Porous SiC and SiC/Cf Ceramic Microspheres Derived from Polyhydromethylsiloxane by Carbothermal Reduction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Analytical Methods
2.3. Preparation of Precursor Polysiloxane Microspheres
2.4. Ceramization of Microspheres
3. Results
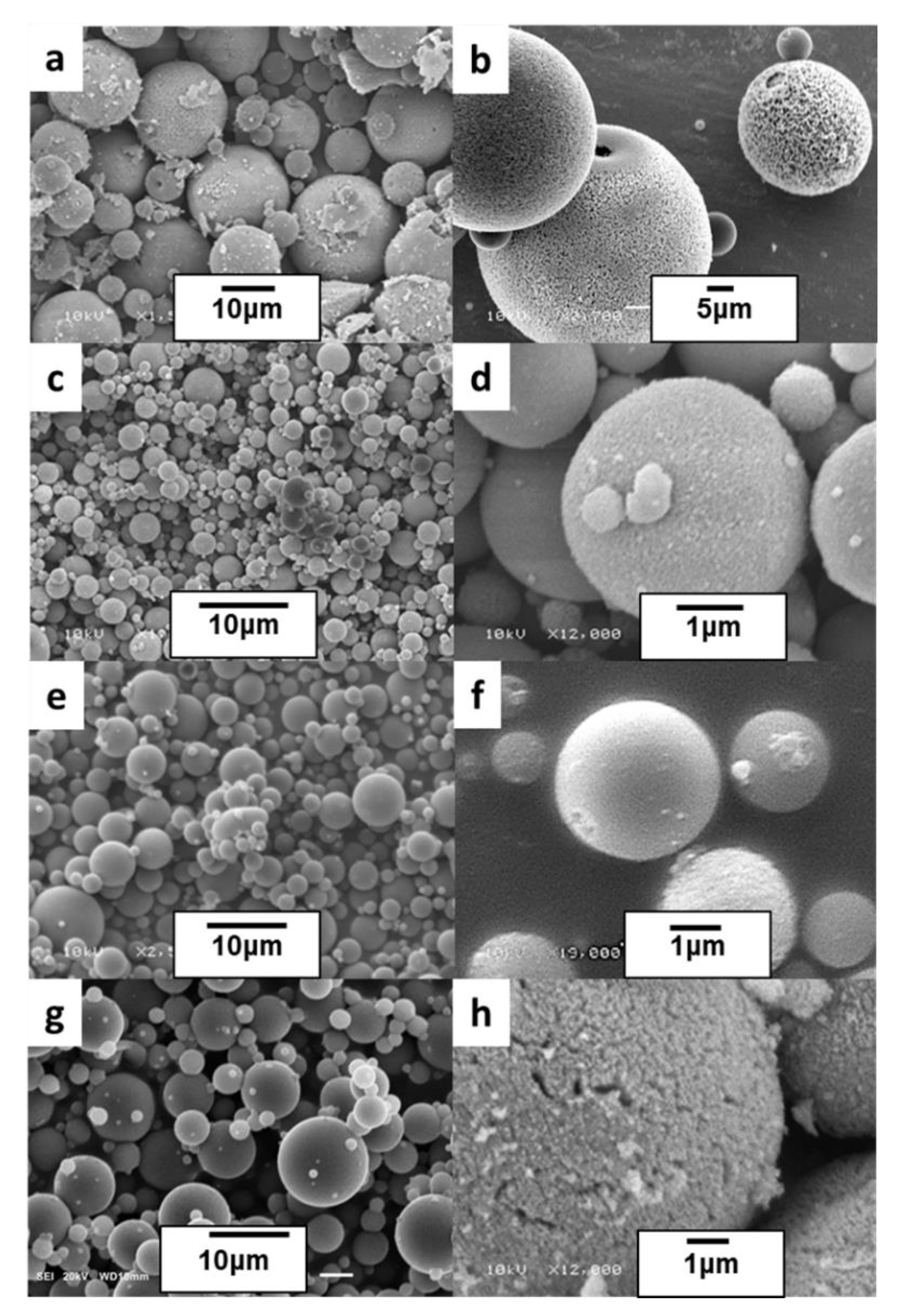
3.1. Precursor Polysiloxane Microspheres
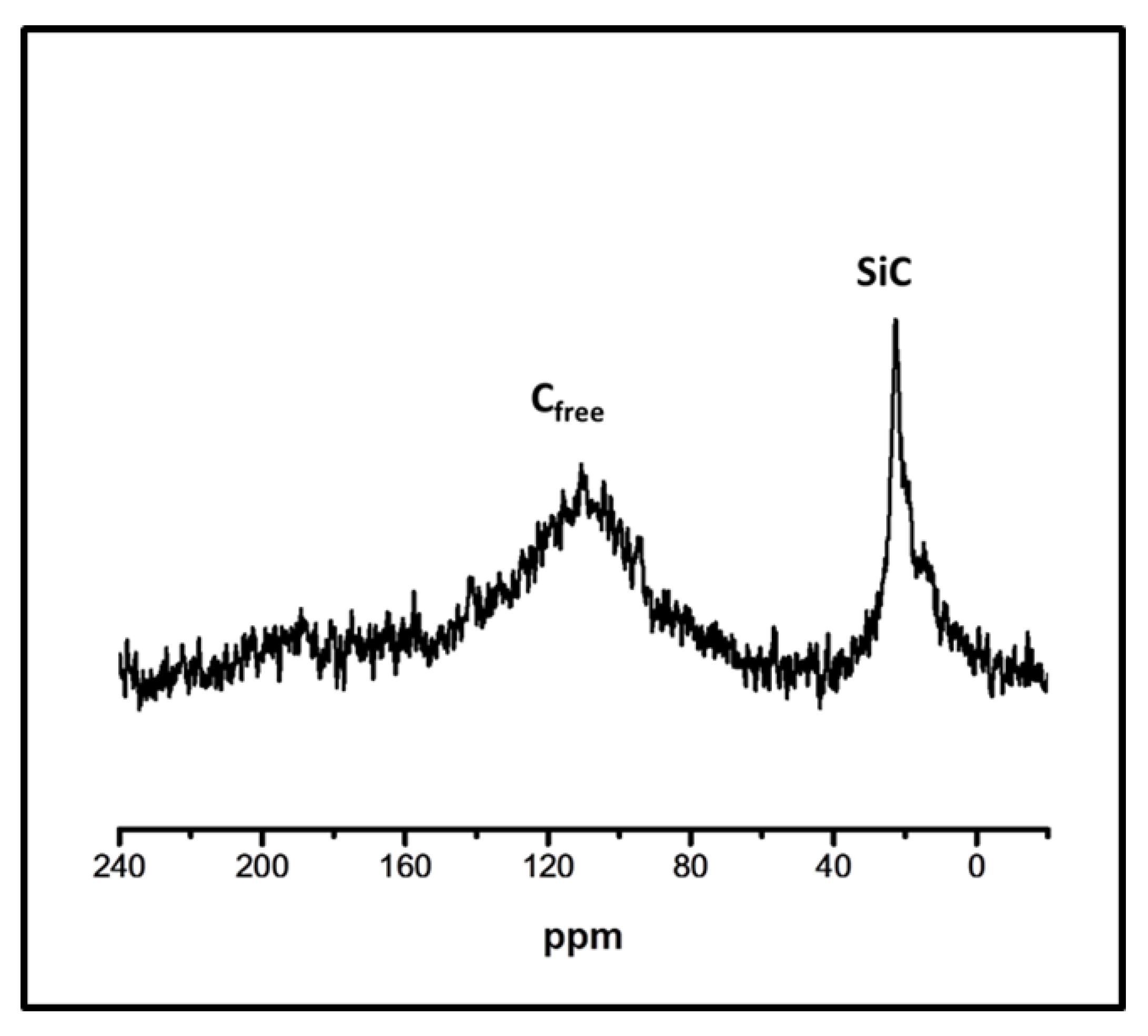
3.2. Ceramic SiC and SiC/Cf Microspheres
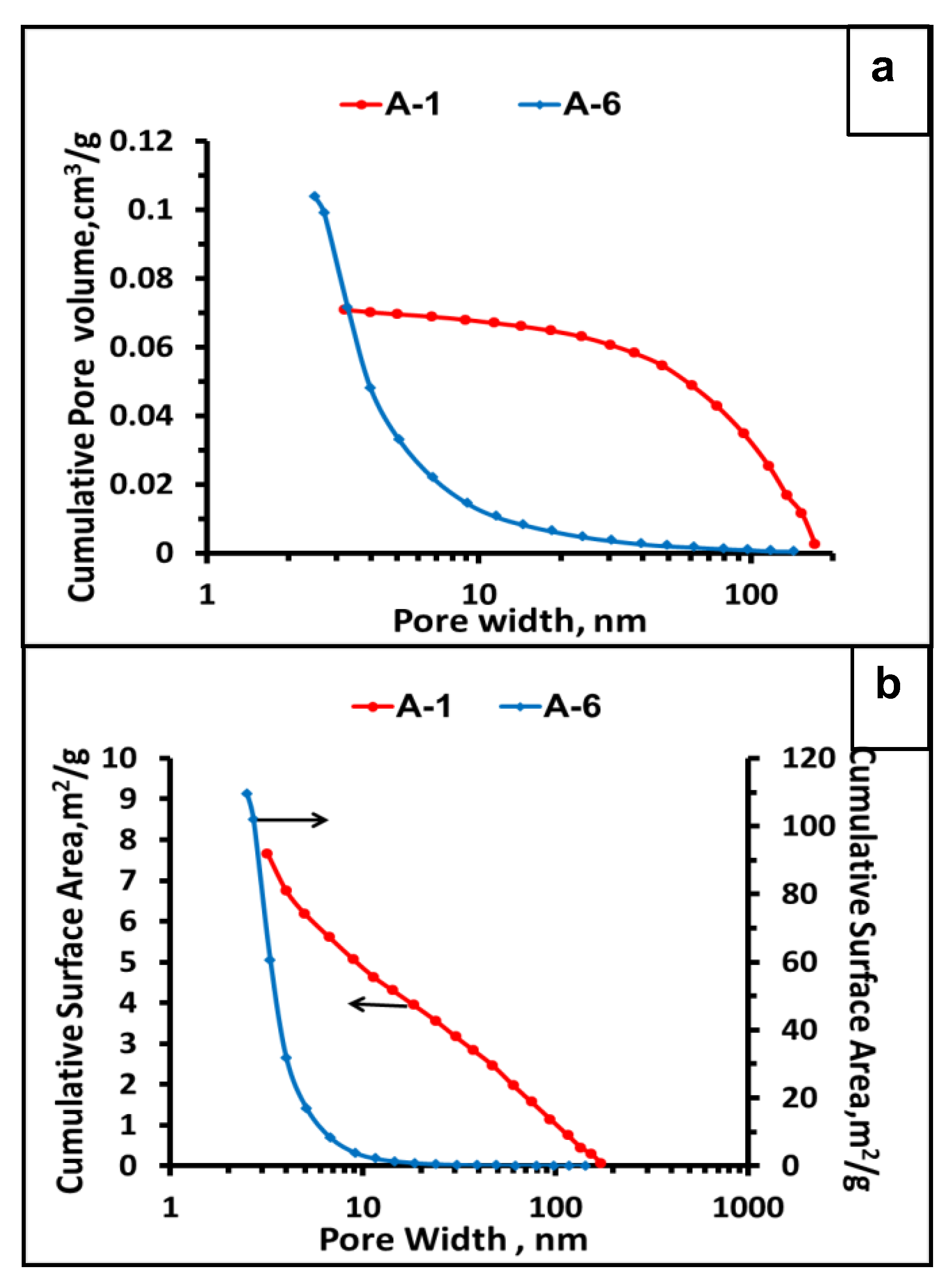
3.3. Porosity by N2 Adsorption
3.4. Porosity by Mercury Intrusion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Naslain, R.R. SiC matrix composites: Tough ceramics for thermostructural applications in different fields. In Engineered Ceramics: Current Status and Future Prospects, Part II Advanced Ceramics and Matrix Composites; Ohji, T., Singh, M., Eds.; American Ceramic Society and John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 142–159. [Google Scholar] [CrossRef]
- Kimoto, T.; Cooper, J.A. Fundamentals of Silicon Carbide Technology: Growth, Characterization, Devices, and Applications; John Wiley & Sons: Singapore, 2014; pp. 1–37. [Google Scholar]
- Khodaei, M.; Yaghobizadeh, O.; Alhosseini, S.H.N.; Esmaeeli, S.; Mousavi, S.R. The effect of oxide, carbide, nitride and boride additives on properties of pressureless sintered SiC: A review. J. Eur. Ceram. Soc. 2019, 39, 2215–2231. [Google Scholar] [CrossRef]
- Ionescu, E.; Bernard, S.; Lucas, R.; Kroll, P.; Ushakov, S.; Navrotsky, A.; Riedel, R. Polymer-Derived Ultra-High Temperature Ceramics (UHTCs) and Related Materials. Adv. Eng. Mater. 2019, 21, 1900269. [Google Scholar] [CrossRef] [Green Version]
- Murayama, N.; Hirao, K.; Sando, M.; Tsuchiya, T.; Yamaguch, H. High-temperature electro-ceramics and their application to SiC power modules. Ceram. Int. 2018, 44, 3523–3530. [Google Scholar] [CrossRef]
- Abderrazak, H.; Hmida, E.S.B.H. Silicon carbide: Synthesis and properties. In Properties and Applications of Silicon Carbide; InTECH: Rijeka, Croatia, 2011; pp. 361–388. [Google Scholar]
