Microstructure and Electrochemical Behavior of a 3D-Printed Ti-6Al-4V Alloy
Abstract
:1. Introduction
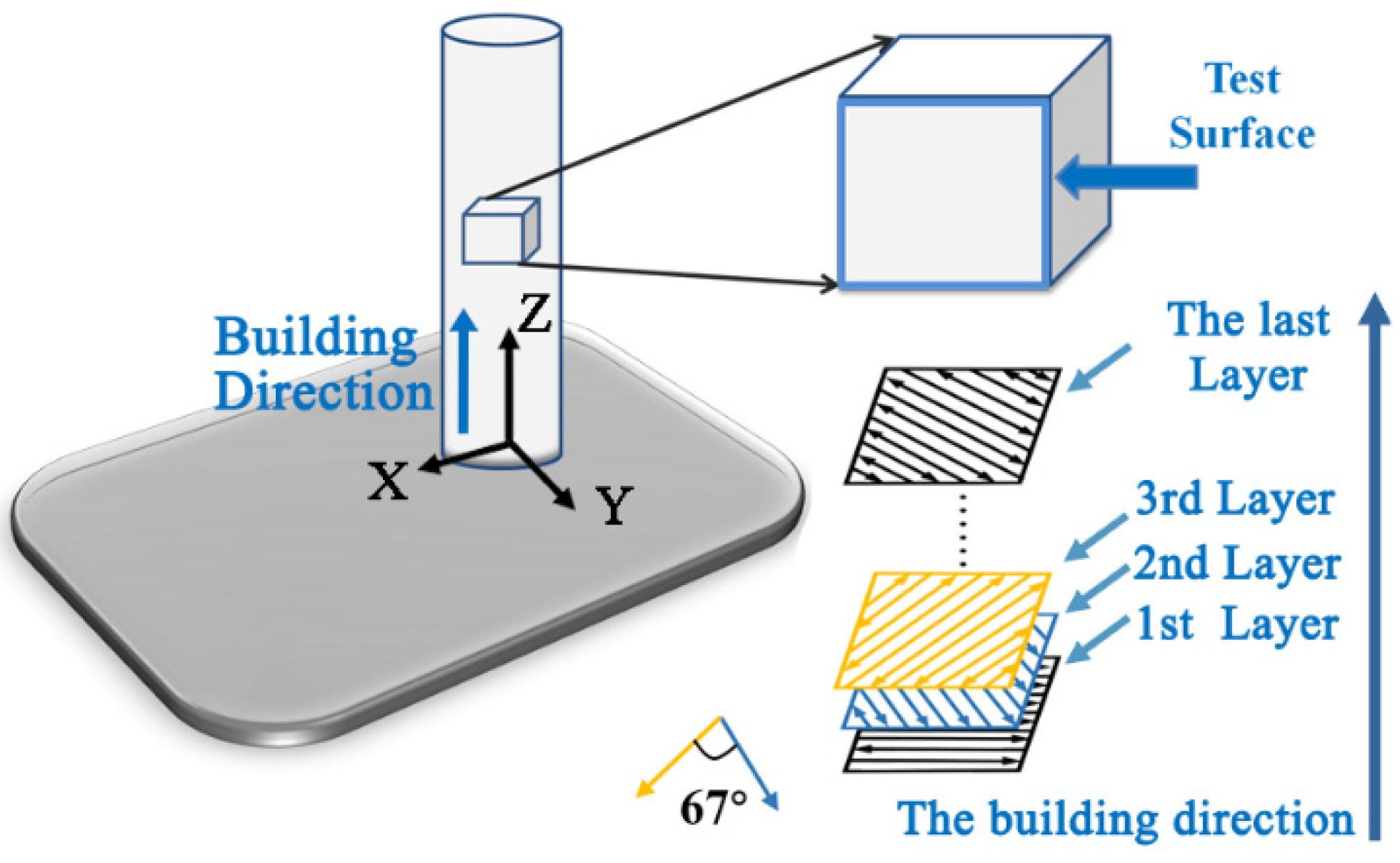
2. Materials and Methods
3. Results
3.1. Microstructure and Phase Analysis
3.1.1. Metallographic Observations
3.1.2. Transmission Electron Microscopy (TEM) Observations
3.1.3. X-ray Diffraction (XRD) Analysis
3.2. Electrochemical Analysis
3.2.1. Potentiodynamic Polarization
3.2.2. Electrochemical Impedance Spectroscopy (EIS)
4. Discussion
5. Conclusions
- The three fabrication methods led to different microstructures although all contained α + β phases to a varying extent. The SLM-manufactured alloy consisted mainly of fine acicular α’ martensite with a large number of twins and some prior β columnar grains, while the EBM-manufactured alloy was composed of columnar α + β structures with rod-shaped prior β grains in the building direction and fine α platelets. The microstructure of the ISF alloy exhibited a typical duplex α + β equiaxial shaped morphology. Furthermore, some small pores were observed in both SLM- and EBM-manufactured samples.
