On the Deposition Process of Ceramic Layer Thin Films for Low-Carbon Steel Pipe Protection
Abstract
1. Introduction
2. Methodology
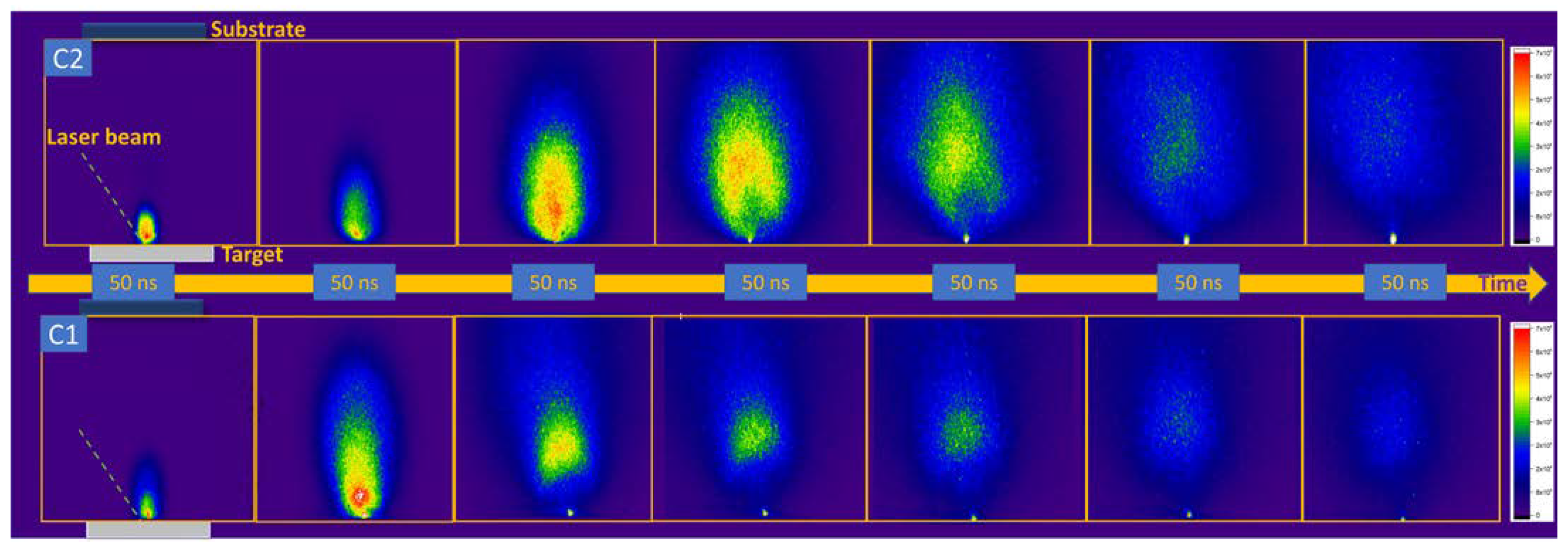
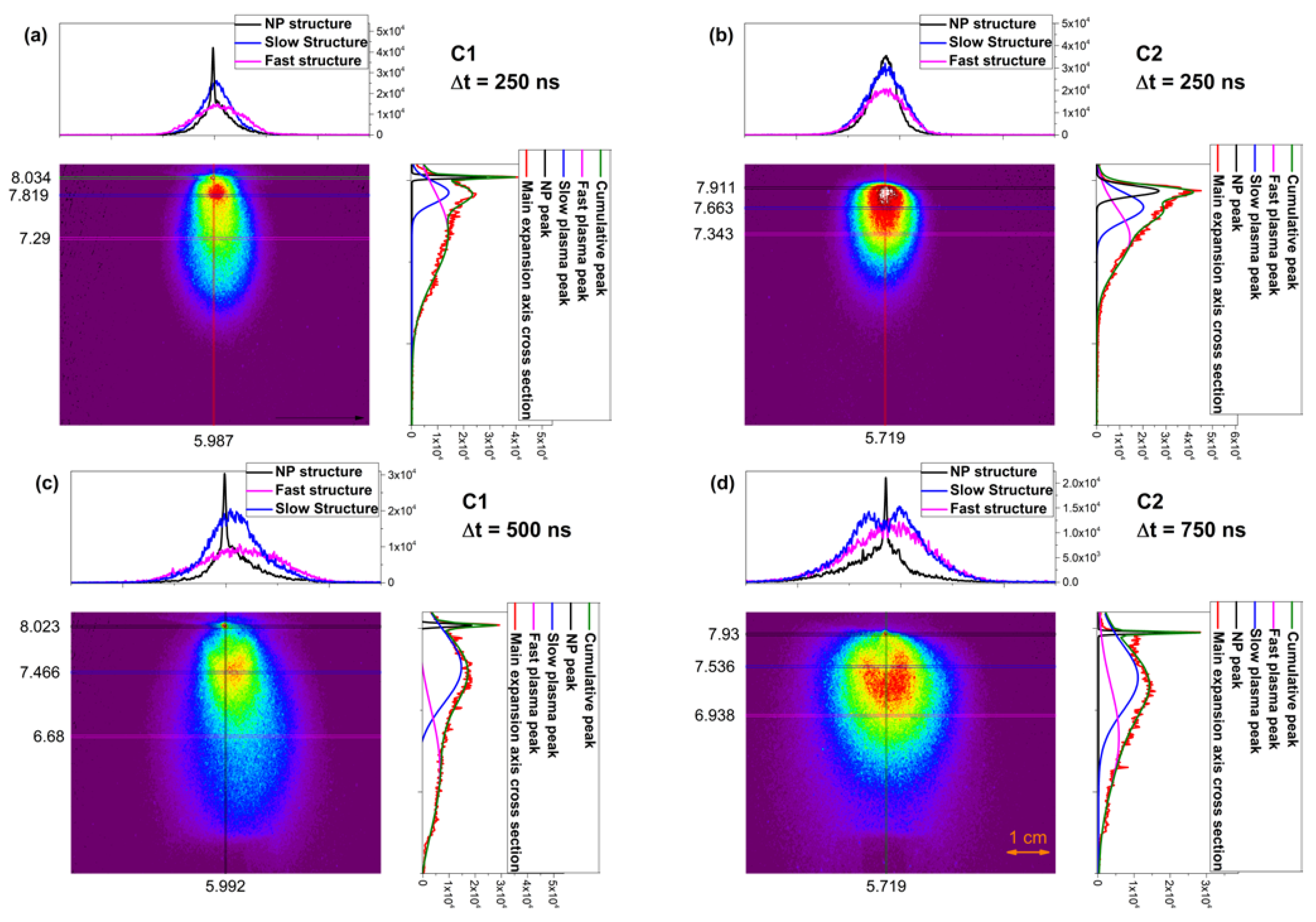
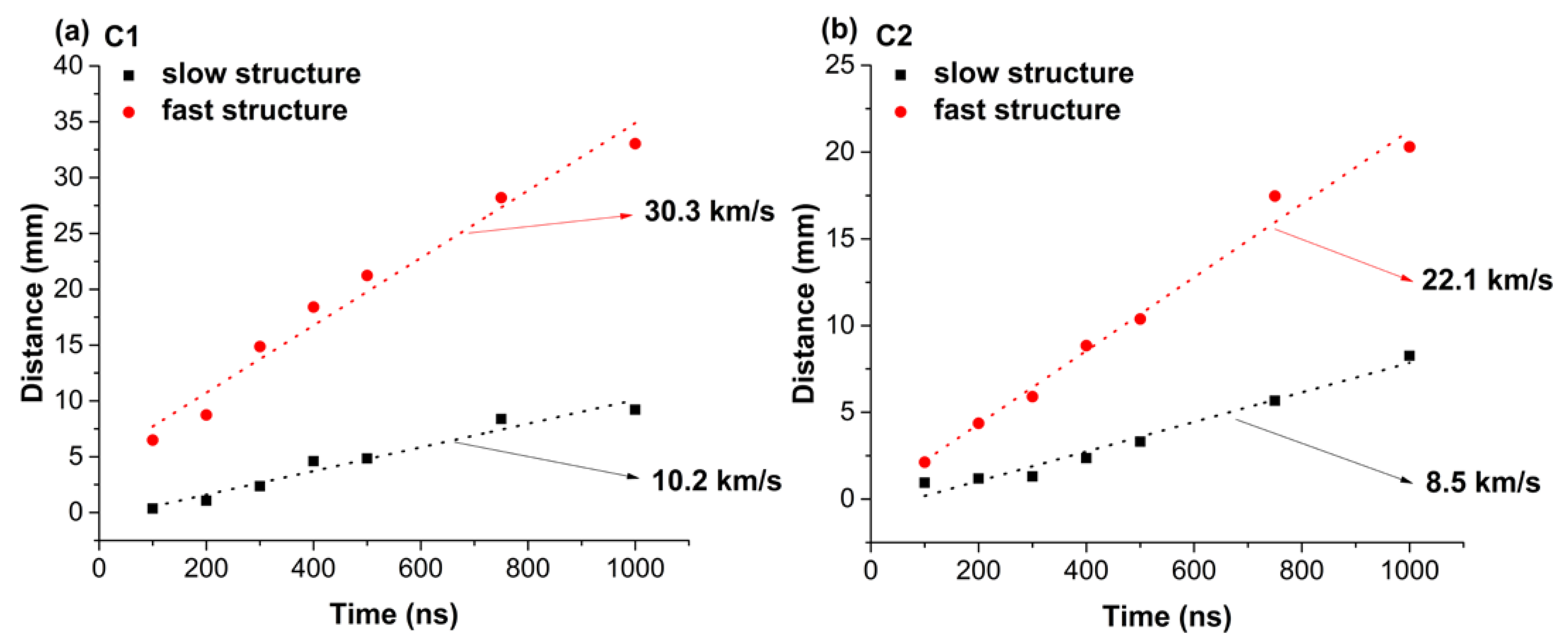
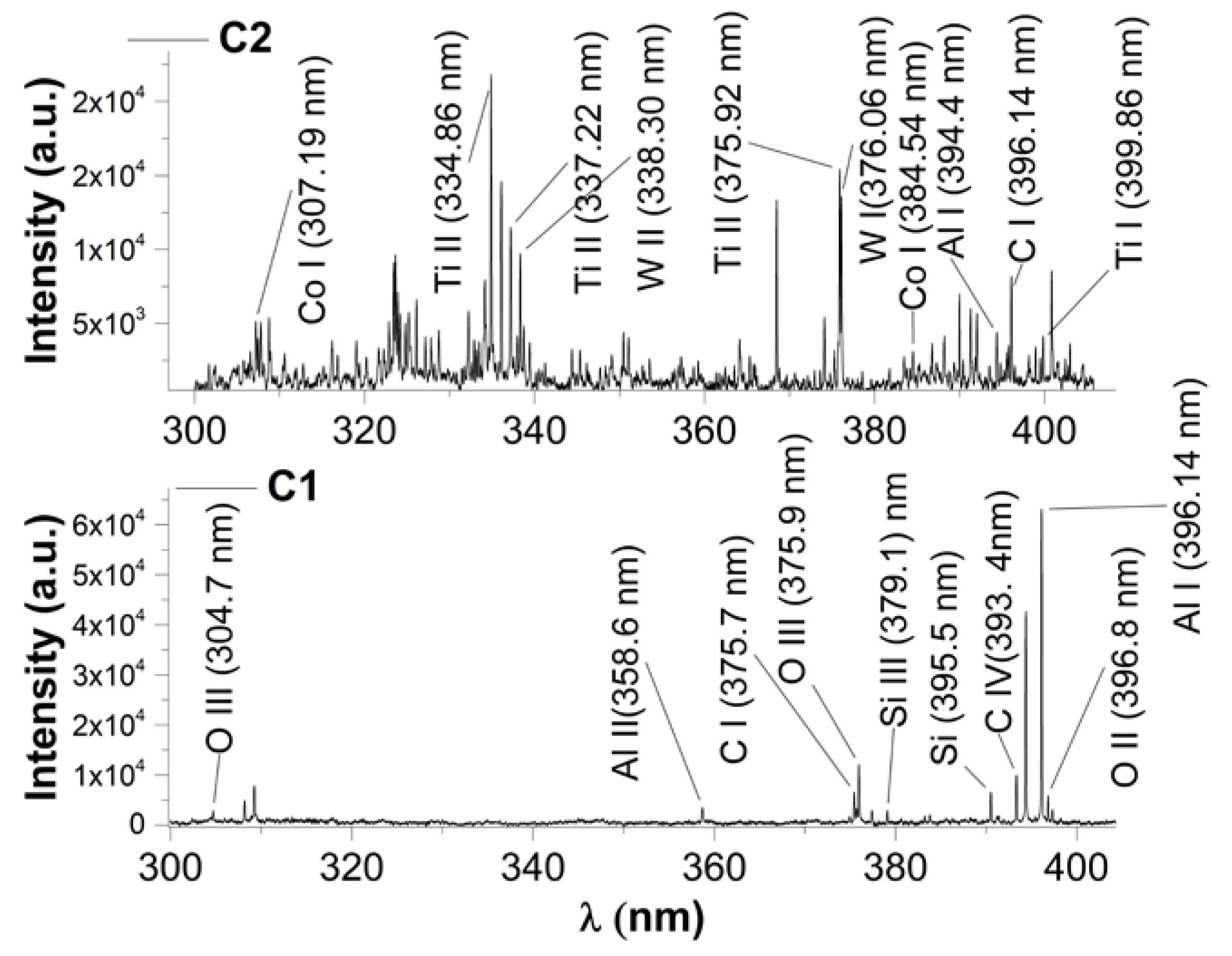
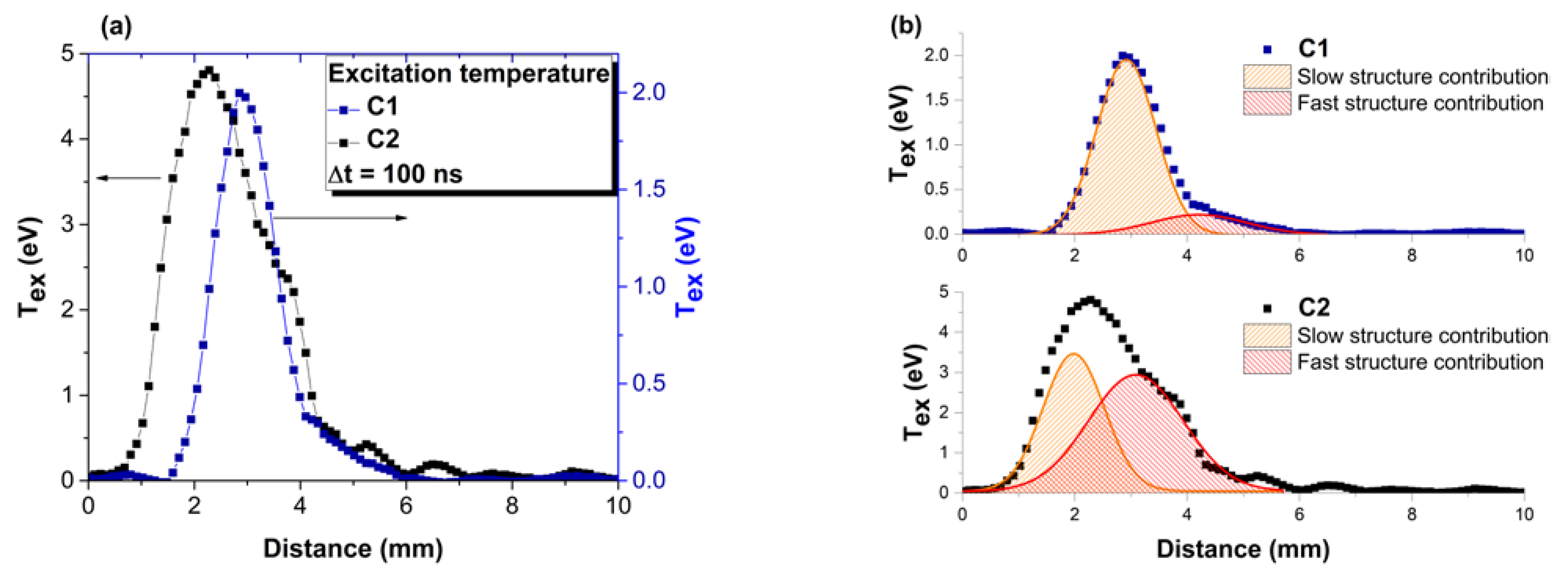
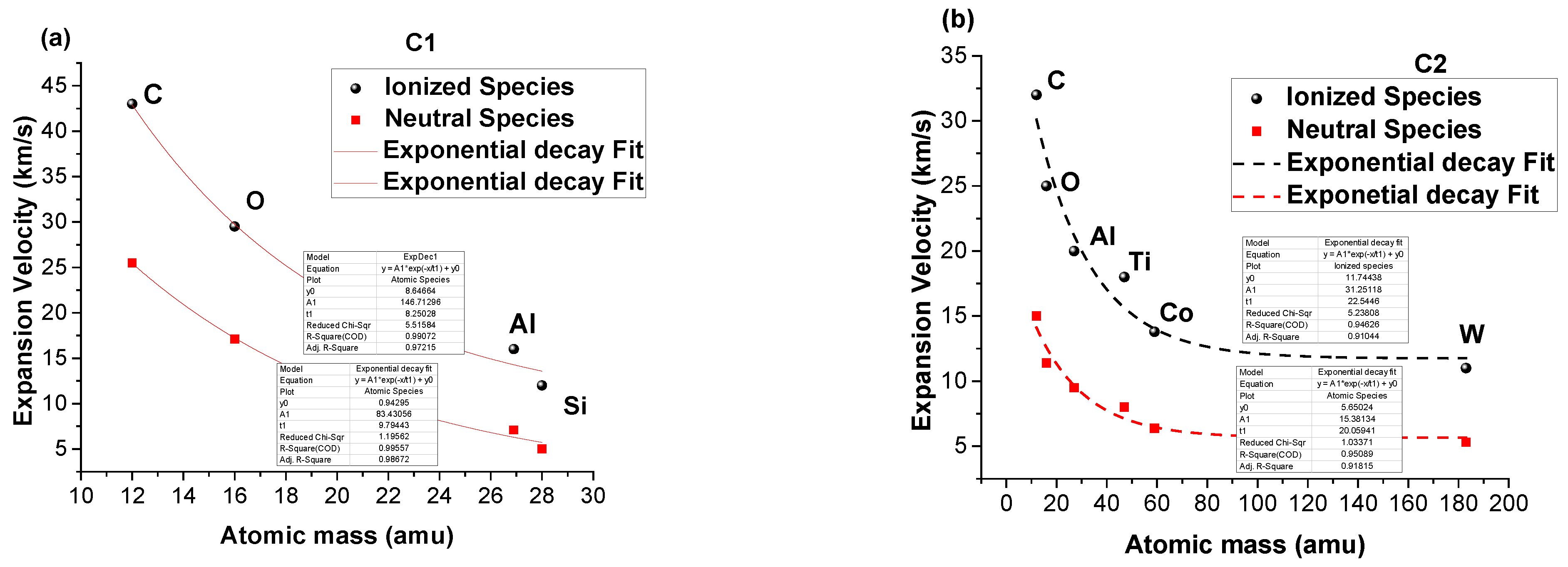
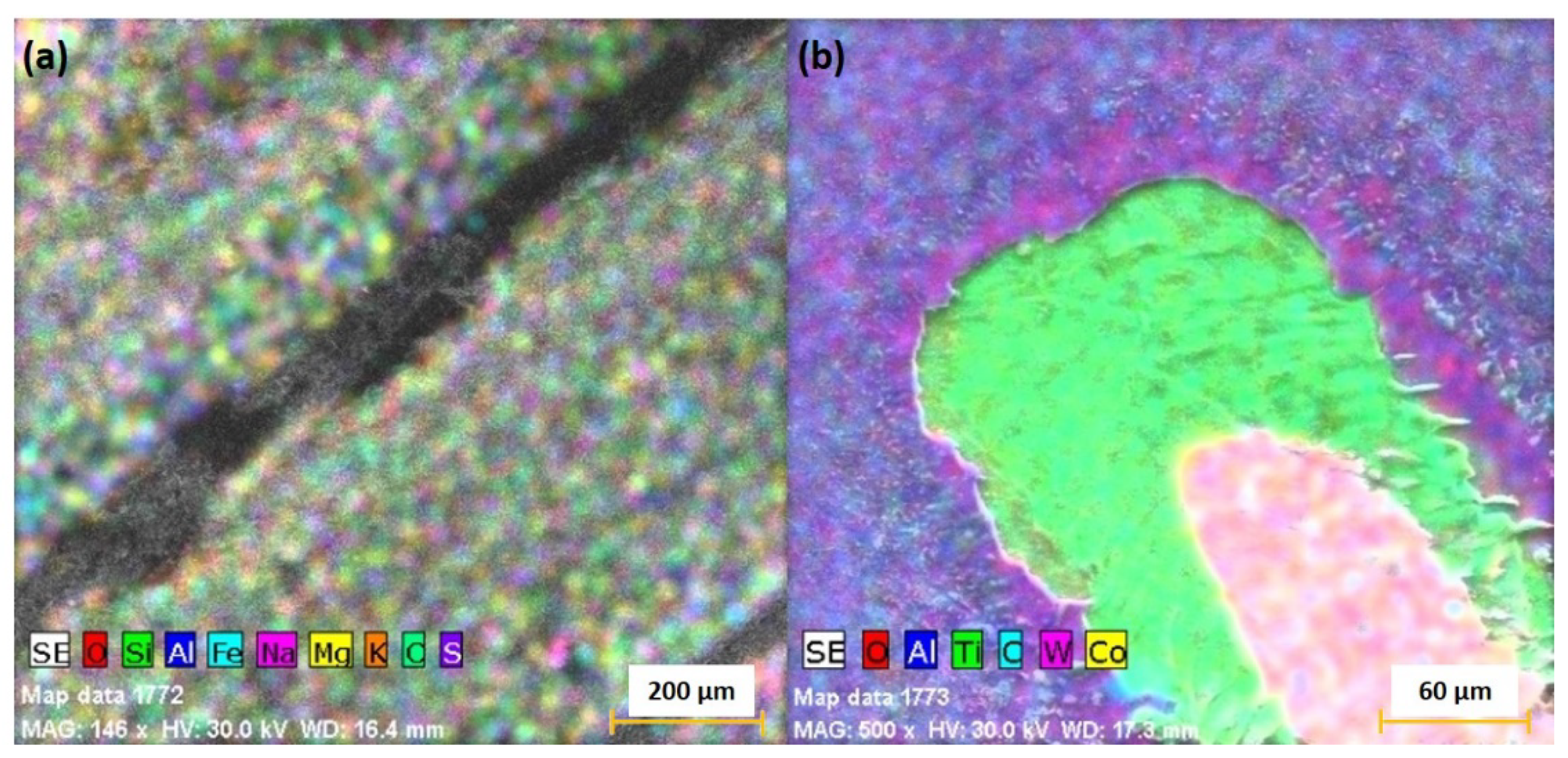
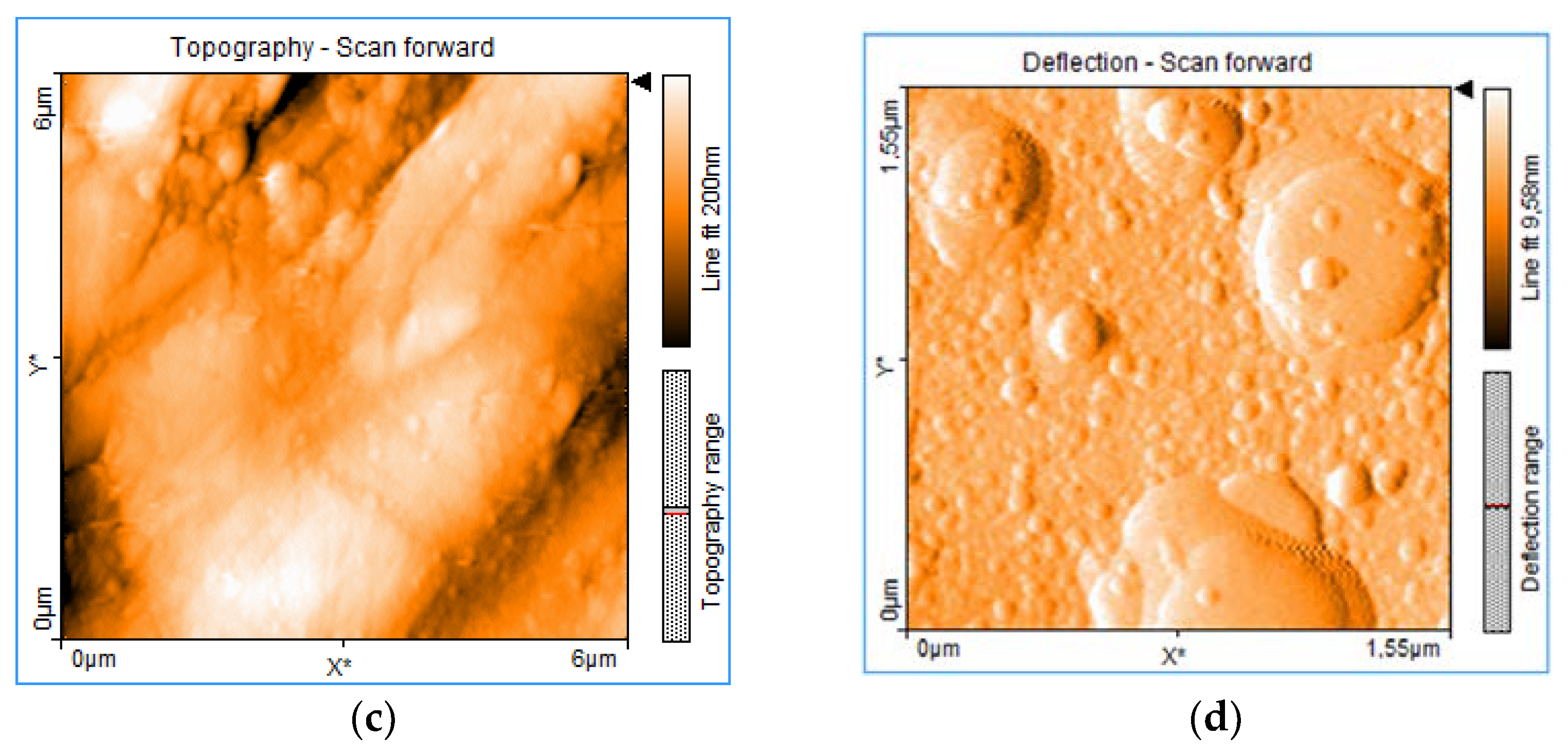
3. Real-Time Monitoring during PLD of Ceramic Protective Layer
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yun, W.B.; Wang, S.; He, L.; Zhang, X.X.; Huixin, L.; Minxu, L. Unraveling the effect of H2S on the corrosion behavior of high strengthsulfur-resistant steel in CO2/H2S/Cl. J. Nat. Gas Sci. Eng. 2022, 100, 104477. [Google Scholar]
- Yan, L.; Wenwen, S.; Anqing, F.; Junfeng, X.; Yaorong, F.; Zhenquan, B.; Chengxian, Y.; Qingwei, M.; Nan, J.; Xianren, K. Combined effect of hydrogen embrittlement and corrosion on the cracking behaviour of C110 low alloy steel in O2-contaminated H2S environment. Corros. Sci. 2022, 194, 109926. [Google Scholar]
- Zaharia, M.G.; Stanciu, S.; Cimpoesu, R.; Ionita, I.; Cimpoesu, N. Preliminary results on effect of H2S on P265GH commercial material for natural gases and petroleum transportation. Appl. Surf. Sci. 2018, 438, 20–32. [Google Scholar] [CrossRef]