- Naglieri, V.; Colombo, P. Ceramic microspheres with controlled porosity by emulsion-ice templating. J. Eur. Ceram. Soc. 2017, 37, 2559–2568. [Google Scholar] [CrossRef]
- Sun, D.; Chen, F.; Gao, Y.; Wang, Y. Synthesis of cross-linked polymer microspheres and pyrolysis conversion to polymer-derived ceramics. Int. J. Appl. Ceram. Technol. 2017, 14, 992–998. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, L.; Luo, C.; Kong, J. Polymer-derived ceramic microspheres with controlled morphology via novel phase separation-assisted pyrolysis. J. Am. Ceram. Soc. 2016, 99, 1485–1493. [Google Scholar] [CrossRef]
- Kang, W.; Liu, H.; Li, J.; Hu, S.; Zhou, C. Fabrication of size-controlled SiC/Si3N4 ceramic microspheres with enhanced extinction property. Ceram. Int. 2015, 41, 10775–10781. [Google Scholar] [CrossRef]
- Feng, Y.; Yu, Z.; Schuch, J.; Tao, S.; Wiehl, L.; Fasel, C.; Jaegermann, W.; Riedel, R. Nowotny phase Mo3+2xSi3C0.6 dispersed in a porous SiC/C matrix: A novel catalyst for hydrogen evolution reaction. J. Am. Ceram. Soc. 2019, 103, 508–519. [Google Scholar] [CrossRef] [Green Version]
- Keller, N.; Pham-Huu, C.; Roy, S.; Ledoux, M.J.; Estournes, C.; Guille, J. Influence of the preparation conditions on the synthesis of high surface area SiC for use as a heterogeneous catalyst support. J. Mater. Sci. 1999, 34, 3189–3202. [Google Scholar] [CrossRef]
- Krasnyi, B.L.; Ikonnikov, K.I.; Vartanyan, M.A.; Rodimov, O.I. Preparation of Porous Permeable Ceramic Based on Silicon Carbide for Hot Flue Gas Filtration. Refract. Ind. Ceram. 2019, 60, 355–361. [Google Scholar] [CrossRef]
- Ghavami, R.; Salimi, A.; Navaee, A. SiC nanoparticles-modified glassy carbon electrodes for simultaneous determination of purine and pyrimidine DNA bases. Biosens. Bioelectron. 2011, 26, 3864–3869. [Google Scholar] [CrossRef]
- Cuiyan, L.; Zhao, X.; Haibo, O.; Liyuan, C.; Jianfeng, H.; Yijun, L. Preparation, Adsorption Properties and Microwave-Assisted Regeneration of Porous C/SiC Ceramics with a Hierarchical Structure. Appl. Compos. Mater. 2020, 27, 131–148. [Google Scholar] [CrossRef]