- The equiaxed α + β microstructure of ISF sample demonstrated a better corrosion resistance than the acicular martensitic α′ + β and lamellar α + β microstructures of AM samples manufactured via SLM and EBM, respectively. This was attributed to the special benefit of the equiaxed α grains along with the randomly distributed β phase, where the fraction of phase interface was lower than that of the lamellar α + β microstructure of the EBM-manufactured alloy, leading to fewer microgalvanic cells. Additionally, the higher amount of β phase present in the ISF sample can also improve the corrosion resistance.
Author Contributions
Funding
Conflicts of Interest
References
- Xu, W.; Liu, E.W.; Pateras, A.; Qian, M.; Brandt, M. In situ tailoring microstructure in additively manufactured Ti-6Al-4V for superior mechanical performance. Acta Mater. 2017, 125, 390–400. [Google Scholar] [CrossRef]
- Wang, J.L.; Liu, R.L.; Majumdar, T.; Mantri, S.A.; Ravi, V.A.; Banerjee, R.; Birbilis, N. A closer look at the in vitro electrochemical characterisation of titanium alloys for biomedical applications using in-situ methods. Acta Biomater. 2017, 54, 469–478. [Google Scholar] [CrossRef]
- Bobbio, L.D.; Otis, R.A.; Borgonia, J.P.; Dillon, R.P.; Shapiro, A.A.; Liu, Z.K.; Beese, A.M. Additive manufacturing of a functionally graded material from Ti-6Al-4V to Invar: Experimental characterization and thermodynamic calculations. Acta Mater. 2017, 127, 133–142. [Google Scholar] [CrossRef]
- Dai, N.; Zhang, L.-C.; Zhang, J.; Chen, Q.; Wu, M. Corrosion behavior of selective laser melted Ti-6Al-4V alloy in NaCl solution. Corros. Sci. 2016, 102, 484–489. [Google Scholar] [CrossRef]
- Zhang, Z.X.; Qu, S.J.; Feng, A.H.; Shen, J.; Chen, D.L. Hot deformation behavior of Ti-6Al-4V alloy: Effect of initial microstructure. J. Alloys Compd. 2017, 718, 170–181. [Google Scholar] [CrossRef]
- Dai, N.W.; Zhang, L.C.; Zhang, J.X.; Zhang, X.; Ni, Q.Z.; Chen, Y.; Wu, M.L.; Yang, C. Distinction in corrosion resistance of selective laser melted Ti-6Al-4V alloy on different planes. Corros. Sci. 2016, 111, 703–710. [Google Scholar] [CrossRef] [Green Version]
- Bai, Y.; Gai, X.; Li, S.J.; Zhang, L.C.; Liu, Y.J.; Hao, Y.L.; Zhang, X.; Yang, R.; Gao, Y.B. Improved corrosion behaviour of electron beam melted Ti-6Al-4V alloy in phosphate buffered saline. Corros. Sci. 2017, 123, 289–296. [Google Scholar] [CrossRef]
- Gong, X.J.; Cui, Y.J.; Wei, D.X.; Liu, B.; Liu, R.P.; Nie, Y.; Li, Y.P. Building direction dependence of corrosion resistance property of Ti-6Al-4V alloy fabricated by electron beam melting. Corros. Sci. 2017, 127, 101–109. [Google Scholar] [CrossRef]
- Vazquez, M.R.A.; Amaya, J.M.S.; Boukha, Z.; Botana, F.J. Microstructure, microhardness and corrosion resistance of remelted TiG2 and Ti6Al4V by a high power diode laser. Corros. Sci. 2012, 56, 36–48. [Google Scholar] [CrossRef]
- Dalmau, A.; Pina, V.G.; Devesa, F.; Amigo, V.; Munoz, A.I. Influence of fabrication process on electronchemical and surface properties of Ti-6Al-4V alloy for medical applications. Electrochim. Acta 2013, 95, 102–111. [Google Scholar] [CrossRef]
