Electrospun Nisin-Loaded Poly(ε-caprolactone)-Based Active Food Packaging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Polymer Solutions for Electrospinning
2.2.2. Viscosity of Solutions
2.2.3. Electrospinning
2.2.4. Scanning Electron Microscopy
2.2.5. Transmission Electron Microscopy
2.2.6. Attenuated Total Reflectance—Fourier Transform Infrared Spectroscopy
2.2.7. Wettability
2.2.8. Mechanical Properties
2.2.9. Thermogravimetric Analysis
2.2.10. Antibacterial Activity
2.2.11. Suitability Observation of Nisin-Loaded PCL for Food Packaging in Real-Time
3. Results and Discussion
3.1. Analysis of the Morphology of the Investigated Samples
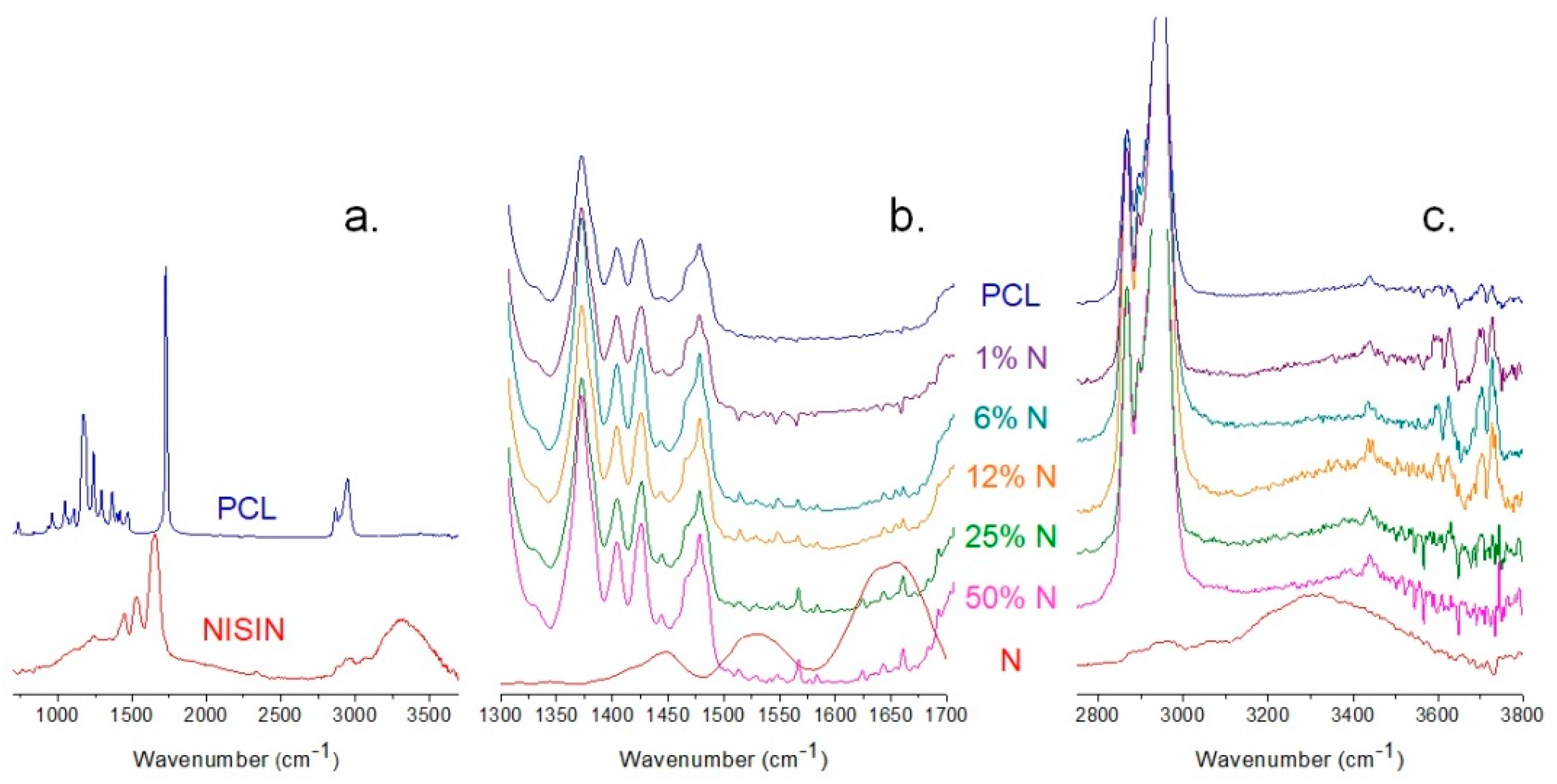
3.2. ATR-FTIR Analysis of PCL and Fibrous Composites PCL/N
3.3. Analysis of Wettability
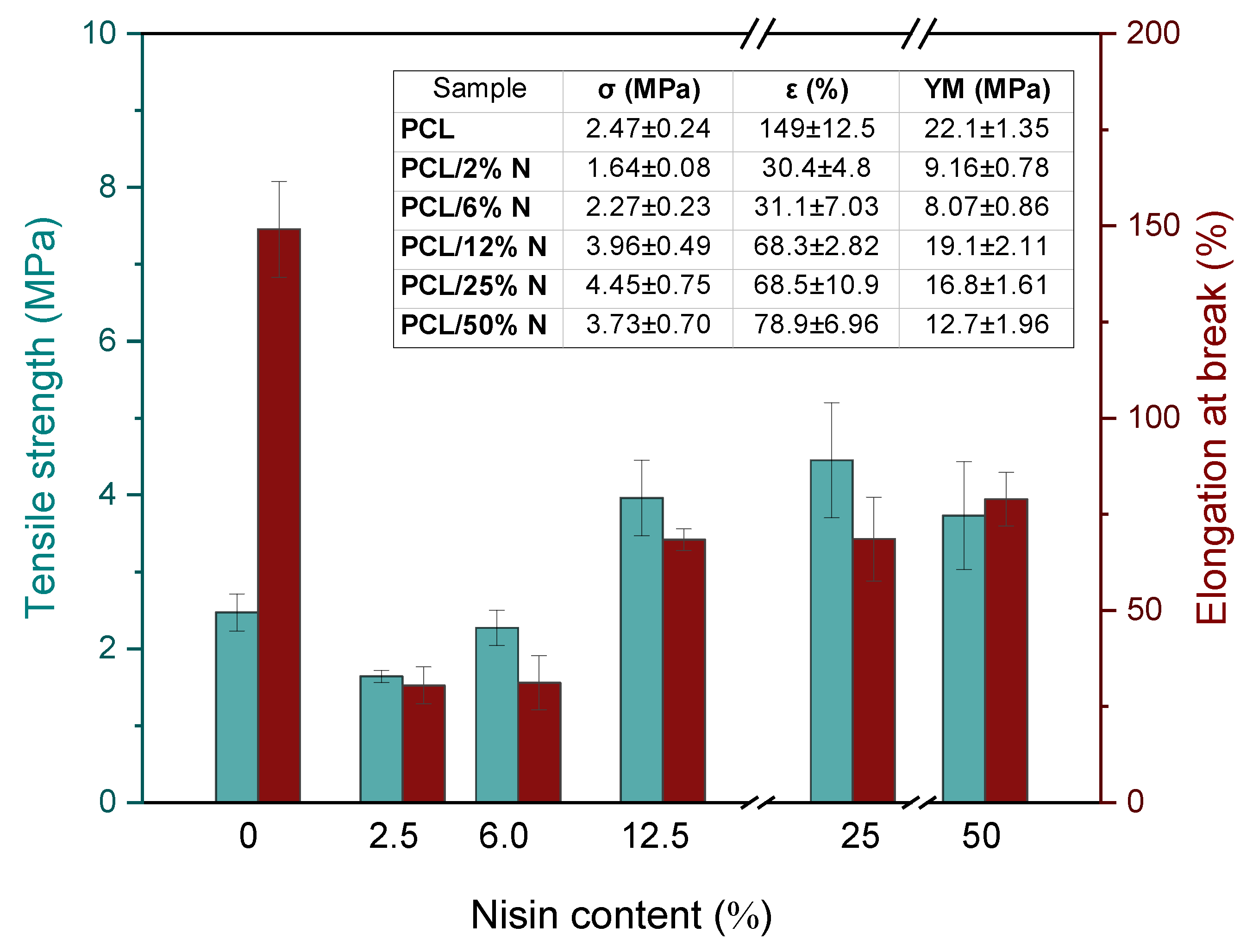
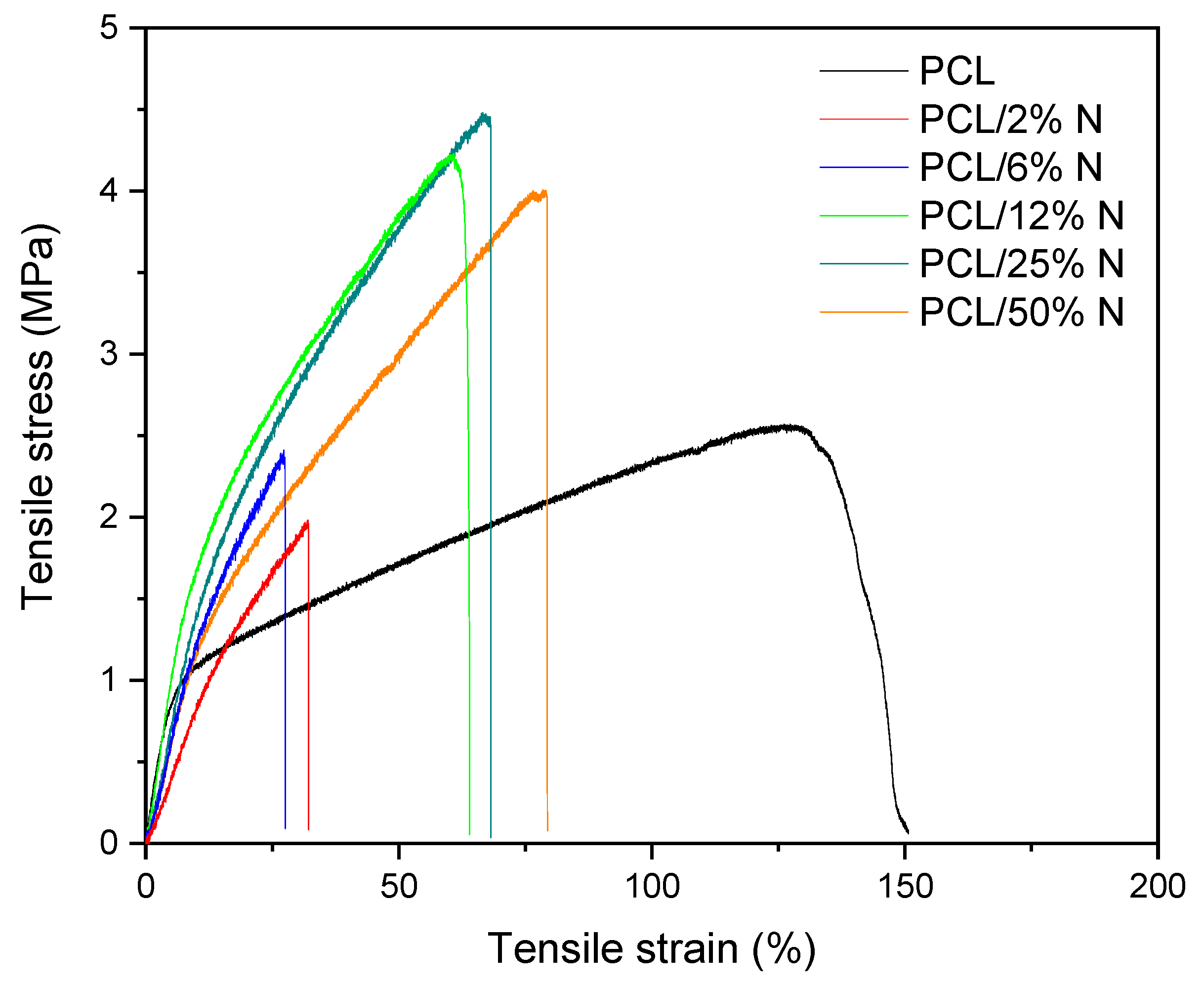
3.4. Mechanical Analysis
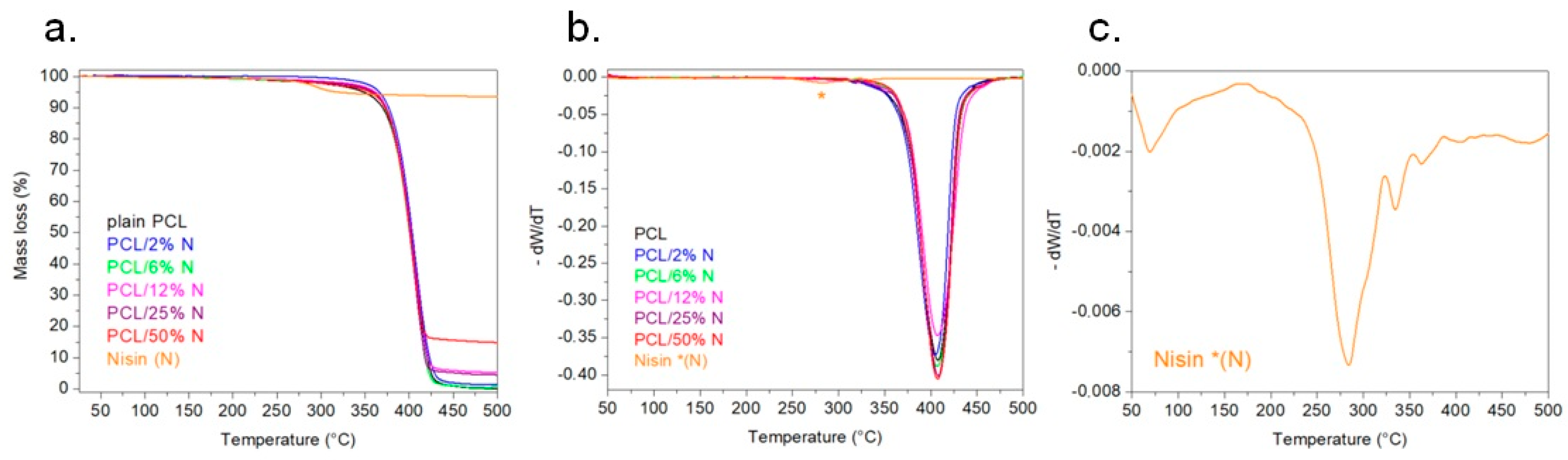
3.5. Thermogravimetry Analysis
3.6. Testing for Antibacterial Activity
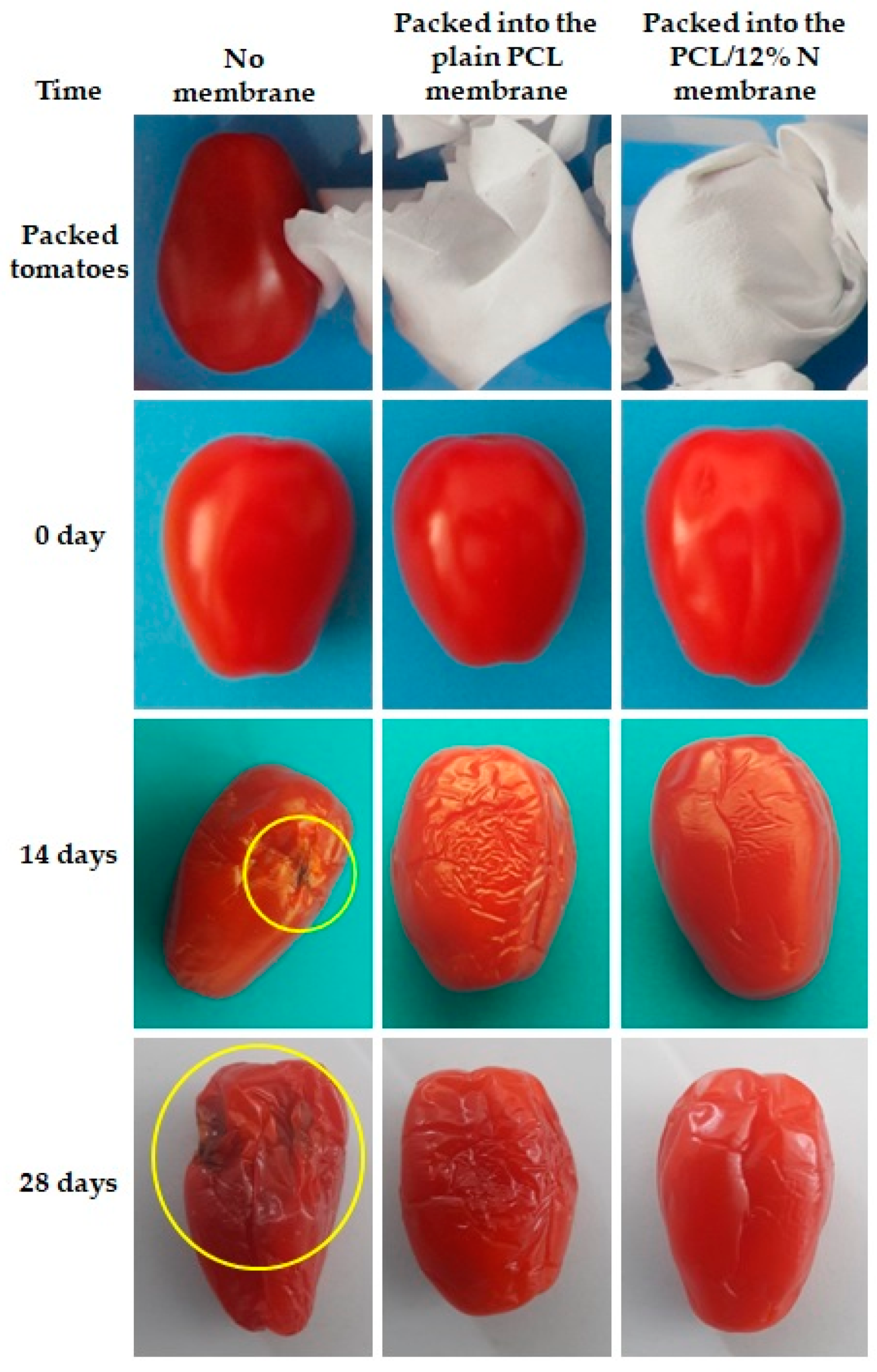
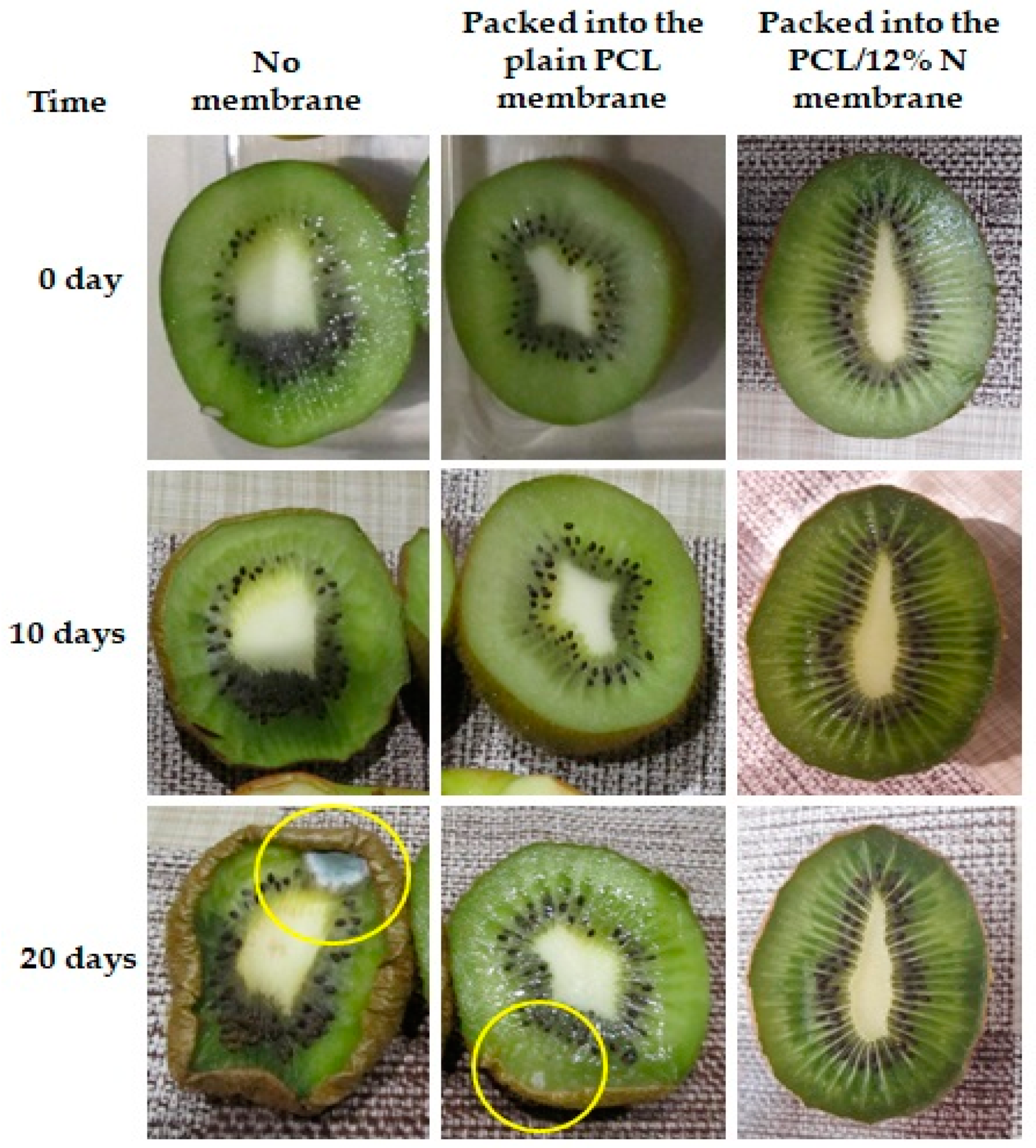
3.7. Suitability Observation of Nisin-Loaded PCL for Food Packaging
- Tomatoes and kiwi were freely placed into an open plastic container;
- Tomatoes and kiwi were covered by nonwoven fabric mate of plain PCL membranes before putting them into an open container and;
- Tomatoes and kiwi were covered by a PCL/12% N nonwoven fabric mat before they were placed in an open container.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Caprile, A.; Rossi, R. International Year of Fruits and Vegetables, at a Glance. 2021. Available online: https://www.europarl.europa.eu/RegData/etudes/ATAG/2021/689367/EPRS_ATA(2021)689367_EN.pdf (accessed on 24 May 2022).
- Kothe, C.I.; Pessoa, J.P.; Malheiros, P.S.; Tondo, E.C. Assessing the growth of Staphilococcus aureus and Esterichia coli on fruits and vegetables. J. Infect. Dev. Ctries. 2019, 13, 480–486. [Google Scholar] [CrossRef]
- Feng, Y.; Li, G.; Lv, X.; Xu, Y.; Wu, Q.; Shi, C.; Li, Q.; Yang, B.; Wang, X.; Xi, M.; et al. Prevalence, distribution, and diversity of Esterichia coli, Staphilococcus aureus, and salmonella in kiwifruit orchards and processing plants. Foodborne Pathog. Dis. 2014, 11, 782–790. [Google Scholar] [CrossRef]
- Ahari, H.; Soufiani, S.P. Smart and active food packaging: Insights in novel food packaging. Front. Microbiol. 2021, 12, 657233. [Google Scholar] [CrossRef]