- Robineau, M.; Deydier, V.; Crusset, D.; Bellefleur, A.; Neff, D.; Vega, E.; Sabot, R.; Jeannin, M.; Refait, P. Formation of Iron Sulfides on Carbon Steel in a Specific Cement Grout Designed for Radioactive Waste Repository and Associated Corrosion Mechanisms. Materials 2021, 14, 3563. [Google Scholar] [CrossRef] [PubMed]
- Nagao, A.; Smith, C.D.; Dadfarnia, M.; Sofronis, P.; Robertson, I.M. The role of hydrogen in hydrogen embrittlement fracture of lath martensitic steel. Acta Mater. 2012, 60, 5182–5189. [Google Scholar] [CrossRef]
- Nagao, A.; Dadfarnia, M.; Somerday, B.P.; Sofronis, P.; Ritchie, R.O. Hydrogen-enhanced-plasticity mediated decohesion for hydrogen-induced intergranular and “quasi-cleavage” fracture of lath martensitic steels. J. Mech. Phys. Solid 2018, 112, 403–430. [Google Scholar] [CrossRef]
- Attarzadeh, N.; Molaei, M.; Babaei, K.; Fattah-Alhosseini, A. New Promising Ceramic Coatings for Corrosion and Wear Protection of Steels: A Review. Surf. Interfaces 2021, 23, 100997. [Google Scholar] [CrossRef]
- Kuzanyan, A.S.; Kuzanyan, A.A. Pulsed Laser Deposition of Large-Area Thin Films and Coatings; IntechOpen: London, UK, 2016; ISBN 978-953-51-2812-0. [Google Scholar]
- Vakulov, Z.; Khakhulin, D.; Zamburg, E.; Mikhaylichenko, A.; Smirnov, V.A.; Tominov, R.; Klimin, V.S.; Ageev, O.A. Towards Scalable Large-Area Pulsed Laser Deposition. Materials 2021, 14, 4854. [Google Scholar] [CrossRef]