- Alvarez, J.F.F.; Gomez, F.J.R.; Bustamante, E.O.; Llongueras, J.G. Study of the electrochemical behavior of a Ti6Al4V alloy modified by heat treatment and chemical conversion. Surf. Coat. Technol. 2017, 315, 498–508. [Google Scholar] [CrossRef]
- Urena, J.; Gordo, E.; Navas, E.R.; Vilaboa, N.; Saldana, L.; Morales, A.J. Electrochemical comparative study on corrosion behavior of conventional and powder metallurgy titanium alloys in physiological conditions. Met. Powder Rep. 2017, 72, 118–123. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.X.; Qu, S.J.; Feng, A.H.; Shen, J. Achieving grain refinement and enhanced mechanical properties in Ti–6Al–4V alloy produced by multidirectional isothermal forging. Mater. Sci. Eng. A 2017, 692, 127–138. [Google Scholar] [CrossRef]
- Kranz, J.; Herzog, D.; Emmelmann, C. Design guidelines for laser additive manufacturing of lightweight structures in TiAl6V4. J. Laser Appl. 2015, 27, S14001. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Chen, Z.; Qu, S.J.; Feng, A.H.; Mi, G.B.; Shen, J.; Huang, X.; Chen, D.L. Microstructure and cyclic deformation behavior of a 3D-printed Ti–6Al–4V alloy. J. Alloys Compd. 2020, 825, 153971. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Chen, Z.; Qu, S.J.; Feng, A.H.; Mi, G.B.; Shen, J.; Huang, X.; Chen, D.L. Multiple a sub-variants and anisotropic mechanical properties of an additively-manufactured Ti-6Al-4V alloy. J. Mater. Sci. Technol. 2021, 70, 113–124. [Google Scholar] [CrossRef]
- Li, C.; Sun, J.; Feng, A.; Wang, H.; Zhang, X.; Zhang, C.; Zhao, F.; Cao, G.; Qu, S.; Chen, D. Active Slip Mode Analysis of an Additively Manufactured Ti-6Al-4V Alloy via In-Grain Misorientation Axis Distribution. Metals 2022, 12, 532. [Google Scholar] [CrossRef]
- Qi, H.; Azer, M.; Singh, P. Adaptive toolpath deposition method for laser net shape manufacturing and repair of turbine compressor airfoils. Int. J. Adv. Manuf. Technol. 2010, 48, 121–131. [Google Scholar] [CrossRef]
- Herzog, D.; Seyda, V.; Wycisk, E.; Emmelmann, C. Additive manufacturing of metals. Acta Mater. 2016, 117, 371–392. [Google Scholar] [CrossRef]
- Jones, N. Three-dimensional printers are opening up new worlds to research. Nature 2012, 487, 22–23. [Google Scholar] [CrossRef]
- Witze, A. NASA to send 3D printer into space. Nature 2014, 513, 156. [Google Scholar] [CrossRef] [Green Version]
- MacDonald, E.; Wicker, R. Multiprocess 3D printing for increasing component functionality. Science 2016, 353, 1512. [Google Scholar] [CrossRef]
- Martin, J.H.; Yahata, B.D.; Hundley, J.M.; Mayer, J.A.; Schaedler, T.A.; Pollock, T.M. 3D printing of high-strength aluminium alloys. Nature 2017, 549, 365–369. [Google Scholar] [CrossRef]