- Mangaraj, S.; Yadav, A.; Bal, L.M.; Dash, S.; Mahanti, N.K. Application of biodegradable polymers in food packaging industry: A comprehensive review. J. Package Technol. Res. 2019, 3, 77–96. [Google Scholar] [CrossRef]
- Nilsen-Nygaard, J.; Fernández, E.N.; Radusin, T.; Rotabakk, B.T.; Sarfraz, J.; Sharmin, N.; Sivertsvik, M.; Sone, I.; Pettersen, M.K. Current status of biobased and biodegradable food packaging materials: Impact on food quality and effect of innovative processing technologies. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1333–1380. [Google Scholar] [CrossRef]
- Copello, L.; Haut, G.; Maillot, J.; Mongodin, F. Moving on from Single-Use Plastics: How is Europe Doing? Assessment of European Countries Transportation of the Single Use Plastics Directive. 2021. Available online: https://www.actu-environnement.com/media/pdf/news-37813-rapport-mise-en-oeuvre-directive-sup.pdf (accessed on 12 May 2022).
- Directive (EU) 2019/904 of the European Parliament and of the Council of 5 June 2019 on the Reduction of the Impact of Certain Plastic Products on the Environment, Single-Use Plastics. 2019. Available online: https://ec.europa.eu/environment/topics/plastics/single-use-plastics_en (accessed on 12 May 2022).
- Yildirim, S.; Röcker, B.; Pettersen, M.K.; Nilsen-Nygaard, J.; Ayhan, Z.; Rutkaite, R.; Radusin, T.; Suminska, P.; Marcos, B.; Coma, V. Active Packaging Applications for Food. Compr. Rev. Food Sci. Food Saf. 2018, 17, 165–199. [Google Scholar] [CrossRef] [Green Version]
- Patil, H.I.; Espinoza, S.M.; Martinez, E.S.M.; Pimentel, R.C.; Ige, P.P. Poly-ε-caprolactone (PCL), a promising polymer for pharmaceutical and biomedical applications: Focus on nanomedicine in cancer. Int. J. Polym. Mater. Polym. Biomater. 2019, 69, 85–126. [Google Scholar]
- Yang, X.; Wang, Y.; Zhou, Y.; Chen, J.; Wan, Q. The Application of Polycaprolactone in Three-Dimensional Printing Scaffolds for Bone Tissue Engineering. Polymers 2021, 13, 2754. [Google Scholar] [CrossRef]
- Bartnikowski, M.; Dargaville, T.R.; Ivanovski, S.; Hutmacher, D.W. Degradation mechanisms of polycaprolactone in the context of chemistry, geometry and environment. Prog. Polym. Sci. 2019, 96, 1–20. [Google Scholar] [CrossRef]
- Cesur, S.; Koroglu, C.; Yalcin, A.T. Antimicrobial and biodegradable food packaging applications of polycaprolactone/orano nanoclay/chitosan polymeric composite films. J. Vinyl Additive Technol. 2017, 24, 376–387. [Google Scholar] [CrossRef]
