Lead Oxide Production in Barton Reactor—Effect of Increased Air Humidity on Lead Oxide Production Parameters
Abstract
:1. Introduction
2. Research Methodology
- Determination of the degree of oxidation (every 2 h) according to an internal procedure;
- Determination of bulk density using an electromagnetic volumeter;
- Determination of the absorption of sulfuric acid;
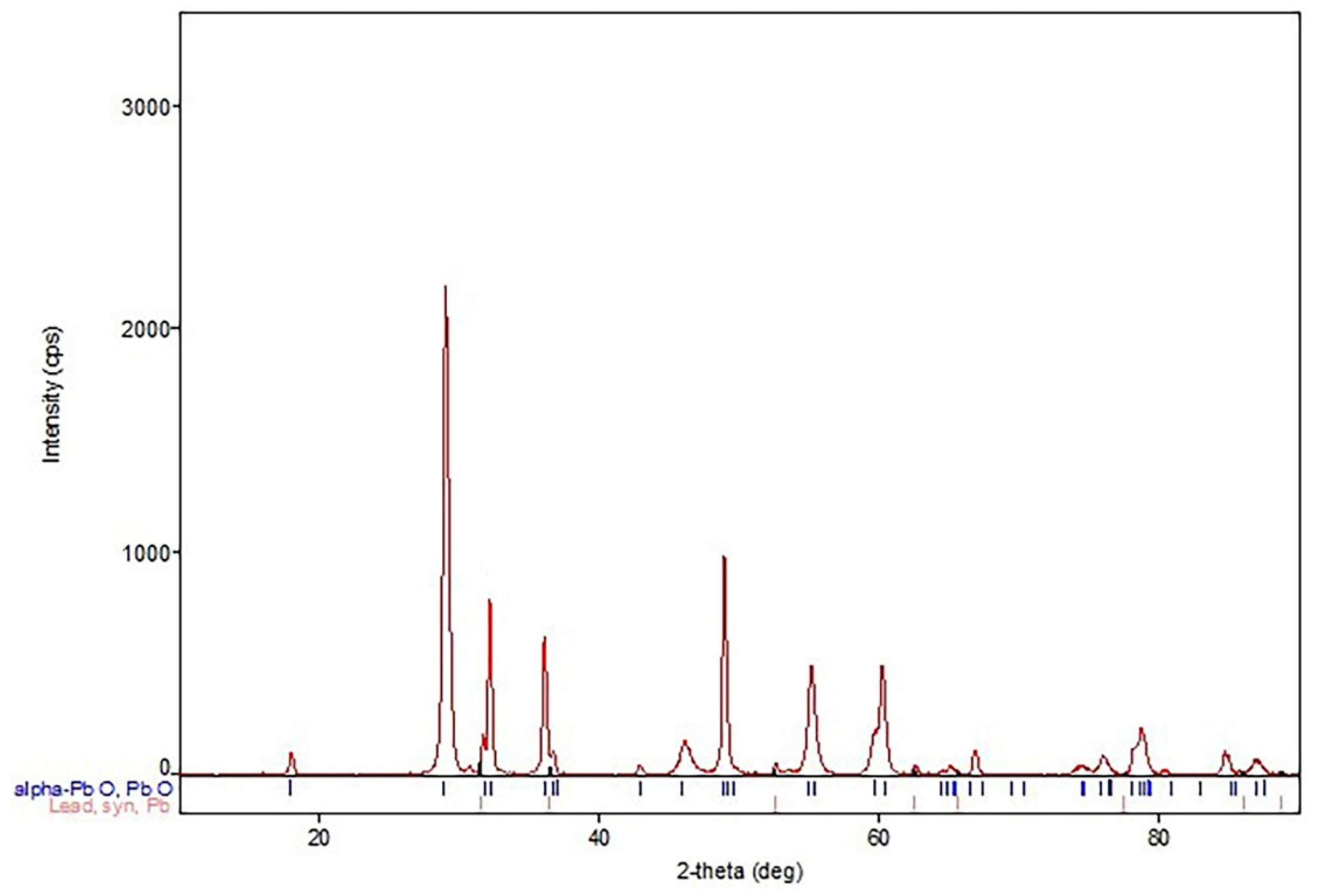
- Phase analysis (XRD).