- Todd, I. No more tears for metal 3D printing. Nature 2017, 549, 342–343. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Liou, F.; Newkirk, J.; Taminger, K.M.B.; Seufzer, W.J. Investigation on Ti6Al4V-V-Cr-Fe-SS316 multi-layers metallic structure fabricated by laser 3D printing. Sci. Rep. 2017, 7, 7977. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.M.; Voisin, T.; McKeown, J.T.; Ye, J.C.; Calta, N.P.; Li, Z.; Zeng, Z.; Zhang, Y.; Chen, W.; Roehling, T.T.; et al. Additively manufactured hierarchical stainless steels with high strength and ductility. Nat. Mater. 2018, 17, 63–70. [Google Scholar] [CrossRef] [Green Version]
- Todd, I. Printing steels. Nat. Mater. 2018, 17, 13–14. [Google Scholar] [CrossRef]
- Wang, P.; Tan, X.P.; Nai, M.L.S.; Tor, S.B.; Wei, J. Spatial and geometrical-based characterization of microstructure and microhardness for an electron beam melted Ti-6Al-4V component. Mater. Des. 2016, 95, 287–295. [Google Scholar] [CrossRef]
- Wysocki, B.; Maj, P.; Sitek, R.; Buhagiar, J.; Kurzydlowski, K.J.; Swieszkowski, W. Laser and electron beam additive manufacturing methods of fabricating titanium bone implants. Appl. Sci. 2017, 7, 657. [Google Scholar] [CrossRef]
- Sanders, G.; Wilk, J.; Marks, S.; Paolicelli, S.A.; Dicaprio, M.; Bucinell, R. The optimization of a porous Ti6Al4V bone construct using additive manufacturing. Am. J. Eng. Appl. Sci. 2017, 10, 13–19. [Google Scholar]
- Dutta, B.; Froes, F.H.S. The additive manufacturing (AM) of titanium alloys. Met. Powder Rep. 2017, 72, 96–106. [Google Scholar] [CrossRef]
- Xu, W.; Brandt, M.; Sun, S.; Elambasseril, J.; Liu, Q.; Latham, K.; Xia, K.; Qian, M. Additive manufacturing of strong and ductile Ti–6Al–4V by selective laser melting via in situ martensite decomposition. Acta Mater. 2015, 85, 74–84. [Google Scholar] [CrossRef]
- Cao, J.; Nash, P. Numerical simulation of the effects of preheating on electron beam additive manufactured Ti-6Al-4V build plate. Mater. Sci. Forum 2016, 879, 274–278. [Google Scholar] [CrossRef]
- Tan, X.P.; Kok, Y.H.; Toh, W.Q.; Tan, Y.J.; Descoins, M.; Mangelinck, D.; Tor, S.B.; Leong, K.F.; Chua, C.K. Revealing martensitic transformation and a/b interface evolution in electron beam melting three-dimensional-printed Ti-6Al-4V. Sci. Rep. 2016, 6, 26039. [Google Scholar] [CrossRef] [Green Version]
- Hrabe, N.; Quinn, T. Effects of processing on microstructure and mechanical properties of a titanium alloy (Ti-6Al-4V) fabricated using electron beam melting (EBM), part 1: Distance from build plate and part size. Mater. Sci. Eng. A 2013, 573, 264–270. [Google Scholar] [CrossRef]
- Hrabe, N.; Quinn, T. Effects of processing on microstructure and mechanical properties of a titanium alloy (Ti-6Al-4V) fabricated using electron beam melting (EBM), Part 2: Energy input, orientation, and location. Mater. Sci. Eng. A 2013, 573, 271–277. [Google Scholar] [CrossRef]
- Zhang, S.; Wei, Q.S.; Cheng, L.Y.; Li, S.; Shi, Y.S. Effects of scan line spacing on pore characteristics and mechanical properties of porous Ti6Al4V implants fabricated by selective laser melting. Mater. Des. 2014, 63, 185–193. [Google Scholar] [CrossRef]