- Lyu, J.S.; Lee, J.S.; Han, J. Development of a biodegradable polycaprolactone film incorporated with an antimicrobial agent via an extrusion process. Sci. Rep. 2019, 9, 20236. [Google Scholar] [CrossRef] [PubMed]
- Meira, S.M.M.; Zehetmeyer, G.; Jardim, Z.A.; Scheibel, J.M.; de Oliveira, R.V.B.; Brandelli, A. Polypropylene/Montmorillonite nanocomposites containing food packaging. Food Bioprocess Technol. 2014, 7, 3349–3357. [Google Scholar] [CrossRef]
- Karam, L.; Jana, C.; Mamede, A.; Boukla, S.; Dhulster, P.; Chihib, N. Nisin-activated hydrophobic and hydrophilic surfaces: Assessment of peptide adsorption and antibacterial activity against some food pathogens. Appl. Microbiol. Biotechnol. 2013, 97, 10321–10328. [Google Scholar] [CrossRef]
- Gumienna, M.; Górna, B. Antimicrobial food packaging with biodegradable polymers and bacteriocins. Molecules 2021, 26, 3735. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Mahalingam, S.; Basnett, P.; Raimi-Abraham, B.; Roy, I.; Craig, D.; Edirisinghe, M. Making nonwoven fibrous poly(ε-caprolactone) constructs for antimicrobial and tissue engineering applications by pressurized melt gyration. Macromol. Mater. Eng. 2016, 301, 922–934. [Google Scholar] [CrossRef] [Green Version]
- Santos, D.M.; Leite, I.S.; Bukzem, A.L.; Santos, R.P.O.; Frollini, E.; Inada, M.N.; Campana-Filho, S.P. Nanostructured electrospun nonwovens of poly(ε-caprolactone)/quaternized chitosan for potential biomedical applications. Carbohydr. Polym. 2018, 186, 110–121. [Google Scholar] [CrossRef]
- Zhao, L.; Duan, G.; Zhang, G.; Yang, H.; He, S.; Jiang, S. Electrospun Functional Materials toward Food Packaging Applications: A Review. Nanomaterials 2020, 10, 150. [Google Scholar] [CrossRef] [Green Version]
- Haichao, L.; Li, H.; Bubakir, M.M.; Yang, W.; Barhoum, A. Engineering nanofibers as electrode and membrane materials for batteries, supercapacitors, and fuel cells. In Handbook of Nanofibers; Barhoum, A., Bechelany, M., Makhlouf, A., Eds.; Springer: Cham, Switzerland, 2019; pp. 1105–1130. [Google Scholar] [CrossRef]
- Liu, Y.; Li, K.; Mohideen, M.M.; Ramakrishna, S. Melt Electrospinning: A green Method to Produce Superfine Fibers; Elsevier Inc.: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and electrospu nanofibers: Methods, materials, and applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef]
- Stojanov, S.; Berlec, A. Electrospun nanofibers as carriers of microorganisms, stems cells, proteins, and nucleic acid in therapeutic and other applications. Front. Bioeng. Biotechnol. 2020, 8, 130. [Google Scholar] [CrossRef]