- Hoffmann-Urlaub, S.; Zhang, Y.; Wang, Z.; Kressdorf, B.; Meyer, T. Fabrication of tin-based halide perovskites by pulsed laser deposition. Appl. Phys. A: Mater. Sci. Processing 2020, 126, 553. [Google Scholar] [CrossRef]
- Canulescu, S.; Döbeli, M.; Yao, X.; Lippert, T.; Amoruso, S.; Schou, J. Nonstoichiometric transfer during laser ablation of metal alloys. Phys. Rev. Mater. 2017, 1, 073402. [Google Scholar] [CrossRef]
- Irimiciuc, S.A.; Chertopalov, S.; Lancok, J.; Craciun, V. Langmuir Probe Technique for Plasma Characterization during Pulsed Laser Deposition Process. Coatings 2021, 11, 762. [Google Scholar] [CrossRef]
- Doggett, B.; Lunney, J.G. Langmuir probe characterization of laser ablation plasmas. J. Appl. Phys. 2009, 105, 033306. [Google Scholar] [CrossRef]
- Kautz, E.J.; Phillips, M.C.; Zelenyuk, A.; Harilal, S.S. Oxidation in laser-generated metal plumes. Phys. Plasmas 2022, 29, 053509. [Google Scholar]
- Irimiciuc, S.A.; Hodoroaba, B.C.; Bulai, G.; Gurlui, S.; Craciun, V. Multiple structure formation and molecule dynamics in transient plasmas generated by laser ablation of graphite. Spectrochim. Acta Part B At. Spectrosc. 2020, 165, 105774. [Google Scholar] [CrossRef]
- Metarapi, D.; van Elteren, J.T.; Sala, M.; Vogel-Milkus, K.; Arcon, I.; Selih, V.S.; Kolar, M.; Hocevar, S.B. Laser ablation-single-particle-inductively coupled plasma mass spectrometry as a multimodality bioimaging tool in nano-based omics. Environ. Sci. Nano 2021, 8, 647–656. