. Total Environ. 2021, 793, 148519. [Google Scholar] [CrossRef]
- Zhang, R.; Zeng, K.; Zhang, T. Enhanced visible-light-driven photocatalytic activity of Bi2WO6-BiSI Z-scheme heterojunction photocatalysts for tetracycline degradation. Int. J. Environ. Anal. Chem. 2020, 8, 1–16. [Google Scholar] [CrossRef]
- Prasad, A.S.; Kumar, S.N.; Maheswari, M.A.; Prabhakaran, D. Monolithic heterojunctions of CeO2/La2O3/TiO2 nanocomposites as visible-light capturing photoactive materials for fast and efficient clean-up of persistent pharmaceutical pollutants. Bull. Mater. Sci. 2021, 44, 99. [Google Scholar] [CrossRef]
- Rajkumar, M.; Arunpandian, M.; Leeladevi, K.; Rameshkumar, P.; Arunachalam, S. Fabrication of pebble stone-like PbMoO4 nanostructure: Focus on photocatalysis, photoluminescence and electron density distribution analysis. Phys. B Condens. Matter. 2021, 620, 413222. [Google Scholar] [CrossRef]
- Ciğeroğlu, Z.; Şahin, S.; Kazan, E.S. One-pot green preparation of deep eutectic solvent-assisted ZnO/GO nanocomposite for cefixime trihydrate photocatalytic degradation under UV-A irradiation. Biomass Convers. Biorefin. 2021. [Google Scholar] [CrossRef]
- Baaloudj, O.; Assadi, A.A.; Azizi, M.; Kenfoud, H.; Trari, M.; Amrane, A.; Assadi, A.A.; Nasrallah, N. Synthesis and Characterization of ZnBi2O4 Nanoparticles: Photocatalytic Performance for Antibiotic Removal under Different Light Sources. Appl. Sci. 2021, 11, 3975. [Google Scholar] [CrossRef]
- Almasi, F.; Dehghanifard, E.; Mohammadi Kalhori, E. Apllication of photocatalitic process using Fe3O4/TiO2 nanocomposite coreshell on the removal of Cefixim antibiotic from aqueous solutions. J. Environ. Health Eng. 2020, 7, 384–400. [Google Scholar] [CrossRef]
- Jahanshahi, R.; Sobhani, S.; Sansano, J.M. High Performance Magnetically Separable G-C3N4/γ-Fe2O3/TiO2 Nanocomposite with Boosted Photocatalytic Capability towards the Cefixime Trihydrate Degradation under Visible-Light. ChemSelect 2020, 5, 10114–10127. [Google Scholar] [CrossRef]
- Assadi, M.H.N.; Gutiérrez Moreno, J.J.; Hanaor, D.A.H.; Katayama-Yoshida, H. Exceptionally high saturation magnetisation in Eu-doped magnetite stabilised by spin–orbit interaction. Phys. Chem. Chem. Phys. 2021, 23, 20129–20137. [Google Scholar] [CrossRef]
- Assadi, M.H.N.; Katayama-Yoshida, H. Covalency a pathway for achieving high magnetisation in TMFe2O4 compounds. J. Phys. Soc. Jpn. 2019, 88, 044706. [Google Scholar] [CrossRef]
- Berger, R.J.F.; Rettenwander, D.; Spirk, S.; Wolf, C.; Patzschke, M.; Ertl, M.; Monkowius, U.; Mitzel, N.W. Relativistic effects in triphenylbismuth and their influence on molecular structure and spectroscopic properties. Phys. Chem. Chem. Phys. 2012, 14, 15520–15524. [Google Scholar] [CrossRef]
- Li, S.; Lin, Y.-H.; Zhang, B.-P.; Nan, C.-W.; Wang, Y. Photocatalytic and magnetic behaviors observed in nanostructured BiFeO3 particles. J. Appl. Phys. 2009, 105, 056105. [Google Scholar] [CrossRef]
- Quan, Z.; Liu, W.; Hu, H.; Xu, S.; Sebo, B.; Fang, G.; Li, M.; Zhao, X. Microstructure, electrical and magnetic properties of Ce-doped BiFeO3 thin films. J. Appl. Phys. 2008, 104, 084106. [Google Scholar] [CrossRef]