- Zhang, Y.; Ullah, I.; Zhang, W.; Ou, H.; Domingos, M.; Gloria, A.; Zhou, J.; Li, W.; Zhang, X. Preparation of electrospun nanofibrous polycaprolactone scaffolds using nontoxic ethylene carbonate and glacial acetic acid solvent system. J. Appl. Polym. Sci. 2020, 137, 48387. [Google Scholar] [CrossRef]
- Cheremisinoff, N. Industrial Solvents Handbook, 2nd ed.; Marcel Dekker Inc.: New York, NY, USA, 2008; pp. 51–53. [Google Scholar]
- Liverani, L.; Boccaccini, A.R. Versatile production of poly(epsilon-caprolactone) fibers by electrospinning using benign solvents. Nanomaterials 2016, 6, 75. [Google Scholar] [CrossRef] [PubMed]
- Marsella, J.A. Dimethylformamide. In Kirk-Othmer Encyclopedia of Chemical Technology; Updated by Staff; Wiley: Hoboken, NJ, USA, 2013. [Google Scholar] [CrossRef]
- Opálková Šišková, A.; Bučková, M.; Kroneková, Z.; Kleinová, A.; Nagy, Š.; Rydz, J.; Opálek, A.; Sláviková, M.; Eckstein Andiscová, A. The drug-loaded electrospun poly(ε-capolactone) mats for therapeutic application. Nanomaterials 2021, 11, 922. [Google Scholar] [CrossRef] [PubMed]
- Mosher, C.Z.; Brudnicki, P.A.P.; Gong, Z.; Childs, H.R.; Lee, S.W.; Antrobus, R.M.; Fang, E.C.; Schiros, T.N.; Lu, H.H. Green electrospinning for biomaterials and biofabrication. Biofabrication 2021, 13, 035049. [Google Scholar] [CrossRef] [PubMed]
- Abel, S.B.; Liverani, L.; Boccaccini, A.R.; Abraham, G.A. Effect of benign solvents composition on poly(ε-caprolactone) electrospun fiber properties. Mater. Lett. 2019, 245, 86–89. [Google Scholar] [CrossRef]
- Dodero, A.; Alloisio, M.; Castellano, M.; Vicini, S. Multilayer alginate-polycaprolactone electrospun membranes as skin wound patches with drug delivery abilities. ACS Appl. Mater. Interfaces 2020, 12, 31162–31171. [Google Scholar] [CrossRef]
- Mailley, D.; Hébraud, A.; Schlatter, G. A review on the impact of humidity during electrospinning: From the nanofiber structure engineering to the applications. Macromol. Mater. Eng. 2021, 306, 2100115. [Google Scholar] [CrossRef]
- De Vrieze, S.; Van Camp, T.; Nelvig, A.; Hangtröm, B.; Westbroek, P.; De Clerk, K. The effect of temperature and humidity on electrospinning. J. Mater. Sci. 2009, 44, 1357–1362. [Google Scholar] [CrossRef]
- Rydz, J.; Šišková, A.; Eckstein Andicsová, A. Microscopic techniques in materials science: Current trends in the area of blends, composites, and hybrid materials. Adv. Mater. Sci. Eng. 2019, 2019, 9072958. [Google Scholar] [CrossRef] [Green Version]