- Simonelli, M.; Tse, Y.Y.; Tuck, C. Effect of the build orientation on the mechanical properties and fracture modes of SLM Ti-6Al-4V. Mater. Sci. Eng. A 2014, 616, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Ren, Y.M.; Lin, X.; Yang, H.O.; Tan, H.; Chen, J.; Jian, Z.Y.; Li, J.Q.; Huang, W.D. Microstructural feature of Ti-6Al-4V manufactured via high power laser directed energy deposition under low-cycle fatigue. J. Mater. Sci. Technol. 2021, 83, 18–33. [Google Scholar] [CrossRef]
- Zhang, S.; Ma, Y.; Huang, S.; Youssef, S.S.; Qi, M.; Wang, H.; Qiu, J.; Lei, J.; Yang, R. Temperature-gradient induced microstructure evolution in heat-affected zone of electron beam welded Ti-6Al-4V titanium alloy. J. Mater. Sci. Technol. 2019, 35, 1681–1690. [Google Scholar] [CrossRef]
- Sun, J.; Qi, M.; Zhang, J.; Li, X.; Wang, H.; Ma, Y.; Xu, D.; Lei, J.; Yang, R. Formation mechanism of α lamellae during β→α transformation in polycrystalline dual-phase Ti alloys. J. Mater. Sci. Technol. 2021, 71, 98–108. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, K.; Yang, Y.; Wang, H.; Zhu, Y.; Huang, A. Grain boundary α-phase precipitation and coarsening: Comparing laser powder bed fusion with as-cast Ti-6Al-4V. Scr. Mater. 2022, 207, 114261. [Google Scholar] [CrossRef]
- Murr, L.E.; Gaytan, S.M.; Ramirez, D.A.; Martinez, E.; Hernandez, J.; Amato, K.N.; Shindo, P.W.; Medina, F.R.; Wicker, R.B. Metal fabrication by additive manufacturing using laser and electron beam melting technologies. J. Mater. Sci. Technol. 2012, 28, 1–14. [Google Scholar] [CrossRef]
- Zhai, Y.W.; Galarraga, H.; Lados, D.A. Microstructure Evolution, Tensile Properties, and Fatigue Damage Mechanisms in Ti-6Al-4V Alloys Fabricated by Two Additive Manufacturing Techniques. Procedia Eng. 2015, 114, 658–666. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.L.; Li, S.J.; Zhang, M.; Liu, Y.D.; Sercombe, T.B.; Wang, S.G.; Hao, Y.L.; Yang, R.; Murr, L.E. Comparison of the microstructures and mechanical properties of Ti-6Al-4V fabricated by selective laser melting and electron beam melting. Mater. Des. 2016, 95, 21–31. [Google Scholar] [CrossRef]
- Zhang, L.C.; Liu, Y.J.; Li, S.J.; Hao, Y.L. Additive manufacturing of titanium alloys by electron beam melting: A review. Adv. Eng. Mater. 2018, 20, 1700842. [Google Scholar] [CrossRef]
- Fojt, J.; Fousova, M.; Jablonska, E.; Joska, L.; Hybasek, V.; Pruchova, E.; Vojtech, D.; Ruml, T. Corrosion behaviour and cell interaction of Ti-6Al-4V alloy prepared by two techniques of 3D printing. Mater. Sci. Eng. C 2018, 93, 911–920. [Google Scholar] [CrossRef]
- Murr, L.E.; Quinones, S.A.; Gaytan, S.M.; Lopez, M.I.; Rodela, A.; Martinez, E.Y.; Hernandez, D.H.; Martinez, E.; Medina, F.; Wicker, R.B. Microstructure and mechanical behavior of Ti-6Al-4V produced by rapid-layer manufacturing, for biomedical applications. J. Mech. Behav. Biomed. Mater. 2009, 2, 20–32. [Google Scholar] [CrossRef]
- Safdar, A.; Wei, L.Y.; Snis, A.; Lai, Z. Evaluatioin of microstructural development in electron beam melted Ti-6Al-4V. Mater. Charact. 2012, 65, 8–15. [Google Scholar] [CrossRef]