- Shcherban, N.D.; Filonenko, S.M.; Yaremov, P.S.; Sergiienko, S.i.A.; Ilyin, V.G.; Murzin, D.Y. Carbothermal synthesis of porous silicon carbide using mesoporous silicas. J. Mat. Sci. 2017, 52, 3917–3926. [Google Scholar] [CrossRef]
- Pastila, P.H.; Helanti, V.; Nikkila, A.P.; Mantyla, T.A. Effect of Crystallization on Creep of Clay Bonded SiC filters. Ceram. Eng. Sci. Proc. 1998, 19, 37–44. [Google Scholar] [CrossRef]
- Fukushima, M.; Zhou, Y.; Yoshizawa, Y.I. Fabrication and microstructural characterization of porous SiC membrane supports with Al2O3–Y2O3 additives. J. Membr. Sci. 2009, 339, 78–84. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, P.; Zhou, W.; Luo, H.; Li, Z.; Chen, W.; Li, Y. Microstructures, dielectric response and microwave absorption properties of polycarbosilane derived SiC powders. Ceram. Int. 2018, 44, 3606–3613. [Google Scholar] [CrossRef]
- Mahmoodi, M.; Ghazanfari, L. Fundamentals of biomedical applications of biomorphic SiC. In Silicon Carbide Synthesis and Properties; InTECH: Rijeka, Croatia, 2011; pp. 297–343. [Google Scholar]
- Zhang, Q.; Gou, Y.; Wang, J.; Wang, H.; Jian, K.; Wang, Y. Preparation and characterization of polymer-derived Zr/Si/C multiphase ceramics and microspheres with electromagnetic wave absorbing capabilities. J. Eur. Ceram. Soc. 2017, 37, 1909–1916. [Google Scholar] [CrossRef]
- Liu, X.; Zhu, X.; Pan, D. Solutions for the problems of silicon–carbon anode materials for lithium-ion batteries. R. Soc. Open Sci. 2018, 5, 172370. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.; Kim, J. Redox deposition of birnessite-type manganese oxide on silicon carbide microspheres for use as supercapacitor electrodes. ACS Appl. Mater. Int. 2014, 6, 9036–9045. [Google Scholar] [CrossRef]
- Ma, J.; Li, G.; Chu, Z.; Li, X.; Li, Y.; Hu, T. Microstructure and growth mechanism of multi-layer graphene standing on polycrystalline SiC microspheres. Carbon 2014, 69, 634–637. [Google Scholar] [CrossRef]