- Halabi, M.; Mann-Lahav, M.; Beilin, V.; Shter, G.E.; Elishav, O.; Grader, G.S.; Dekel, D.R. Electrospun anion-conducting ionomer fibers-effect of humidity on final properties. Polymers 2020, 12, 1020. [Google Scholar] [CrossRef]
- Huang, S.; Liu, G.; Han, G.; Cheng, W.; Fu, Z.; Wu, Q.; Wang, Q. Effect of experimental parameters on morphological, mechanical and hydrophobic properties of electrospun polystyrene fibers. Materials 2015, 8, 2718–2734. [Google Scholar] [CrossRef] [Green Version]
- Korycka, P.; Mirek, A.; Kramek-Romanowska, K.; Grzeczkowicz, M.; Lewińska, D. Effect of electrospinning process variables on the size of polymer fibers and bead-on-string structures established with a 23 factorial design. Beilstein J. Nanotechnol. 2018, 9, 2466–2478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.; Zou, L.; Lu, H.; Kang, T. Effect of different solvent systems on PHBV/PEO electrospun fibers. RSC Adv. 2017, 7, 4000–4010. [Google Scholar] [CrossRef] [Green Version]
- Jamnongkan, T.; Kaewpirom, S.; Wattanakornsiri, A.; Mongkholrattanasit, R. Effect of ZnO concentration on the diameter of electrospun fibers from poly(vinyl alcohol) composited with ZnO nanoparticles. Key Eng. Mater. 2017, 759, 81–85. [Google Scholar] [CrossRef]
- Asran, A.S.; Salama, M.; Popescu, C.; Michler, G.H. Solvent influences the morphology and mechanical properties of electrospun poly(L-lactic acid) scaffold for tissue engineering applications. Macromol. Symp. 2010, 294, 153–161. [Google Scholar] [CrossRef]
- Qin, X.; Wu, D. Effect of different solvents on poly (caprolactone) (PCL) electrospun nonwoven membranes. J. Therm. Anal. Calorim. 2012, 107, 1007–1013. [Google Scholar] [CrossRef]
- Mosnáčková, K.; Opálková Šišková, A.; Kleinová, A.; Danko, M.; Mosnáček, J. Properties and degradation of novel fully biodegradable PLA/PHB blends filled with keratin. Int. J. Mol. Sci. 2020, 21, 9678. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, Z.; Zou, H.; Zhang, X.; Sheng, R.; Zhang, Y.; Zhang, B.; Li, C.; Qi, Y. An enhanced antibacterial nanoflowers AgPW@PDA@Nisin constructed from polyoxometalate and nisin. J. Inorg. Biochem. 2020, 212, 111212. [Google Scholar] [CrossRef]
- Report of the Scientific Committee on Food, Risk Profile on the Microbiological Contamination of Fruits and Vegetables Eaten Raw. 2002. Available online: https://ec.europa.eu/food/system/files/2020-12/sci-com_scf_out125_en.pdf (accessed on 11 May 2022).