- Choi, T.; Lee, S.; Choi, Y.J.; Kiryukhin, V.; Cheong, S.W. Switchable Ferroelectric Diode and Photovoltaic Effect in BiFeO3. Science 2009, 324, 63. [Google Scholar] [CrossRef]
- Cheong, S.-W.; Mostovoy, M. Multiferroics: A magnetic twist for ferroelectricity. Nat. Mater. 2007, 6, 13–20. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.; Pandey, V.; Kotnala, R.K.; Pandey, D. Direct Evidence for Multiferroic Magnetoelectric Coupling in 0.9 BiFeO3-0.1 BaTiO3. Phys. Rev. Lett. 2008, 101, 247602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peñalva, J.; Lazo, A. Synthesis of Bismuth Ferrite BiFeO3 by solution combustion method. J. Phys. Conf. Ser. 2018, 1143, 012025. [Google Scholar] [CrossRef]
- Comyn, T.P.; Kanguwe, D.F.; He, J.; Brown, A.P. Synthesis of bismuth ferrite lead titanate nano-powders and ceramics using chemical co-precipitation. J. Eur. Ceram. Soc. 2008, 28, 2233–2238. [Google Scholar] [CrossRef]
- Maleki, H.; Haselpour, M.; Fathi, R. The effect of calcination conditions on structural and magnetic behavior of bismuth ferrite synthesized by co-precipitation method. J. Mater. Sci.-Mater. Electron. 2018, 29, 4320–4326. [Google Scholar] [CrossRef]
- Szafraniak-Wiza, I.; Andrzejewski, B.; Hilczer, B. Magnetic Properties of Bismuth Ferrite Nanopowder Obtained by Mechanochemical Synthesis. Acta Phys. Pol. A 2014, 126, 1029. [Google Scholar] [CrossRef]
- Ghosh, S.; Dasgupta, S.; Sen, A.; Sekhar Maiti, H. Low-Temperature Synthesis of Nanosized Bismuth Ferrite by Soft Chemical Route. J. Am. Ceram. Soc. 2005, 88, 1349–1352. [Google Scholar] [CrossRef]
- Tsai, C.-J.; Yang, C.-Y.; Liao, Y.-C.; Chueh, Y.-L. Hydrothermally grown bismuth ferrites: Controllable phases and morphologies in a mixed KOH/NaOH mineralizer. J. Mater. Chem. 2012, 22, 17432–17436. [Google Scholar] [CrossRef]
- Wang, Y.P.; Zhou, L.; Zhang, M.F.; Chen, X.Y.; Liu, J.-M.; Liu, Z.G. Room-temperature saturated ferroelectric polarization in BiFeO3 ceramics synthesized by rapid liquid phase sintering. Appl. Phys. Lett. 2004, 84, 1731–1733. [Google Scholar] [CrossRef]
- Kumar, M.M.; Palkar, V.R.; Srinivas, K.; Suryanarayana, S.V. Ferroelectricity in a pure BiFeO3 ceramic. Appl. Phys. Lett. 2000, 76, 2764–2766. [Google Scholar] [CrossRef]
- Pradhan, A.K.; Zhang, K.; Hunter, D.; Dadson, J.B.; Loiutts, G.B.; Bhattacharya, P.; Katiyar, R.; Zhang, J.; Sellmyer, D.J.; Roy, U.N.; et al. Magnetic and electrical properties of single-phase multiferroic BiFeO3. J. Appl. Phys. 2005, 97, 093903. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.K.; Kim, Y.K.; Funakubo, H.; Ishiwara, H. Epitaxial BiFeO3 thin films fabricated by chemical solution deposition. Appl. Phys. Lett. 2006, 88, 162904. [Google Scholar] [CrossRef]
- Jia, D.-C.; Xu, J.-H.; Ke, H.; Wang, W.; Zhou, Y. Structure and multiferroic properties of BiFeO3 powders. J. Eur. Ceram. Soc. 2009, 29, 3099–3103. [Google Scholar] [CrossRef]
- Xie, S.H.; Li, J.Y.; Proksch, R.; Liu, Y.M.; Zhou, Y.C.; Liu, Y.Y.; Ou, Y.; Lan, L.N.; Qiao, Y. Nanocrystalline multiferroic BiFeO3 ultrafine fibers by sol-gel based electrospinning. Appl. Phys. Lett. 2008, 93, 222904. [Google Scholar] [CrossRef]