3. Results and Discussion
4. Conclusions
- The basic parameter deciding on the course of lead oxidation process and influencing the properties of produced lead oxide is the humidity of air supplied to the Barton reactor chamber. High humidity of approx. 60% results in high quality oxide and has a positive impact on the processing parameters such as load on the reactor, air draft, amount of processed lead and process efficiency. These processing parameters affect the cost of producing lead oxide.
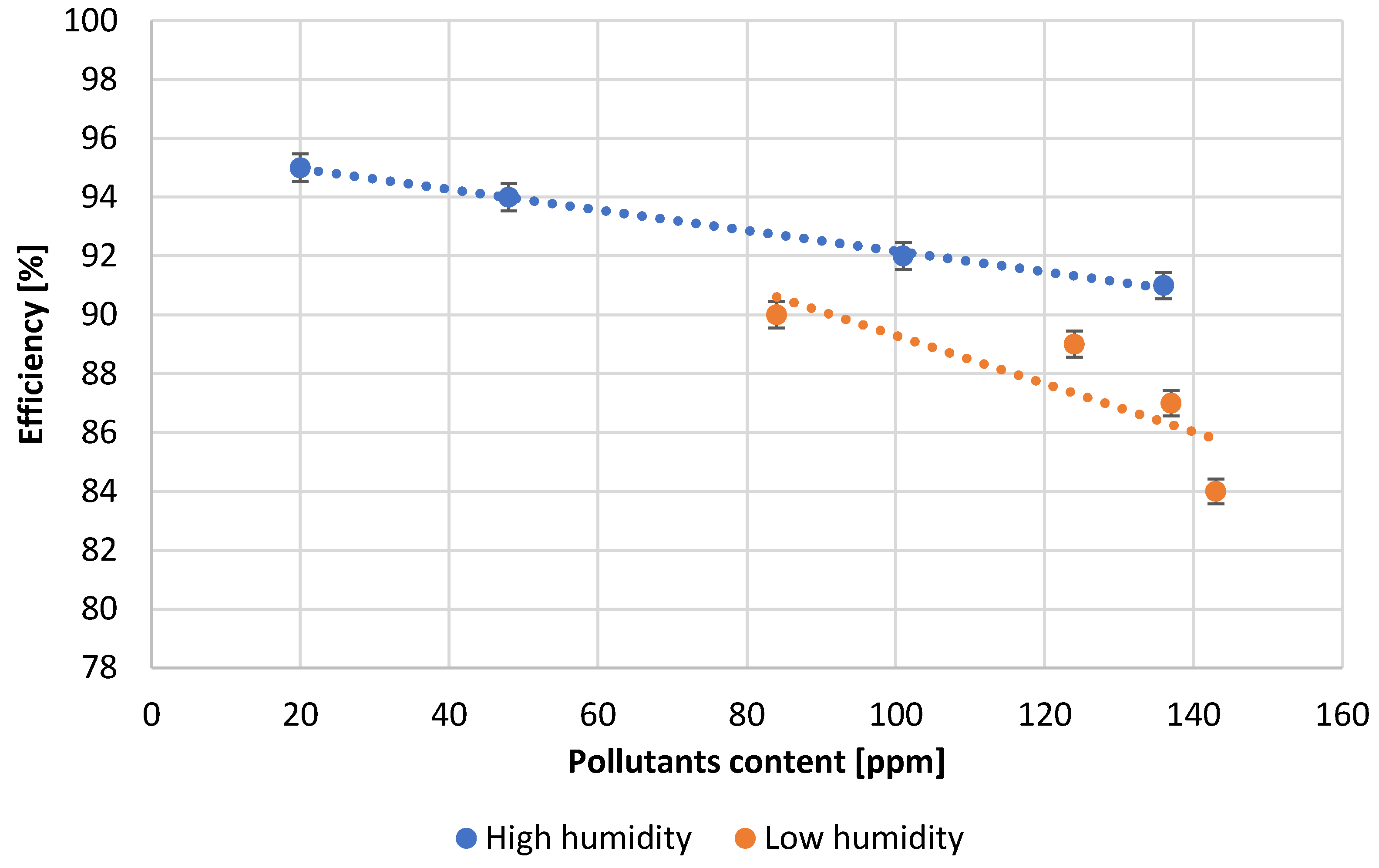
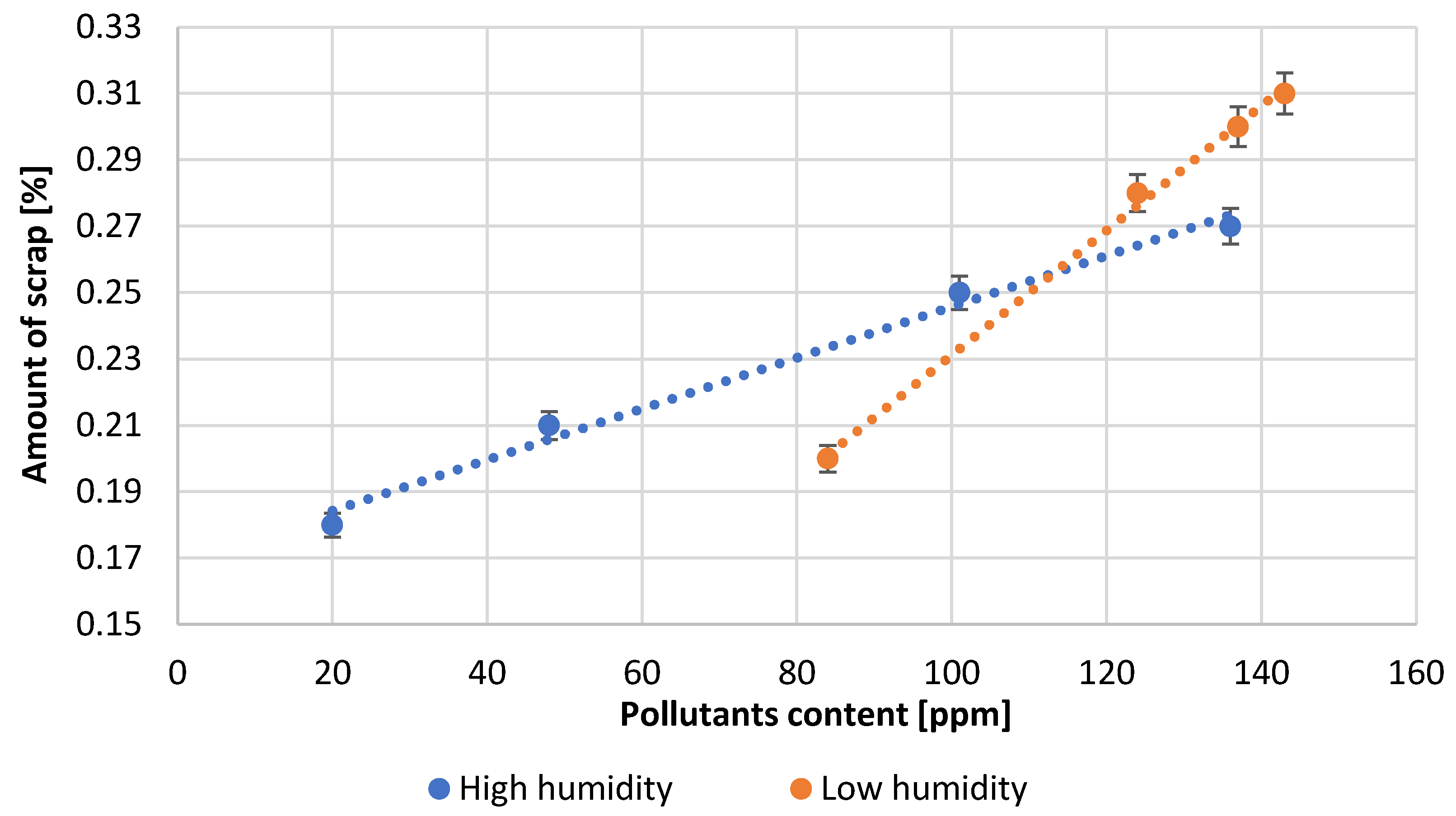
- The influence of the content of lead impurities on the process of lead oxidation and the quality of the obtained product was observed. In the studied range of impurities’ content, their influence is particularly significant on the reactor load, process efficiency, the amount of lead processed per time unit and the amount of scrap produced. An increase in the content of impurities has a negative effect on each of the parameters mentioned above.