- Hashimi, M.; Ullah, S.; Ullah, A.; Saito, Y.; Haider, M.K.; Bie, X.; Wada, K.; Kim, I.S. Carboxyl cellulose (CMC) based electrospun composite nanofiber mats for food packaging. Polymers 2021, 13, 302. [Google Scholar] [CrossRef]
- Göksen, G.; Fabra, M.J.; Pérez-Cataluña, A.; Ekiz, H.I.; Sanchez, G.; López-Rubio, A. Biodegradable active food packaging structures based on hybrid cross-linked electrospun polyvinyl alcohol fibers containing essential oils and their application in the preservation of chicken breast fillets. Food Packag. Shelf Life 2021, 27, 100613. [Google Scholar] [CrossRef]
- Surendhiran, D.; Cui, H.; Lin, L. Encapsulation of phlorotannin in alginate/PEO blended nanofibers to preserve chicken meat from Salmonella contaminations. Food Packag. Shelf Life 2019, 21, 100346. [Google Scholar] [CrossRef]
- Forghani, S.; Almasi, H.; Moradi, M. Electrospun nanofibers as food freshness and time-temperature indicators: A new approach in food intelligent packaging. Innov. Food Sci. Emerg. Technol. 2021, 73, 102804. [Google Scholar] [CrossRef]
- Siracusa, V. Food Packaging Permeability Behaviour: A Report. Int. J. Polym. Sci. 2012, 2012, 302029. [Google Scholar] [CrossRef]
- Mauriello, G.; De Luca, E.; La Storia, A.; Villani, F.; Ercolini, D. Antimicrobial activity of a nisin-activated plastic film for food packaging. Lett. Appl. Microbiol. 2005, 41, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, H.; Wu, M.; Ma, J.; Lu, P. Bio-based antimicrobial packaging from sugarcane bagasse nanocellulose/nisin hybrid films. Int. J. Biol. Macromol. 2020, 161, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Jin, T.; Liu, L.; Zhang, H.; Hicks, K. Antimicrobial activity of nisin incorporated in pectin and polylactic acid composite films against Listeria monocytogenes. Int. J. Food Sci. Technol. 2009, 44, 322–329. [Google Scholar] [CrossRef]
- Wu, H.; Teng, C.; Liu, B.; Wang, J. Characterization and long term antimucrobial activity of the nisin anchored cellulose films. Int. J. Biol. Macromol. 2018, 113, 487–493. [Google Scholar] [CrossRef]
- Meral, R.; Alav, A.; Karakas, C.; Dertli, E.; Yilmaz, M.T.; Ceylan, Z. Effect of electrospun nisin and curcumin loaded nanomats on the microbial quality, hardness and sensory characteristics of rainbow trout fillet. LWT 2019, 113, 108292. [Google Scholar] [CrossRef]
- Ceylan, Z. Use of characterized chitosan nanoparticles integrated in poly(vinyl alcohol) nanofibers as an alternative nanoscale material for fish balls. J. Food Saf. 2018, 38, e12551. [Google Scholar] [CrossRef]
- Perumal, A.B.; Huang, L.; Nambiar, R.B.; He, Y.; Li, X.; Sellamuthu, P.S. Application of essential oils in packaging films for the preservation of fruits and vegetables: A review. Food Chem. 2022, 375, 131810. [Google Scholar] [CrossRef]