- Gouze, B.; Cervantes-Diaz, K.B.; Nardin, T.; Diat, O.; Cambedouzou, J. Highly crystalline silicon carbide of controlled mesoporosity. Mat. Chem. Phys. 2020, 250, 123208. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, H.; Li, J.; Kang, W.; Chu, P. A novel SiCN@ TiO2 core–shell ceramic microspheres derived from a polymeric precursor. Ceram. Int. 2016, 42, 7135–7140. [Google Scholar] [CrossRef]
- Sokolowski, K.; Zambrzycki, M.; Fraczek-Szczypta, A.; Blazewicz, S. Ceramic coating formation during carbothermic reaction of polysiloxanes with carbon and graphite materials. Mat. Chem. Phys. 2019, 238, 121908. [Google Scholar] [CrossRef]
- Duan, L.; Ma, Q.; Chen, Z. Fabrication and CO2 capture performance of silicon carbide derived carbons from polysiloxane. Microporous Mesoporous Mater. 2015, 203, C24–C31. [Google Scholar] [CrossRef]
- Kim, J.H.E.Y.W.; Park, C.B.; Wang, C. Effect of forming methods on porosity and compressive strength of polysiloxane-derived porous silicon carbide ceramics. J. Ceram. Soc. Jpn. 2012, 120, 199–203. [Google Scholar] [CrossRef] [Green Version]
- Eom, J.H.; Kim, Y.W. Effect of additives on mechanical properties of macroporous silicon carbide ceramics. Met. Mater. Int. 2010, 16, 399–405. [Google Scholar] [CrossRef]
- Jang, D.H.; Kim, Y.W.; Song, I.H.; Kim, H.D.; Park, C.B. Processing of highly porous, open-cell, microcellular silicon carbide ceramics by expansion method using expandable microspheres. J. Ceram. Soc. Jpn. 2006, 114, 549–553. [Google Scholar] [CrossRef] [Green Version]
- Du, B.; Hong, C.; Wang, A.; Zhou, S.; Qu, Q.; Zhou, S.; Zhang, X. Preparation and structural evolution of SiOC preceramic aerogel during high-temperature treatment. Ceram. Int. 2018, 44, 563–570. [Google Scholar] [CrossRef]
- Kim, Y.W.; Eom, J.H.; Wang, C.; Park, C.B. Processing of porous silicon carbide ceramics from carbon-filled polysiloxane by extrusion and carbothermal reduction. J. Am. Ceram. Soc. 2008, 91, 1361–1364. [Google Scholar] [CrossRef]