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Liu, C.; Yu, Y.; Zarkadoula, E.; Yoon, M.; Puretzky, A.A.; Liang, L.; Kong, X.; Gu, Y.; Strasser, A.; et al. Low Energy Implantation into Transition-Metal Dichalcogenide Monolayers to Form Janus Structures. ACS Nano 2020, 14, 3906. [Google Scholar] [CrossRef]
- Giuffredi, G.; Mezzetti, A.; Perego, A.; Mazzolini, P.; Prato, M.; Fumagalli, F.; Lin, Y.C.; Liu, C.; Ivanov, I.N.; Belianinov, A.; et al. Non-Equilibrium Synthesis of Highly Active Nanostructured, Oxygen-Incorporated Amorphous Molybdenum Sulfide HER Electrocatalyst. Small 2020, 16, 2004047. [Google Scholar]
- Spadaro, M.C.; Fazio, E.; Neri, F.; Trusso, S.; Ossi, P.M. On the role of the ablated mass on the propagation of a laser-generated plasma in an ambient gas. EPL 2015, 109, 25002. [Google Scholar] [CrossRef]
- Chen, J.; Döbeli, M.; Wokaun, A.; Lippert, T. Plasma interactions with the N2O background gas: Enhancing the oxidization of alkaline-earth species for pulsed laser deposition. J. Appl. Phys. 2018, 124, 085308. [Google Scholar]
- Yao, X.; Schneider, C.W.; Lippert, T.; Wokaun, A. Manipulation of ion energies in pulsed laser deposition to improve film growth. Appl. Phys. A Mater. Sci. Processing 2019, 125, 344. [Google Scholar] [CrossRef]
- Ojeda-G-P, A.; Döbeli, M.; Lippert, T. Influence of Plume Properties on Thin Film Composition in Pulsed Laser Deposition. Adv. Mater. Interfaces 2018, 5, 1701062. [Google Scholar] [CrossRef]