- Jovanovic, M.T.; Tadic, S.; Zec, S.; Miskovic, Z.; Bobic, I. The effect of annealing temperatures and cooling rates on microstructure and mechanical properties of investment cast Ti-6Al-4V alloy. Mater. Des. 2006, 27, 192–199. [Google Scholar] [CrossRef]
- Song, D.; Ma, A.B.; Jiang, J.H.; Lin, P.H.; Yang, D.H.; Fan, J.F. Corrosion behaviour of bulk ultra-fine grained AZ91D magnesium alloy fabricated by euqal-channel angular pressing. Corros. Sci. 2011, 53, 362–373. [Google Scholar] [CrossRef]
- Mythili, R.; Shankar, A.R.; Saroja, S.; Raju, V.R.; Vijayalakshmi, M.; Dayal, R.K.; Raghunathan, V.S.; Balasubramaniam, R. Influence of microstructure on corrosion behavior of Ti-5%Ta-1.8%Nb alloy. J. Mater. Sci. 2007, 42, 5924–5935. [Google Scholar] [CrossRef]
- Martin, E.; Azzi, M.; Salishchev, G.A.; Szpunar, J. Influence of microstructure and texture on the corrosion and tribocorrosion behavior of Ti-6Al-4V. Tribol. Int. 2010, 43, 918–924. [Google Scholar] [CrossRef]
- Chen, J.R.; Tsai, W.T. In situ corrosion monitoring of Ti-6Al-4V alloy in H2SO4/HCl mixed solution using electrochemical AFM. Electrochim. Acta 2011, 56, 1746–1751. [Google Scholar] [CrossRef]
- Aung, N.N.; Zhou, W. Effect of grain size and twins on corrosion behaviour of AZ31B magnesium alloy. Corros. Sci. 2010, 52, 589–594. [Google Scholar] [CrossRef]










| Ti-6Al-4V | Ti | Al | V | Fe | C | H | O | N |
|---|---|---|---|---|---|---|---|---|
| SLM | Bal. | 5.50–6.75 | 3.50–4.50 | <0.30 | <0.08 | <0.015 | <0.20 | <0.05 |
| EBM | Bal. | 6.40 | 4.12 | 0.18 | 0.01 | 0.003 | 0.14 | 0.01 |
| ISF | Bal. | 5.50–6.75 | 3.50–4.50 | <0.30 | <0.08 | <0.015 | <0.20 | <0.05 |
| Samples | Rs (Ω·cm2) | Rf (kΩ·cm2) | CPE1 (F·cm−2) | n1 | Rct (MΩ·cm2) | CPE2 (F·cm−2) | n2 | χ2 × 10−4 |
|---|---|---|---|---|---|---|---|---|
| SLM | 12.3 ± 0.1 | 14.0 ± 0.9 | 59.02 ± 0.41 | 0.99 | 0.69 ± 0.04 | 14.87 ± 0.16 | 0.78 | 6.51 |
| EBM | 11.5 ± 0.2 | 18.8 ± 0.6 | 96.61 ± 0.93 | 0.98 | 0.89 ± 0.08 | 20.29 ± 0.25 | 0.82 | 6.03 |
| ISF | 10.9 ± 0.7 | 48.3 ± 1.1 | 113.91 ± 1.80 | 0.92 | 2.08 ± 0.16 | 41.27 ± 0.39 | 0.94 | 2.84 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Z.; Chen, Z.; Qu, D.; Qu, S.; Wang, H.; Zhao, F.; Zhang, C.; Feng, A.; Chen, D. Microstructure and Electrochemical Behavior of a 3D-Printed Ti-6Al-4V Alloy. Materials 2022, 15, 4473. https://doi.org/10.3390/ma15134473
Yu Z, Chen Z, Qu D, Qu S, Wang H, Zhao F, Zhang C, Feng A, Chen D. Microstructure and Electrochemical Behavior of a 3D-Printed Ti-6Al-4V Alloy. Materials. 2022; 15(13):4473. https://doi.org/10.3390/ma15134473
Chicago/Turabian StyleYu, Zhijun, Zhuo Chen, Dongdong Qu, Shoujiang Qu, Hao Wang, Fu Zhao, Chaoqun Zhang, Aihan Feng, and Daolun Chen. 2022. "Microstructure and Electrochemical Behavior of a 3D-Printed Ti-6Al-4V Alloy" Materials 15, no. 13: 4473. https://doi.org/10.3390/ma15134473
APA StyleYu, Z., Chen, Z., Qu, D., Qu, S., Wang, H., Zhao, F., Zhang, C., Feng, A., & Chen, D. (2022). Microstructure and Electrochemical Behavior of a 3D-Printed Ti-6Al-4V Alloy. Materials, 15(13), 4473. https://doi.org/10.3390/ma15134473