- Jin, G.Q.; Guo, X.Y. Synthesis and characterization of mesoporous silicon carbide. Microporous Mesoporous Mater. 2003, 60, 207–212. [Google Scholar] [CrossRef]
- Fortuniak, W.; Pospiech, P.; Mizerska, U.; Chojnowski, J.; Slomkowski, S.; Nyczyk-Malinowska, A.; Lach, R.; Hasik, M. SiCO ceramic microspheres produced by emulsion processing and pyrolysis of polysiloxanes of various structures. Ceram. Int. 2016, 42, 11654–11665. [Google Scholar] [CrossRef]
- Chojnowski, J.; Slomkowski, S.; Fortuniak, W.; Mizerska, U.; Pospiech, P. Hydrophilic polysiloxane microspheres and ceramic SiOC microspheres derived from them. J. Inorg. Organomet. Polym. Mater. 2020, 30, 56–68. [Google Scholar] [CrossRef] [Green Version]
- Mizerska, U.; Fortuniak, W.; Rubinsztajn, S.; Chojnowski, J. Impact of cross-linkers on the structure and hydrophilic-hydrophobic properties of polyhydromethylsiloxane derived microspheres. Polym. Adv. Technol. 2021, 32, 3967–3974. [Google Scholar] [CrossRef]
- Burns, G.T.; Taylor, R.B.; Xu, Y.; Zangvil, A.; Zank, G.A. High-temperature chemistry of the conversion of siloxanes to silicon carbide. Chem. Mater. 1992, 4, 1313–1323. [Google Scholar] [CrossRef]
- Mazo, M.A.; Tamayo, A.; Rubio, F.; Soriano, D.; Rubio, J. Effect of processing on the structural characteristics of sintered silicon oxycarbide materials. J. Non-Cryst. Solids 2014, 391, 23–31. [Google Scholar] [CrossRef]
- Godoy, N.V.; Pereira, J.L.; Duarte, E.H.; Tarley, C.R.T.; Segatelli, M.G. Influence of activated charcoal on the structural and morphological characteristics of ceramic based on silicon oxycarbide (SiOC): A promising approach to obtain a new electrochemical sensing platform. Mat. Chem. Phys. 2016, 175, 33–45. [Google Scholar] [CrossRef]
- Kleebe, H.J.; Blum, Y.D. SiOC ceramic with high excess free carbon. J. Eur. Ceram. Soc. 2008, 28, 1037–1042. [Google Scholar] [CrossRef]