- Pryds, N.; Schou, J.; Linderoth, S. The spatial thickness distribution of metal films produced by large area pulsed laser deposition. Appl. Surf. Sci. 2007, 253, 8231–8234. [Google Scholar] [CrossRef]
- Amoruso, S.; Toftmann, B.; Schou, J. Broadening and attenuation of UV laser ablation plumes in background gases. Appl. Surf. Sci. 2005, 248, 323–328. [Google Scholar] [CrossRef]
- Amoruso, S.; Schou, J.; Lunney, J.G. Multiple-scattering effects in laser ablation plume propagation in gases. Europhys. Lett. 2006, 76, 436–442. [Google Scholar] [CrossRef][Green Version]
- Amoruso, S.; Schou, J.; Lunney, J.G. Influence of the atomic mass of the background gas on laser ablation plume propagation. Appl. Phys. A 2008, 92, 907–911. [Google Scholar] [CrossRef]
- Toftmann, B.; Doggett, B.; Budtz-Jørgensen, C.; Schou, J.; Lunney, J.G. Femtosecond ultraviolet laser ablation of silver and comparison with nanosecond ablation. J. Appl. Physics 2013, 113, 083304. [Google Scholar] [CrossRef]
- Mantecón, R.; Rufo-Martín, C.; Castellanos, R.; Diaz-Alvarez, J. Experimental assessment of thermal gradients and layout effects on the mechanical performance of components manufactured by fused deposition modeling. Rapid Prototyp. J. 2022; ahead-of-print. [Google Scholar] [CrossRef]
- Dayam, S.; Tandon, P.; Priyadarshi, S. Development of paste extrusion-based metal additive manufacturing process. Rapid Prototyp. J. 2022; ahead-of-print. [Google Scholar] [CrossRef]