- Parvez, M.M.; Haque, M.E.; Akter, M.; Ferdous, H. Synthesis of Bismuth Ferrite Nanoparticles by Modified Pechini Sol-Gel Method. Int. J. Sci. Eng. Investig. 2020, 9, 35–38. [Google Scholar]
- Farhadi, S.; Zaidi, M. Bismuth ferrite (BiFeO3) nanopowder prepared by sucrose-assisted combustion method: A novel and reusable heterogeneous catalyst for acetylation of amines, alcohols and phenols under solvent-free conditions. J. Mol. Catal. A Chem. 2009, 299, 18–25. [Google Scholar] [CrossRef]
- Al-Madanat, O.; AlSalka, Y.; Ramadan, W.; Bahnemann, D.W. TiO2 Photocatalysis for the Transformation of Aromatic Water Pollutants into Fuels. Catalysts 2021, 11, 317. [Google Scholar] [CrossRef]
- Soltani, T.; Entezari, M.H. Photolysis and photocatalysis of methylene blue by ferrite bismuth nanoparticles under sunlight irradiation. J. Mol. Catal. A Chem. 2013, 377, 197–203. [Google Scholar] [CrossRef]
- Liu, H.; Guo, Y.; Guo, B.; Zhang, D. Synthesis and visible-light photocatalysis capability of BiFeO3–(Na0.5Bi0.5)TiO3 nanopowders by a sol–gel method. Solid State Sci. 2013, 19, 69–72. [Google Scholar] [CrossRef]
- Soltani, T.; Entezari, M.H. Solar-Fenton catalytic degradation of phenolic compounds by impure bismuth ferrite nanoparticles synthesized via ultrasound. Chem. Eng. J. 2014, 251, 207–216. [Google Scholar] [CrossRef]
- Phadatare, M.R.; Salunkhe, A.B.; Khot, V.M.; Sathish, C.I.; Dhawale, D.S.; Pawar, S.H. Thermodynamic, structural and magnetic studies of NiFe2O4 nanoparticles prepared by combustion method: Effect of fuel. J. Alloy. Compd. 2013, 546, 314–319. [Google Scholar] [CrossRef]
- Manzoor, A.; Afzal, A.M.; Umair, M.; Ali, A.; Rizwan, M.; Yaqoob, M.Z. Synthesis and characterization of Bismuth ferrite (BiFeO3) nanoparticles by solution evaporation method. J. Magn. Magn. Mater. 2015, 393, 269–272. [Google Scholar] [CrossRef]
- Rojas-George, G.; Silva, J.; Castañeda, R.; Lardizábal, D.; Graeve, O.A.; Fuentes, L.; Reyes-Rojas, A. Modifications in the rhombohedral degree of distortion and magnetic properties of Ba-doped BiFeO3 as a function of synthesis methodology. Mater. Chem. Phys. 2014, 146, 73–81. [Google Scholar] [CrossRef]
- Kharel, P.; Talebi, S.; Ramachandran, B.; Dixit, A.; Naik, V.M.; Sahana, M.B.; Sudakar, C.; Naik, R.; Rao, M.S.R.; Lawes, G. Structural, magnetic, and electrical studies on polycrystalline transition-metal-doped BiFeO3 thin films. J. Phys. Condens. Matter 2008, 21, 036001. [Google Scholar] [CrossRef] [PubMed]
- Karoblis, D.; Griesiute, D.; Mazeika, K.; Baltrunas, D.; Karpinsky, D.V.; Lukowiak, A.; Gluchowski, P.; Raudonis, R.; Katelnikovas, A.; Zarkov, A.; et al. A facile synthesis and characterization of highly crystalline submicro-sized BiFeO3. Materials 2020, 13, 3035. [Google Scholar] [CrossRef]
- Imtiaz, A.; Farrukh, M.A.; Khaleeq-ur-rahman, M.; Adnan, R. Micelle-Assisted Synthesis of Al2O3·CaO Nanocatalyst: Optical Properties and Their Applications in Photodegradation of 2,4,6-Trinitrophenol. Sci. World J. 2013, 2013, 641420. [Google Scholar] [CrossRef] [Green Version]