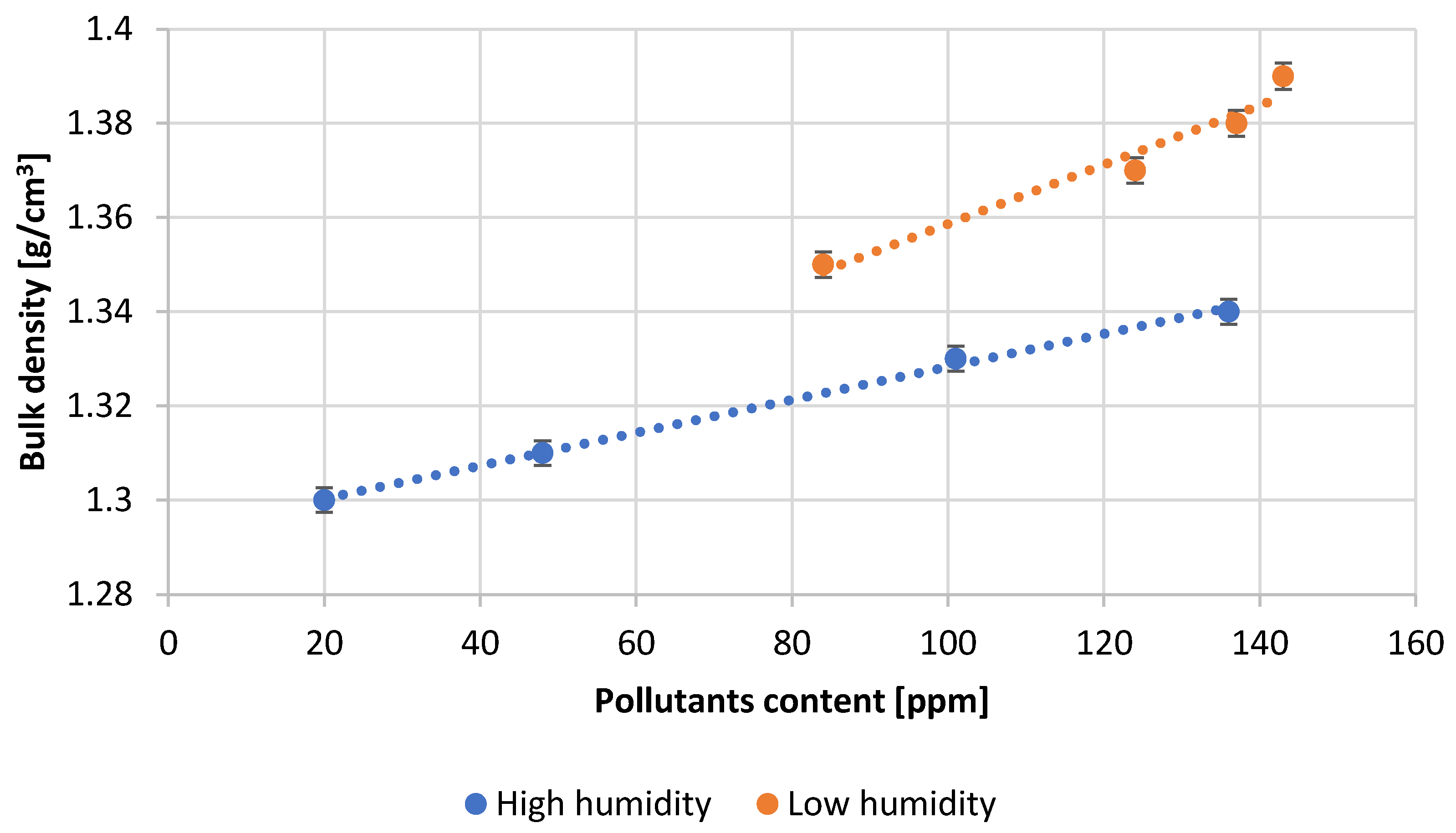
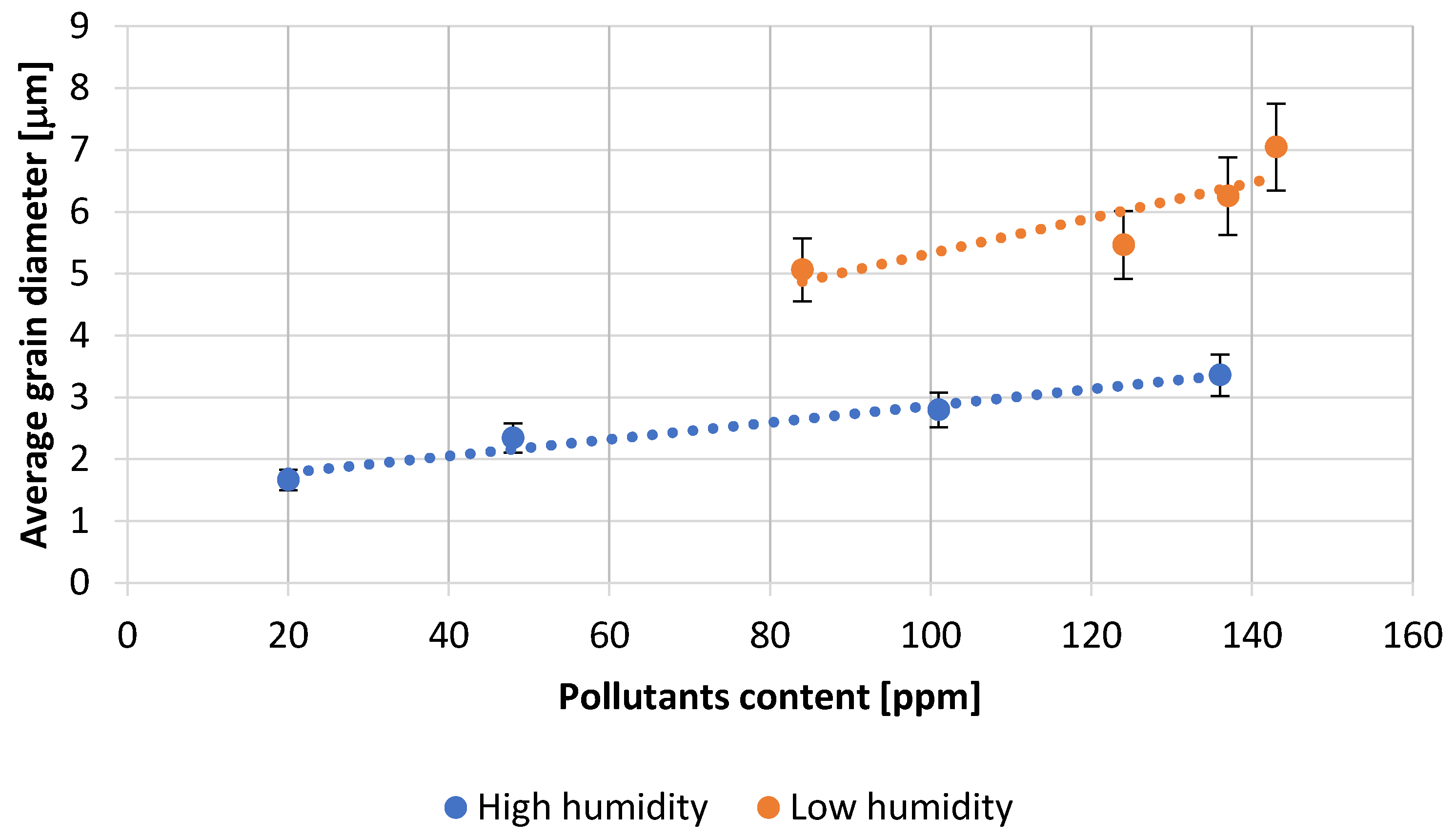
- Microscopic analysis of the lead oxide obtained confirms the conclusions formulated earlier. The process carried out under conditions of high humidity of the air fed to the reactor results in obtaining lead oxide grains of small dimensions and strongly expanded surface.