- Khorasani, M.; Loy, J.; Ghasemi, A.H.; Sharabian, E.; Leary, M.; Mirafzal, H.; Cochrane, P.; Rolfe, B.; Gibson, I. A review of Industry 4.0 and additive manufacturing synergy. Rapid Prototyp. J. 2022; ahead-of-print. [Google Scholar] [CrossRef]
- Irimiciuc, S.; More-Chevalier, J.; Chertpalov, S.; Fekete, L.; Novotný, M.; Havlová, S.; Poupon, M.; Zikmund, T.; Kůsová, K.; Lančok, J. In-situ plasma monitoring by optical emission spectroscopy during pulsed laser deposition of doped Lu2O3. Appl. Phys. B Lasers Opt. 2021, 127, 140. [Google Scholar] [CrossRef]
- Irimiciuc, S.A.; Chertopalov, S.; Bulíř, J.; Vondracek, M.; Fekete, L.; Jiricek, P.; Novotný, M.; Craciun, V.; Lancok, J. Insight into the plasma oxidation process during pulsed laser deposition. Plasma Processes Polym. 2021, 19, e2100102. [Google Scholar] [CrossRef]
- Irimiciuc, S.A.; Chertopalov, S.; Bulíř, J.; Fekete, L.; Vondráček, M.; Novotný, M.; Craciun, V.; Lancok, J. In situ optical and electrical analysis of transient plasmas generated by ns-laser ablation for Ag nanostructured film production. Vacuum 2021, 193, 110528. [Google Scholar] [CrossRef]
- De Giacomo, A.; Shakhatov, V.A.; De Pascale, O. Optical emission spectroscopy and modelling of plasma produced by laser ablation of titanium oxide. Spectrochim. Acta-Part B At. Spectrosc. 2001, 56, 753–776. [Google Scholar] [CrossRef]