- Gao, T.; Chen, Z.; Huang, Q.; Niu, F.; Huang, X.; Qin, L.; Huang, Y. A Review: Preparation of Bismuth Ferrite Nanoparticles and Its Applications in Visible-Light Induced Photocatalyses. Rev. Adv. Mater. Sci. 2015, 40, 97–109. [Google Scholar]
- Karthikeyan, K.; Thirumoorthi, A. Synthesis, Characterization and Photoluminescence Behaviour of Bismuth Ferrites. Int. J. Appl. Adv. Sci. Res. 2017, 2, 51–56. [Google Scholar] [CrossRef]
- Wu, H.; Xue, P.; Lu, Y.; Zhu, X. Microstructural, optical and magnetic characterizations of BiFeO3 multiferroic nanoparticles synthesized via a sol-gel process. J. Alloy. Compd. 2018, 731, 471–477. [Google Scholar] [CrossRef]
- Delfard, N.B.; Maleki, H.; Badizi, A.M.; Taraz, M. Enhanced Structural, Optical, and Multiferroic Properties of Rod-Like Bismuth Iron Oxide Nanoceramics by Dopant Lanthanum. J. Supercond. Nov. Magn. 2020, 33, 1207–1214. [Google Scholar] [CrossRef]
- Ke, H.; Wang, W.; Wang, Y.; Xu, J.; Jia, D.; Lu, Z.; Zhou, Y. Factors controlling pure-phase multiferroic BiFeO3 powders synthesized by chemical co-precipitation. J. Alloy. Compd. 2011, 509, 2192–2197. [Google Scholar] [CrossRef]
- Gao, F.; Yuan, Y.; Wang, K.F.; Chen, X.Y.; Chen, F.; Liu, J.-M.; Ren, Z.F. Preparation and photoabsorption characterization of BiFeO3 nanowires. Appl. Phys. Lett. 2006, 89, 102506. [Google Scholar] [CrossRef]
- Wang, X.; Dou, L.; Yang, L.; Yu, J.; Ding, B. Hierarchical structured MnO2@SiO2 nanofibrous membranes with superb flexibility and enhanced catalytic performance. J. Hazard. Mater. 2017, 324, 203–212. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, X.; Song, J.; Si, Y.; Zhuang, X.; Yu, J.; Ding, B. In situ synthesis of flexible hierarchical TiO2 nanofibrous membranes with enhanced photocatalytic activity. J. Mater. Chem. A 2015, 3, 22136–22144. [Google Scholar] [CrossRef]
- Sahraeian, S.; Rahmanian, O.; Alipour, V. High efficient degradation of Cefixime using UV/TiO2 photocatalytic process: A comparison between photocatalytic and photolytic. Hormozgan Med. J. 2017, 21, 141–149. [Google Scholar] [CrossRef] [Green Version]
- Tang, Y.; Xu, Y.; Qi, C.; Li, X.; Xing, E.; Wang, F.; Kan, Z.; Wang, C.; Tang, J.; Zheng, G.; et al. Size-Dependent Effect of Cu2O Nanocubes in Electrochemical and Photocatalytic Properties. J. Nanosci. Nanotechnol. 2018, 18, 8282–8288. [Google Scholar] [CrossRef]
- Kojima, T.; Sugimoto, H.; Fujii, M. Size-Dependent Photocatalytic Activity of Colloidal Silicon Quantum Dot. J. Phys. Chem. C 2018, 122, 1874–1880. [Google Scholar] [CrossRef]
- Yin, L.; Zhang, D.; Wang, D.; Kong, X.; Huang, J.; Wang, F.; Wu, Y. Size dependent photocatalytic activity of ZnS nanostructures prepared by a facile precipitation method. Mater. Sci. Eng. B 2016, 208, 15–21. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, F.; Wu, X.; Chen, P.; Deng, N. Photodegradation of acetaminophen in TiO2 suspended solution. J. Hazard. Mater. 2008, 157, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Konstantinou, I.K.; Albanis, T.A. TiO2-assisted photocatalytic degradation of azo dyes in aqueous solution: Kinetic and mechanistic investigations: A review. Appl. Catal. B Environ. 2004, 49, 1–14. [Google Scholar] [CrossRef]