- Duran, M.; Aday, M.S.; Zorba, N.N.D.; Temizkan, R.; Büyükcan, M.B.; Caner, C. Potential of antimicrobial active packaging ‘containing natamycin, nisin, pomegranate and grape seed extract in chitosan coating’ to extend shelf life of fresh strawberry. Food Bioprod. Process. 2016, 98, 354–363. [Google Scholar] [CrossRef]
- Jafarzadeh, S.; Nafchi, A.M.; Salehabadi, A.; Oladzad-abbasabadi, N.; Jafari, S.M. Application of bio-nanocomposite films and edible coatings for extending the shelf life of fresh fruits and vegetables. Adv. Colloid Interface Sci. 2021, 291, 102405. [Google Scholar] [CrossRef] [PubMed]
- Resende, N.S.; Gonçalves, G.A.S.; Reis, K.C.; Tonoli, G.H.D.; Boas, E.V.B.V. Chitosan/cellulose nanofibril nanocomposite and its effect on quality of coated strawberries. J. Food. Qual. 2018, 2018, 1727426. [Google Scholar] [CrossRef] [Green Version]
- Wen, P.; Zhu, D.H.; Wu, H.; Zong, M.H.; Jing, Y.R.; Han, S.Y. Encapsulation of cinnamon essential oil in electrospun nanofibrous film for active food packaging. Food Control 2016, 59, 366–376. [Google Scholar] [CrossRef]
- Li, Y.; Dong, Q.; Chen, J.; Li, L. Effects of coaxial electrospun eugenol loaded core-sheath PVP/shellac fibrous films on postharvest quality and shelf life of strawberries. Postharvest Biol. Technol. 2020, 159, 111028. [Google Scholar] [CrossRef]
- Min, T.; Sun, X.; Yuan, Z.; Zhou, L.; Jiao, X.; Zha, J.; Zhu, Z.; Wen, Y. Novel antimicrobial packaging film based on porous poly(lactic acid) nanofiber and polymeric coating for humidity-controlled release of thyme essential oil. LWT 2021, 135, 110034. [Google Scholar] [CrossRef]
- Li, C.; Chen, W.; Siva, S.; Cui, H.; Lin, L. Electrospun phospholipid nanofibers encapsulated with cinnamaldehyde/HP-β-CD inclusion complex as a novel food packaging material. Food Packag. Shelf Life 2021, 28, 100647. [Google Scholar] [CrossRef]
- Ansarifar, E.; Moradinezhad, F. Preservation of strawberry fruit quality via the use of active packaging with encapsulated thyme essential oil in zein nanofiber film. Int. J. Food Sci. Technol. 2021, 56, 4239–4247. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Zhu, Z.; Jiao, X.; Shang, Y.; Wen, Y. Encapsulation of thymol in biodegradable nanofiber via coaxial eletrospinning and applications in fruit preservation. J. Agric. Food Chem. 2019, 67, 1736–1741. [Google Scholar] [CrossRef]
- Fonseca, L.M.; Radünz, M.; Crizel, R.L.; Camargo, T.M.; Gandra, E.A.; Dias, A.R.G. Effect of carvacrol encapsulation in starch-based nanofibers: Thermal resistance and antioxidant and antimicrobial properties. J. Food Process. Preserv. 2021, 45, e15409. [Google Scholar] [CrossRef]
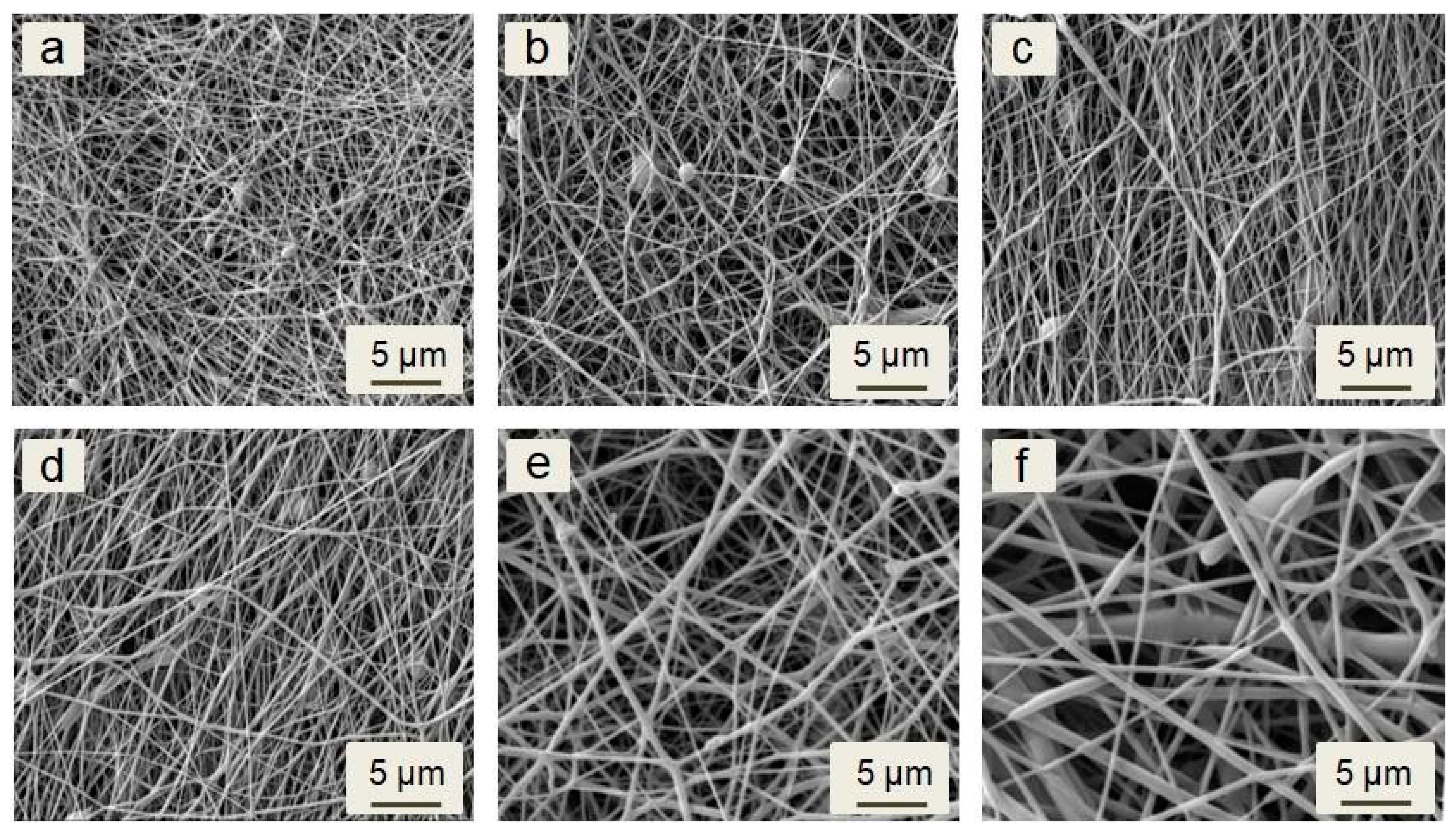

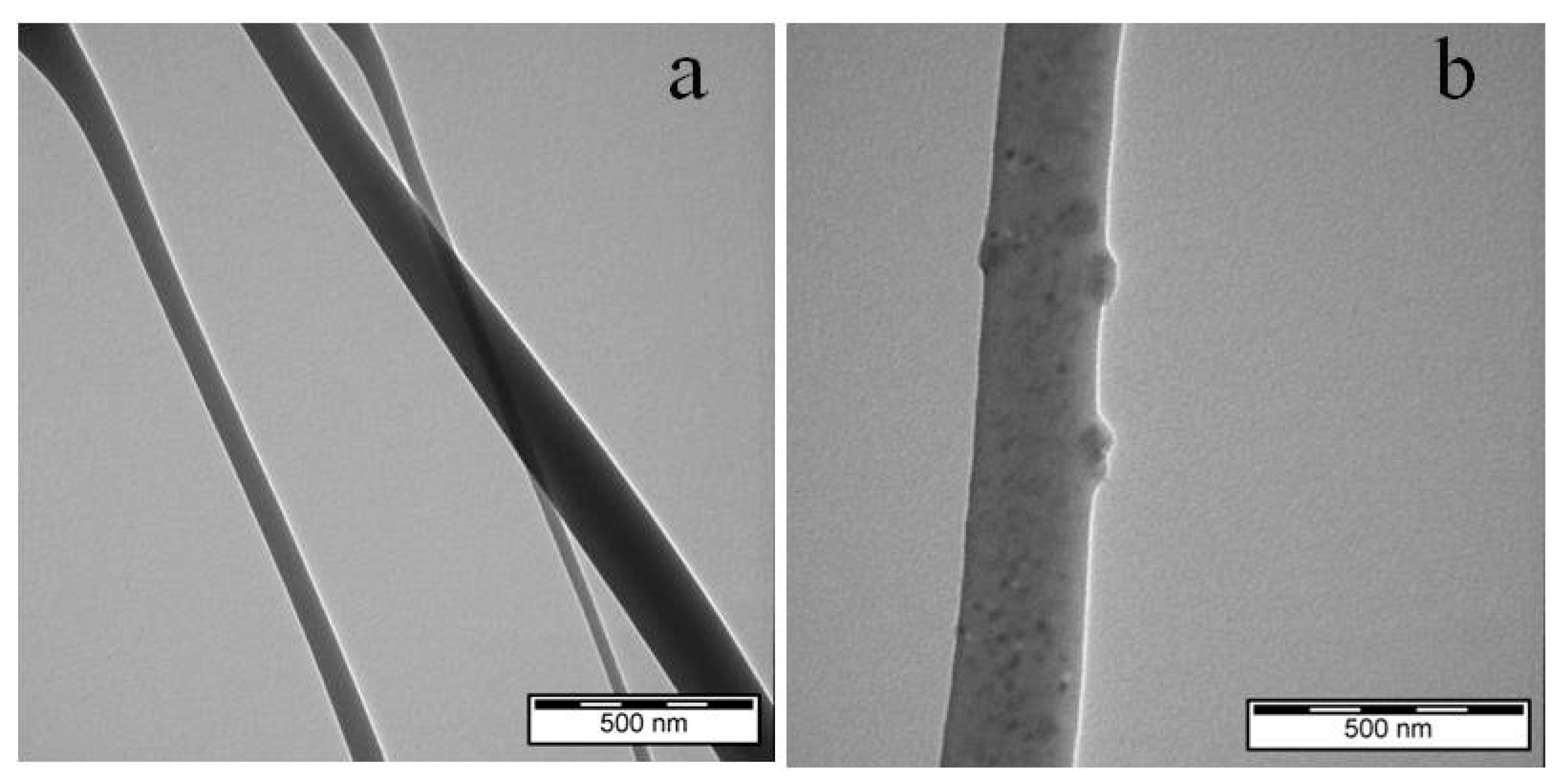