- Irimiciuc, S.; Bulai, G.; Agop, M.; Gurlui, S. Influence of laser-produced plasma parameters on the deposition process: In situ space- and time-resolved optical emission spectroscopy and fractal modeling approach. Appl. Phys. A 2018, 124, 615. [Google Scholar] [CrossRef]
- Irimiciuc, S.A.; Chertopalov, S.; Novotný, M.; Craciun, V.; Lancok, J. Understanding pulsed laser deposition process of copper halides via plasma diagnostics techniques. J. Appl. Physics 2021, 130, 243302. [Google Scholar] [CrossRef]
- Bulgakov, A.; Bulgakova, N.M. Dynamics of laser-induced plume expansion into an ambient gas during film deposition. J. Phys. D Appl. Phys. 1999, 28, 1710–1718. [Google Scholar] [CrossRef]
- Kramida, A.; Ralchenko, Y.; Reader, J.; NIST ASD Team. NIST Atomic Spectra Database Lines Form, NIST Atomic Spectra Database (Ver. 5.2). 2014. Available online: http://physics.nist.gov/asd (accessed on 1 October 2018).
- Canulescu, S.; Papadopoulou, E.L.; Anglos, D.; Lippert, T.; Schneider, C.W.; Wokaun, A. Mechanisms of the laser plume expansion during the ablation of LiMn2O4. J. Appl. Phys. 2009, 105, 063107. [Google Scholar] [CrossRef]
- Cristoforetti, G.; Tognoni, E.; Gizzi, L.A. Thermodynamic equilibrium states in laser-induced plasmas: From the general case to laser-induced breakdown spectroscopy plasmas. Spectrochim. Acta-Part B At. Spectrosc. 2013, 90, 1–22. [Google Scholar] [CrossRef]