- Shokri, M.; Isapour, G.; Shamsvand, S.; Kavousi, B. Photocatalytic degradation of ceftriaxone in aqueous solutions by immobilized TiO2 and ZnO nanoparticles: Investigating operational parameters. J. Mater. Environ. Sci. 2016, 7, 2843–2851. [Google Scholar]
- Pourtaheri, A.; Nezamzadeh-Ejhieh, A. Photocatalytic properties of incorporated NiO onto clinoptilolite nano-particles in the photodegradation process of aqueous solution of cefixime pharmaceutical capsule. Chem. Eng. Res. Des. 2015, 104, 835–843. [Google Scholar] [CrossRef]
- Farzadkia, M.; Rahmani, K.; Gholami, M.; Esrafili, A.; Rahmani, A.; Rahmani, H. Investigation of photocatalytic degradation of clindamycin antibiotic by using nano-ZnO catalysts. Korean J. Chem. Eng. 2014, 31, 2014–2019. [Google Scholar] [CrossRef]
- Srinivas, D.; Suman, M.; Preethi, N.; Sivaneswari, S.; Mounika, B.; Naveen, B.; Kumar, G.H.; Murthy, S.V. Formulation and In-vitro characterization of Floating Microcarriers of Cefixime. Int. J. Adv. Pharm. Biol. Chem. 2014, 3, 626–631. [Google Scholar]
- Peres, M.S.; Maniero, M.G.; Guimarães, J.R. Photocatalytic degradation of ofloxacin and evaluation of the residual antimicrobial activity. Photochem. Photobiol. Sci. 2015, 14, 556–562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andreozzi, R.; Caprio, V.; Insola, A.; Marotta, R. Advanced oxidation processes (AOP) for water purification and recovery. Catal. Today 1999, 53, 51–59. [Google Scholar] [CrossRef]
- Bayarri, B.; Giménez, J.; Curcó, D.; Esplugas, S. Photocatalytic degradation of 2,4-dichlorophenol by TiO2/UV: Kinetics, actinometries and models. Catal. Today 2005, 101, 227–236. [Google Scholar] [CrossRef]
- Vasconcelos, T.G.; Henriques, D.M.; König, A.; Martins, A.F.; Kümmerer, K. Photo-degradation of the antimicrobial ciprofloxacin at high pH: Identification and biodegradability assessment of the primary by-products. Chemosphere 2009, 76, 487–493. [Google Scholar] [CrossRef] [PubMed]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nazir, A.; Latif, S.; Adil, S.F.; Kuniyil, M.; Imran, M.; Hatshan, M.R.; Kanwal, F.; Shaik, B. Photocatalytic Degradation of Cefixime Trihydrate by Bismuth Ferrite Nanoparticles. Materials 2022, 15, 213. https://doi.org/10.3390/ma15010213
Nazir A, Latif S, Adil SF, Kuniyil M, Imran M, Hatshan MR, Kanwal F, Shaik B. Photocatalytic Degradation of Cefixime Trihydrate by Bismuth Ferrite Nanoparticles. Materials. 2022; 15(1):213. https://doi.org/10.3390/ma15010213
Chicago/Turabian StyleNazir, Ammara, Shoomaila Latif, Syed Farooq Adil, Mufsir Kuniyil, Muhammad Imran, Mohammad Rafe Hatshan, Farah Kanwal, and Baji Shaik. 2022. "Photocatalytic Degradation of Cefixime Trihydrate by Bismuth Ferrite Nanoparticles" Materials 15, no. 1: 213. https://doi.org/10.3390/ma15010213
APA StyleNazir, A., Latif, S., Adil, S. F., Kuniyil, M., Imran, M., Hatshan, M. R., Kanwal, F., & Shaik, B. (2022). Photocatalytic Degradation of Cefixime Trihydrate by Bismuth Ferrite Nanoparticles. Materials, 15(1), 213. https://doi.org/10.3390/ma15010213