- Kleebe, H.J.; Gregori, G.; Babonneau, F.; Blum, Y.D.; Queen, D.B.M.; Masse, S. Evolution of C-rich SiOC ceramics. Int. J. Mater. Res. 2006, 97, 699–709. [Google Scholar] [CrossRef]
- Szymanski, W.; Lipa, S.; Fortuniak, W.; Chojnowski, J.; Pospiech, P.; Mizerska, U.; Slomkowski, S.; Nyczyk-Malinowska, A.; Hasik, M. Silicon oxycarbide (SiOC) ceramic microspheres–Structure and mechanical properties by nanoindentation studies. Ceram. Int. 2019, 45, 11946–11954. [Google Scholar] [CrossRef]
- Harder, D.; Jacobson, B.; Myers, N. Oxidation transitions for SiC Part II. Passive-to-active transitions. J. Am. Ceram. Soc. 2013, 96, 606–612. [Google Scholar] [CrossRef]
- Jacobson, N.S.; Lee, K.N.; Fox, D.S. Reactions of silicon carbide and silicon (IV) oxide at elevated temperatures. J. Am. Ceram. Soc. 1992, 5, 1603–1611. [Google Scholar] [CrossRef]
- Seger, M.R.; Maciel, G.E. Quantitative 13C NMR Analysis of Sequence Distributions in Poly(ethylene-co-1-hexene). Anal. Chem. 2004, 76, 5734–5747. [Google Scholar] [CrossRef]
- Ortiz, A.L.; Sanchez-Bajo, F.; Cumbrera, F.L.; Guiberteau, F. X-ray powder diffraction analysis of a silicon carbide-based ceramic. Mat. Lett. 2001, 49, 137–145. [Google Scholar] [CrossRef]
- Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S.; Cançado, L.G.; Jorio, A.; Saito, R. Studying disorder in graphite-based systems by Raman spectroscopy. Phys. Chem. Chem. Phys. 2007, 9, 1276–1290. [Google Scholar] [CrossRef]
- Lucchese, M.M.; Stavale, F.; Ferreira, E.H.M.; Vilani, C.; Moutinho, M.V.O.; Capaz, R.B.; Achete, C.A.; Jorio, A. Quantifying ion-induced defects and Raman relaxation length in graphene. Carbon 2010, 48, 1592–1597. [Google Scholar] [CrossRef]
- Tunistra, F.; Koenig, J.L. Raman spectrum of graphite. J. Phys. Chem. 1970, 53, 1126–1130. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, A.C. Raman spectroscopy of graphene and graphite: Disorder, electron–phonon coupling, doping and nonadiabatic effects. Solid State Commun. 2007, 143, 47–57. [Google Scholar] [CrossRef]