| Sample | PCL (g) | Nisin (g) | DCM (mL) | DMF (mL) | Total Concentration (%) (m/V) |
|---|---|---|---|---|---|
| PCL | 1.5 | - | 5 | 5 | 15.00 |
| PCL/2% N | 1.5 | 0.03 | 5 | 5 | 15.30 |
| PCL/6% N | 1.5 | 0.09 | 5 | 5 | 15.90 |
| PCL/12%N | 1.5 | 0.18 | 5 | 5 | 16.80 |
| PCL/25% N | 1.5 | 0.38 | 5 | 5 | 18.80 |
| PCL/50% N | 1.5 | 0.75 | 5 | 5 | 22.50 |
| Sample | Viscosity (Pa·s) | Avg. dia ± Sd (nm) |
|---|---|---|
| PCL | 0.048 | 89 ± 50 |
| PCL/2% N | 0.049 | 106 ± 47 |
| PCL/6% N | 0.050 | 118 ± 53 |
| PCL/12% N | 0.051 | 123 ± 43 |
| PCL/25% N | 0.057 | 258 ± 122 |
| PCL/50% N | 0.071 | 856 ± 423 |
| Sample | PCL | PCL/2% N | PCL/6% N | PCL/12% N | PCL/25% N | PCL/50% N |
|---|---|---|---|---|---|---|
| Contact angle (°) | 100 ± 5 | 99 ± 7 | 100 ± 6 | 101 ± 5 | 103 ± 4 | 104 ± 5 |
| Tested Microorganism | Amount of Nisin (%) | Number of Bacteria Recovered at 24 h of Contact Time (CFU *·mL−1) | Log. of Number of Bacteria Recovered at 24 h Contact Time (CFU *·mL−1) | Antibacterial Activity R | Reduction (%) |
|---|---|---|---|---|---|
| E. Coli CCM 3988 (G−) | - | 2,300,000 ± 150,000 | 6.36 ± 0.41 | - | - |
| 2 | 1,100,000 ± 40,000 | 6.04 ± 0.22 | 0.32 ± 0.19 | 52.17 ± 3.63 | |
| 6 | 450,000 ± 26,000 | 5.65 ± 0.33 | 0.71 ± 0.08 | 80.43 ± 5.77 | |
| 12 | 200,000 ± 12,000 | 5.30 ± 0.32 | 1.06 ± 0.09 | 91.30 ± 6.00 | |
| 25 | 130,000 ± 9000 | 5.11 ± 0.35 | 1.25 ± 0.06 | 94.35 ± 6.92 | |
| 50 | 120,000 ± 7700 | 5.08 ± 0.33 | 1.28 ± 0.08 | 94.78 ± 6.42 | |
| S. Aureus CCM 3953 (G+) | - | 2,100,000 ± 112,000 | 6.32 ± 0.34 | - | - |
| 2 | 800,000 ± 38,000 | 5.90 ± 0.28 | 0.42 ± 0.06 | 61.90 ± 4.75 | |
| 6 | 300,000 ± 17,500 | 5.48 ± 0.32 | 0.84 ± 0.02 | 85.71 ± 5.83 | |
| 12 | 180,000 ± 9000 | 5.26 ± 0.26 | 1.06 ± 0.08 | 91.43 ± 5.00 | |
| 25 | 130,000 ± 7000 | 5.11 ± 0.28 | 1.21 ± 0.06 | 94.35 ± 5.38 | |
| 50 | 120,000 ± 5500 | 5.08 ± 0.23 | 1.24 ± 0.11 | 94.78 ± 4.58 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Opálková Šišková, A.; Mosnáčková, K.; Musioł, M.; Opálek, A.; Bučková, M.; Rychter, P.; Eckstein Andicsová, A. Electrospun Nisin-Loaded Poly(ε-caprolactone)-Based Active Food Packaging. Materials 2022, 15, 4540. https://doi.org/10.3390/ma15134540
Opálková Šišková A, Mosnáčková K, Musioł M, Opálek A, Bučková M, Rychter P, Eckstein Andicsová A. Electrospun Nisin-Loaded Poly(ε-caprolactone)-Based Active Food Packaging. Materials. 2022; 15(13):4540. https://doi.org/10.3390/ma15134540
Chicago/Turabian StyleOpálková Šišková, Alena, Katarína Mosnáčková, Marta Musioł, Andrej Opálek, Mária Bučková, Piotr Rychter, and Anita Eckstein Andicsová. 2022. "Electrospun Nisin-Loaded Poly(ε-caprolactone)-Based Active Food Packaging" Materials 15, no. 13: 4540. https://doi.org/10.3390/ma15134540