- Irimiciuc, S.A.; Gurlui, S.; Agop, M. Particle distribution in transient plasmas generated by ns-laser ablation on ternary metallic alloys. Appl. Phys. B Lasers Opt. 2019, 125, 190. [Google Scholar] [CrossRef]
- Vitiello, M.; Amoruso, S.; Altucci, C.; de Lisio, C.; Wang, X. The emission of atoms and nanoparticles during femtosecond laser ablation of gold. Appl. Surf. Sci. 2005, 248, 163–166. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Irimiciuc, S.; Zaharia, M.G.; Cimpoesu, R.; Bulai, G.; Gurlui, S.O.; Cimpoesu, N. On the Deposition Process of Ceramic Layer Thin Films for Low-Carbon Steel Pipe Protection. Materials 2022, 15, 4673. https://doi.org/10.3390/ma15134673
Irimiciuc S, Zaharia MG, Cimpoesu R, Bulai G, Gurlui SO, Cimpoesu N. On the Deposition Process of Ceramic Layer Thin Films for Low-Carbon Steel Pipe Protection. Materials. 2022; 15(13):4673. https://doi.org/10.3390/ma15134673
Chicago/Turabian StyleIrimiciuc, Stefan, Marius Gabriel Zaharia, Ramona Cimpoesu, Georgiana Bulai, Silviu Octavian Gurlui, and Nicanor Cimpoesu. 2022. "On the Deposition Process of Ceramic Layer Thin Films for Low-Carbon Steel Pipe Protection" Materials 15, no. 13: 4673. https://doi.org/10.3390/ma15134673
APA StyleIrimiciuc, S., Zaharia, M. G., Cimpoesu, R., Bulai, G., Gurlui, S. O., & Cimpoesu, N. (2022). On the Deposition Process of Ceramic Layer Thin Films for Low-Carbon Steel Pipe Protection. Materials, 15(13), 4673. https://doi.org/10.3390/ma15134673