- IUPAC Physical Chemistry Division, Commision on Colloid and Surface Chemistry, Recommendation for the characterization of porous solids—Technical report. Pure Appl. Chem. 1994, 66, 1739–1758. [CrossRef] [Green Version]
- Lowell, M.; Shields, S.; Thomas, J.E.; Thommes, M.A. Characterization of Porous Solids and Powders: Surface Area, Pore Size and Density; The Kluwer Technology Series; Scarlett, B., Ed.; Springer Science & Business Media: Dordrecht, The Netherlands, 2004; Volume 16, pp. 11–14. [Google Scholar] [CrossRef]

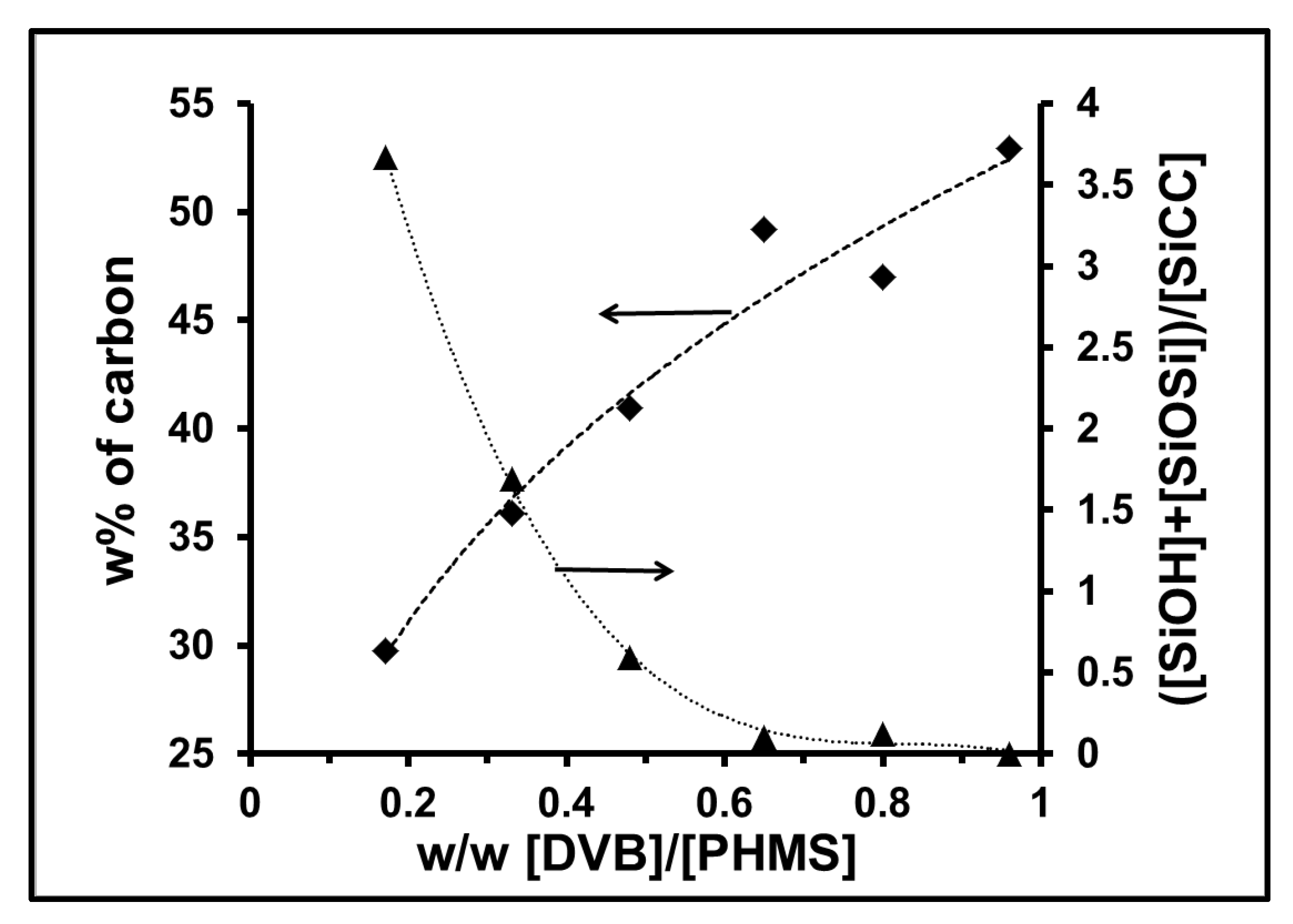
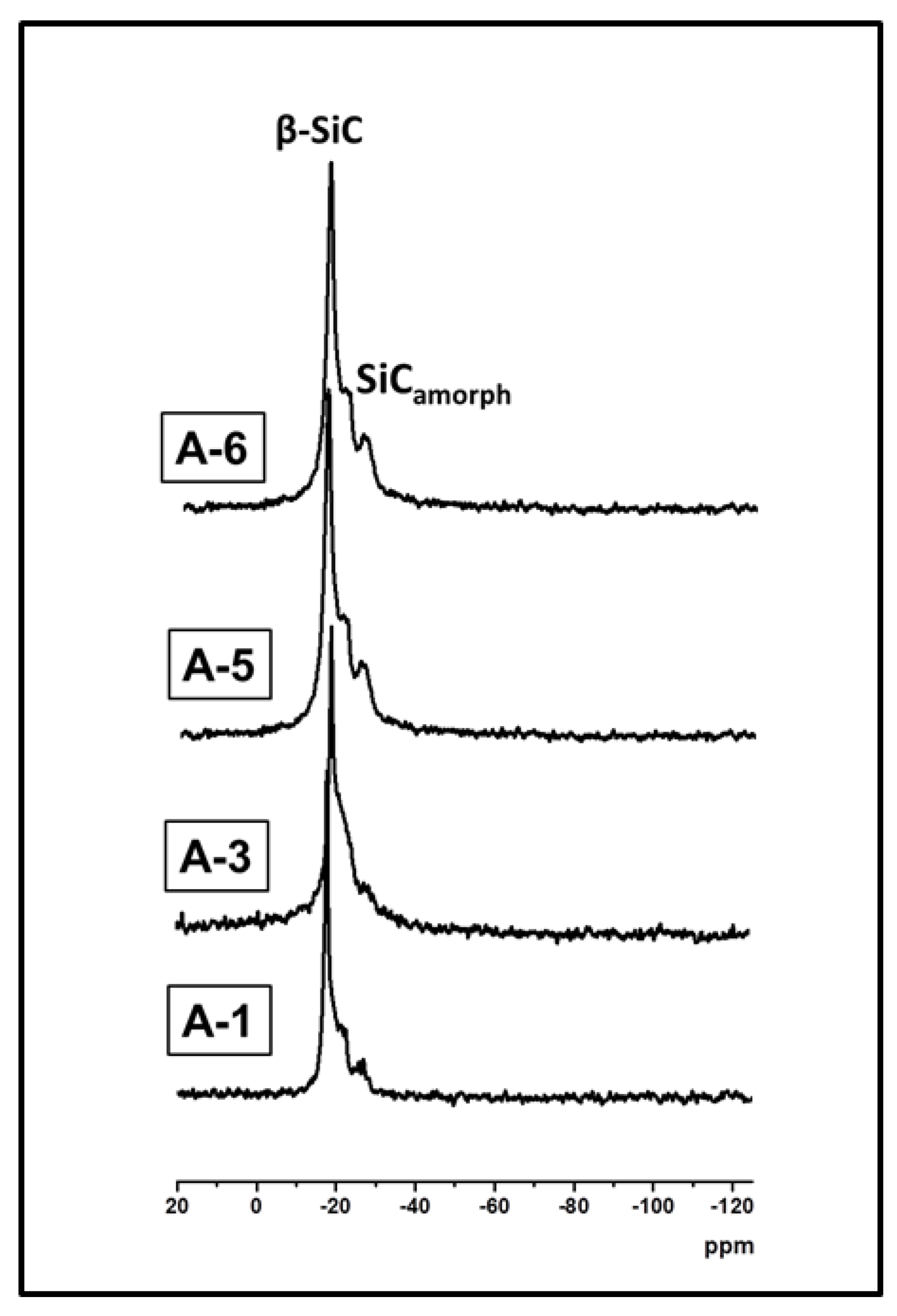
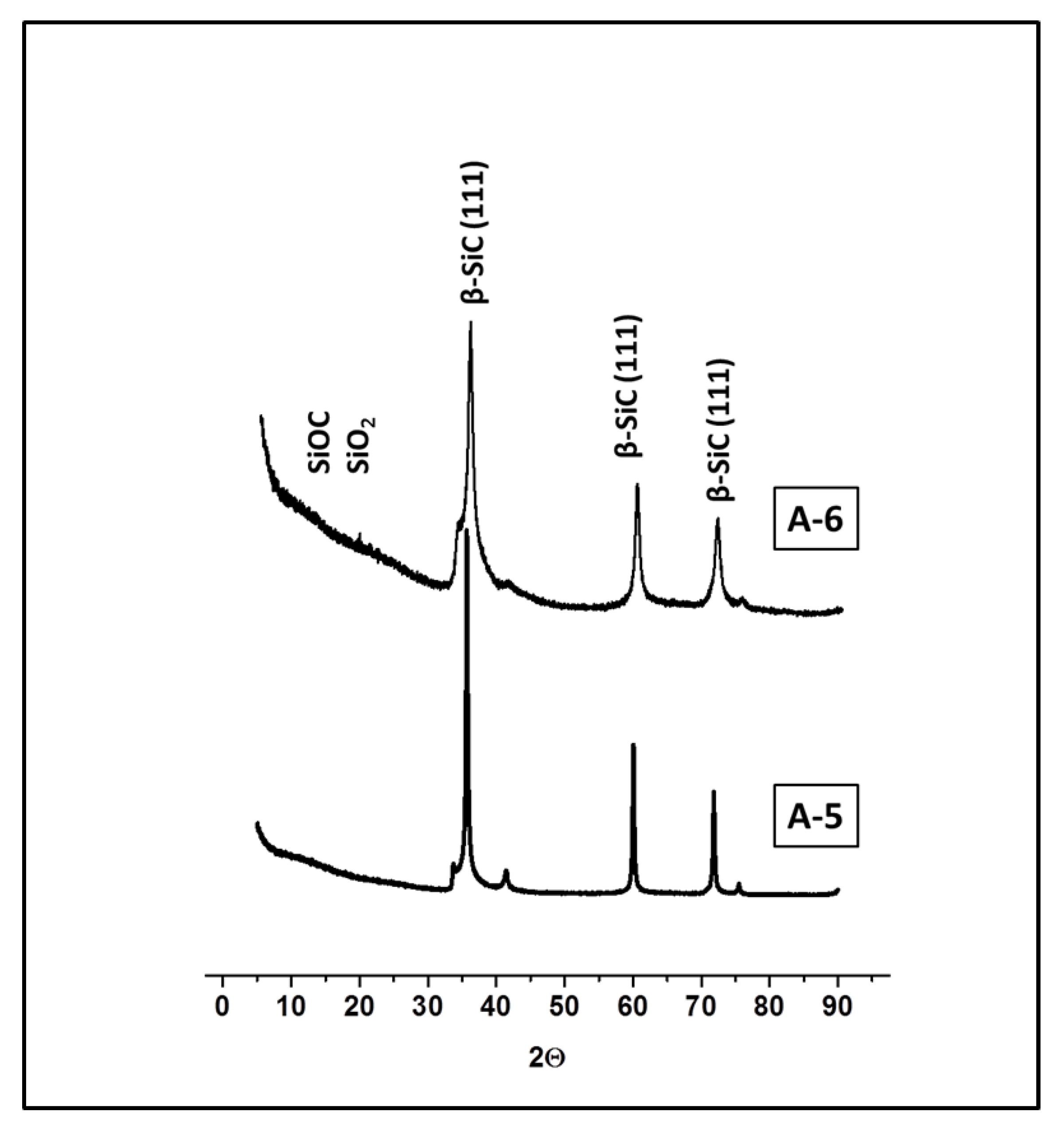
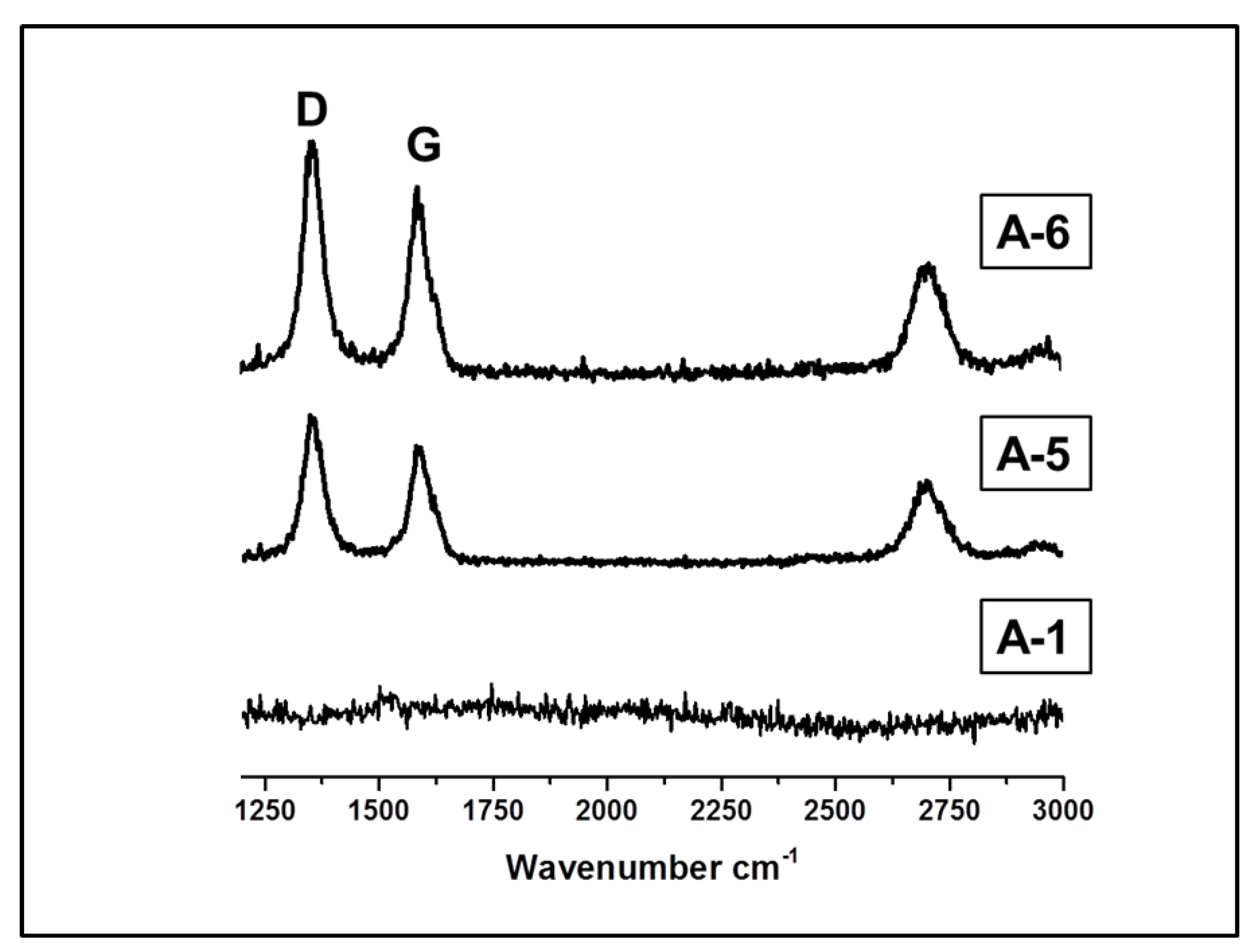
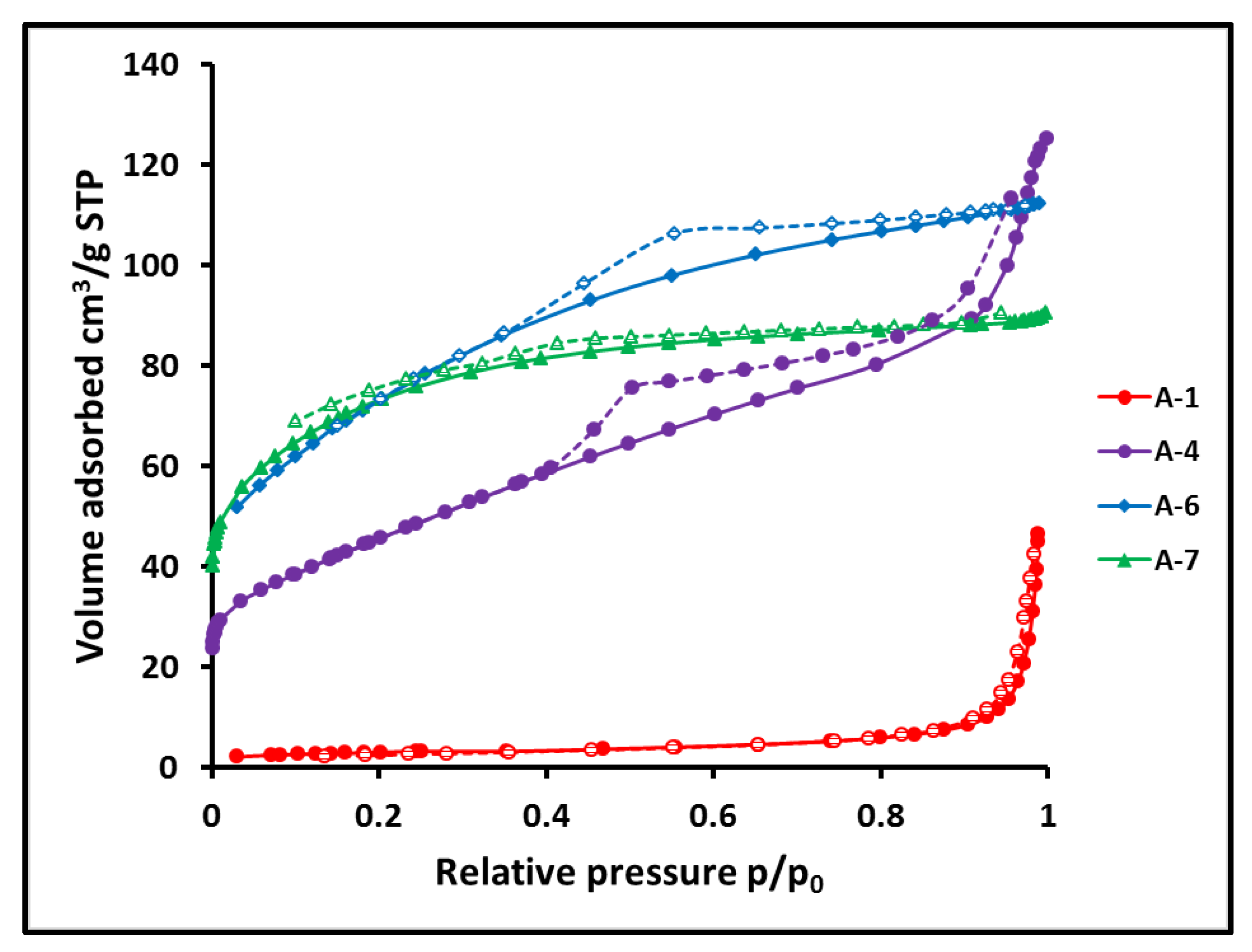

| Sample | Crosslinker | Crosslinker/PHMS w/w | Av. C w% | Av.H w% | ([SiOH] + [SiOSi])/[SiCC] |
|---|---|---|---|---|---|
| PA-1 | DVB | 0.17 | 29.8 | 6.03 | 3.67 |
| PA-2 | DVB | 0.33 | 36.1 | 6.77 | 1.69 |
| PA-3 | DVB | 0.48 | 42.49 | 7.11 | 0.59 |
| PA-4 | DVB | 0.65 | 49.12 | 7.34 | 0.1 |
| PA-5 | DVB | 0.80 | 46.98 | 9.83 | 0.12 |
| PA-6 | DVB | 0.96 | 53.0 | 10.1 | 0 |
| PA-7 | DVB | 0.96 | 55.6 | 9.44 | 0 |
| PB-1 | DVTMDS | 0.22 | 22.67 | 6.42 | 5.29 |
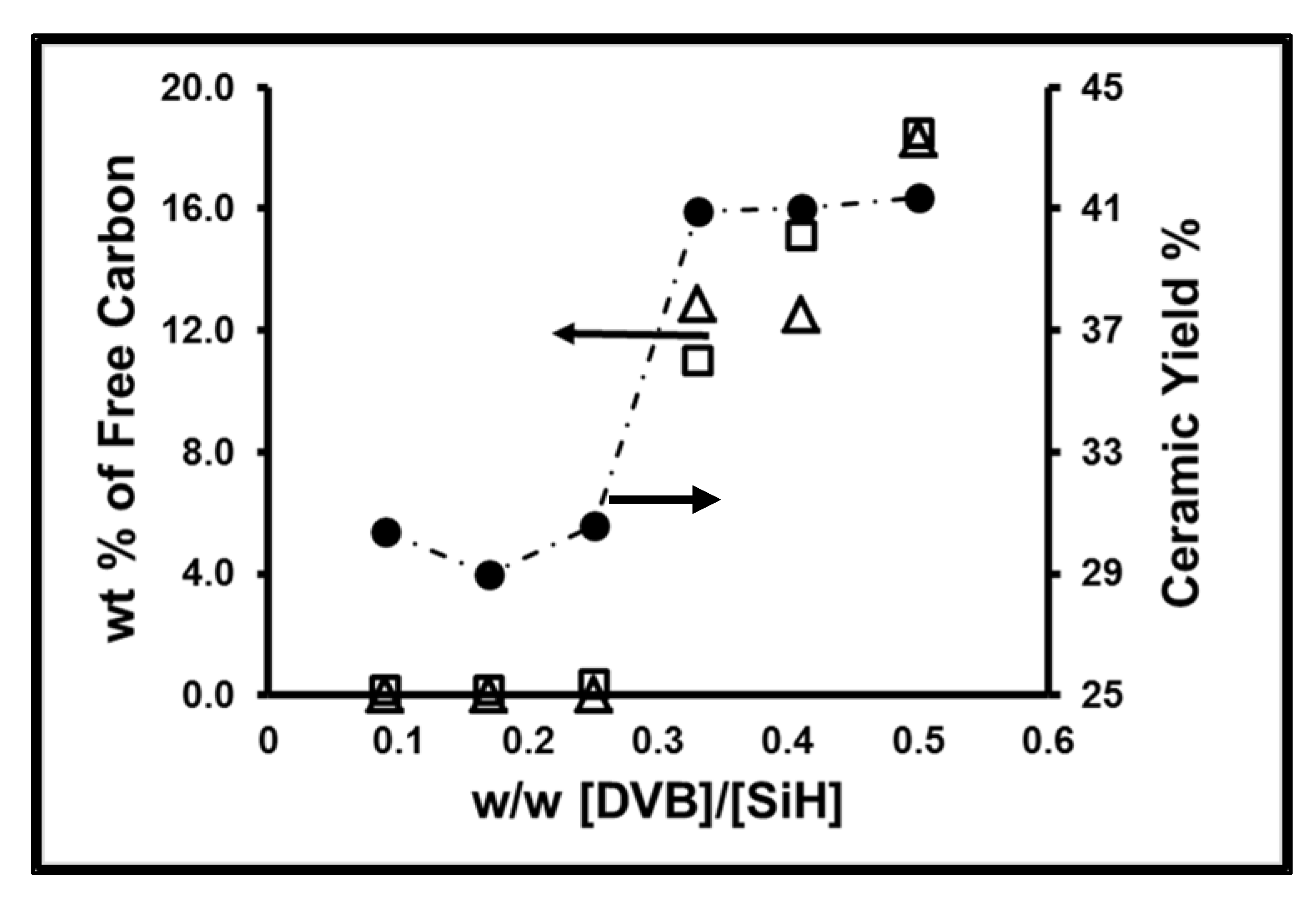
| Ceramized Sample | Color | Ceramic Yield w% | Average Diameter μm | Weight Loss % TGA * | w% Ctotal ** | w% O ** | Calculated w% Cf |
|---|---|---|---|---|---|---|---|
| A-1 | Beige/Green | 30.4 | 7.1 | 0.18 | 28 | 1.12 | 0 |
| A-2 | Beige/Green | 29.0 | 3.5 | 0.17 | 28.2 | 1.54 | 0 |
| A-3 | Beige/Green | 30.6 | 10.4 | 0.34 | 27.3 | 2 | 0 |
| A-4 | Black | 40.9 | 11.1 | 11 | 38 | 1.85 | 11.4 |
| A-5 | Black | 41.0 | 9.5 | 15.1 | 37.9 | 1.6 | 11.2 |
| A-6 | Black | 41.4 | 2.1 | 18.5 | 42 | 1.45 | 17.1 |
| A-7 | Black | 46.3 | 1.2 | 23.5 | 46.6 | 1.50 | 24.9 |
| B-1 | Beige/Green | 8.5 | 4.5 | 0.38 | 30.2 | 1.06 | 0.2 |
| Sample | D Position (FWHM) cm−1 | G Position (FWHM) cm−1 | I(D)/I(G) | La(nm) (ʎ = 488) |
|---|---|---|---|---|
| A-4 | 1356 (55) | 1590 (46) | 1.55 | 2.8 |
| A-5 | 1356 (46) | 1588 (40) | 1.18 | 3.4 |
| A-6 | 1356 (52) | 1589 (43) | 1.47 | 3.0 |
| A-7 | 1350 (53) | 1593 (57) | 2.04 | 2.2 |
| Sample | Nitrogen Gas Adsorption | |||
|---|---|---|---|---|
| BET Surface Area m2/g | BJH Pore Volume cm3/g | BET Av. Pore Width nm | BJH Av.Pore Width nm | |
| A-1 | 10.48 | 0.072 | 21.4 | 28.2 |
| A-2 | 11.24 | 0.058 | 28.2 | 32.0 |
| A-3 | 12.6 | 0.052 | 14.64 | 20.8 |
| A-4 | 164.9 | 0.175 | 4.70 | 5.21 |
| A-5 | 204.6 | 0.195 | 4.16 | 4.59 |
| A-6 | 263. 5 | 0.145 | 2.63 | 3.1 |
| A-7 *) | 347.2 | 0.091 | 2.15 | 2.9 |
| A-7-1 **) | 120.7 | 0.218 | 8.41 | 10.94 |
| A-7-2 ***) | 103.0 | 0.347 | 15.81 | 15.63 |
| B-1 | 53.01 | 0.281 | 20.30 | 21.47 |
| Sample | Total Intrusion (Pore Intrusion) cm3/g | Surface Area m2/g | Av Pore Width nm | Powder Density g/cm3 | Skeletal Density g/cm3 | Porosity % |
|---|---|---|---|---|---|---|
| A-1 | 1.53 (0.50) | 24.1 | 83 | 0.495 | 2.03 | 50.5 |
| A-3 | 0.829 (0.39) | 19.5 | 78 | 0.715 | 1.76 | 32.0 |
| A-5 | 0.764 (0.090) | 25.6 | 13.6 | 0.725 | 1.63 | 12.8 |
| B-1 | 1.33 (0.495) | 43.2 | 46 | 0.560 | 2.20 | 52.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mizerska, U.; Fortuniak, W.; Chojnowski, J.; Rubinsztajn, S.; Zakrzewska, J.; Bak-Sypien, I.; Nyczyk-Malinowska, A. Porous SiC and SiC/Cf Ceramic Microspheres Derived from Polyhydromethylsiloxane by Carbothermal Reduction. Materials 2022, 15, 81. https://doi.org/10.3390/ma15010081
Mizerska U, Fortuniak W, Chojnowski J, Rubinsztajn S, Zakrzewska J, Bak-Sypien I, Nyczyk-Malinowska A. Porous SiC and SiC/Cf Ceramic Microspheres Derived from Polyhydromethylsiloxane by Carbothermal Reduction. Materials. 2022; 15(1):81. https://doi.org/10.3390/ma15010081
Chicago/Turabian StyleMizerska, Urszula, Witold Fortuniak, Julian Chojnowski, Slawomir Rubinsztajn, Joanna Zakrzewska, Irena Bak-Sypien, and Anna Nyczyk-Malinowska. 2022. "Porous SiC and SiC/Cf Ceramic Microspheres Derived from Polyhydromethylsiloxane by Carbothermal Reduction" Materials 15, no. 1: 81. https://doi.org/10.3390/ma15010081