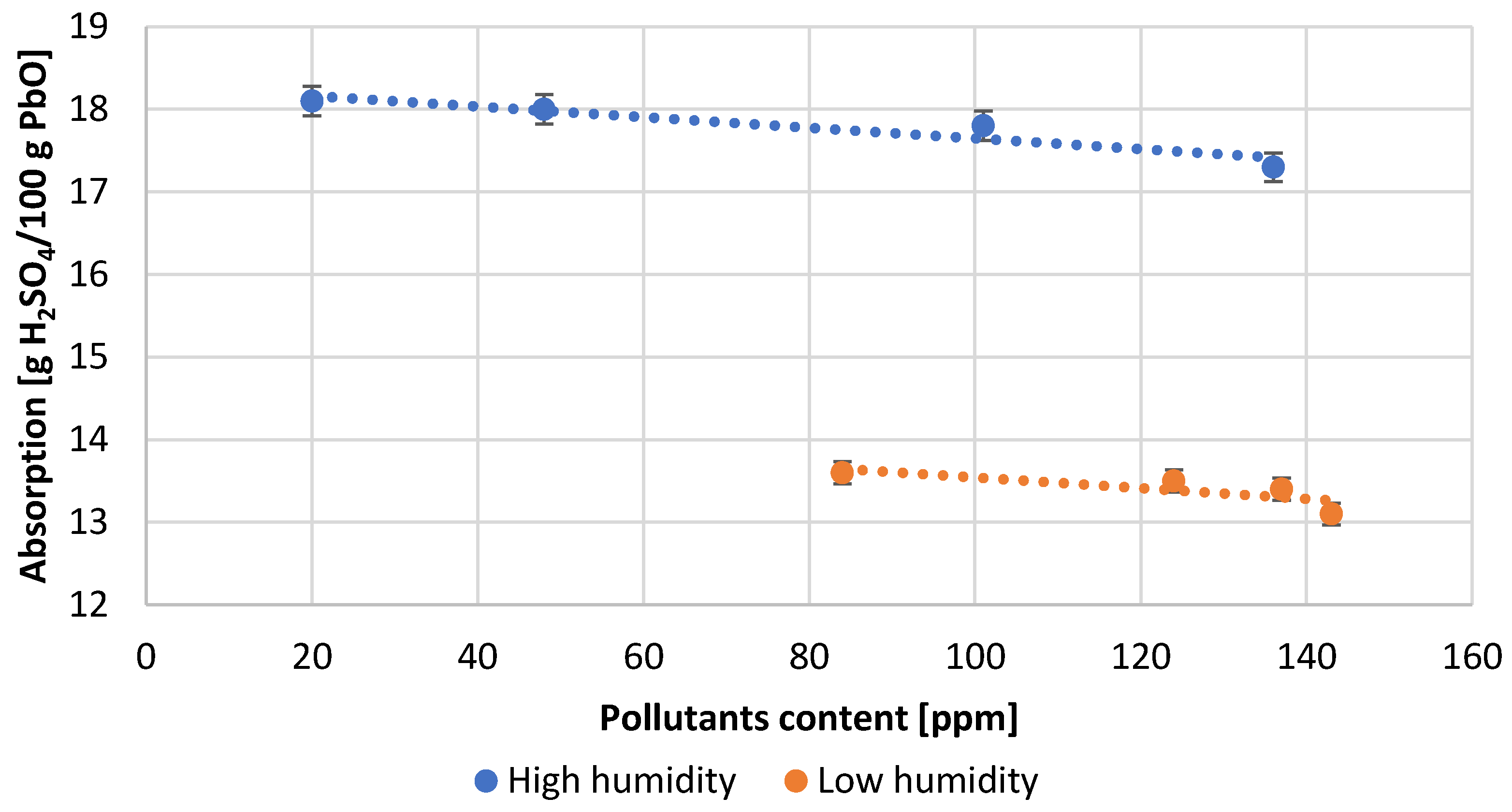
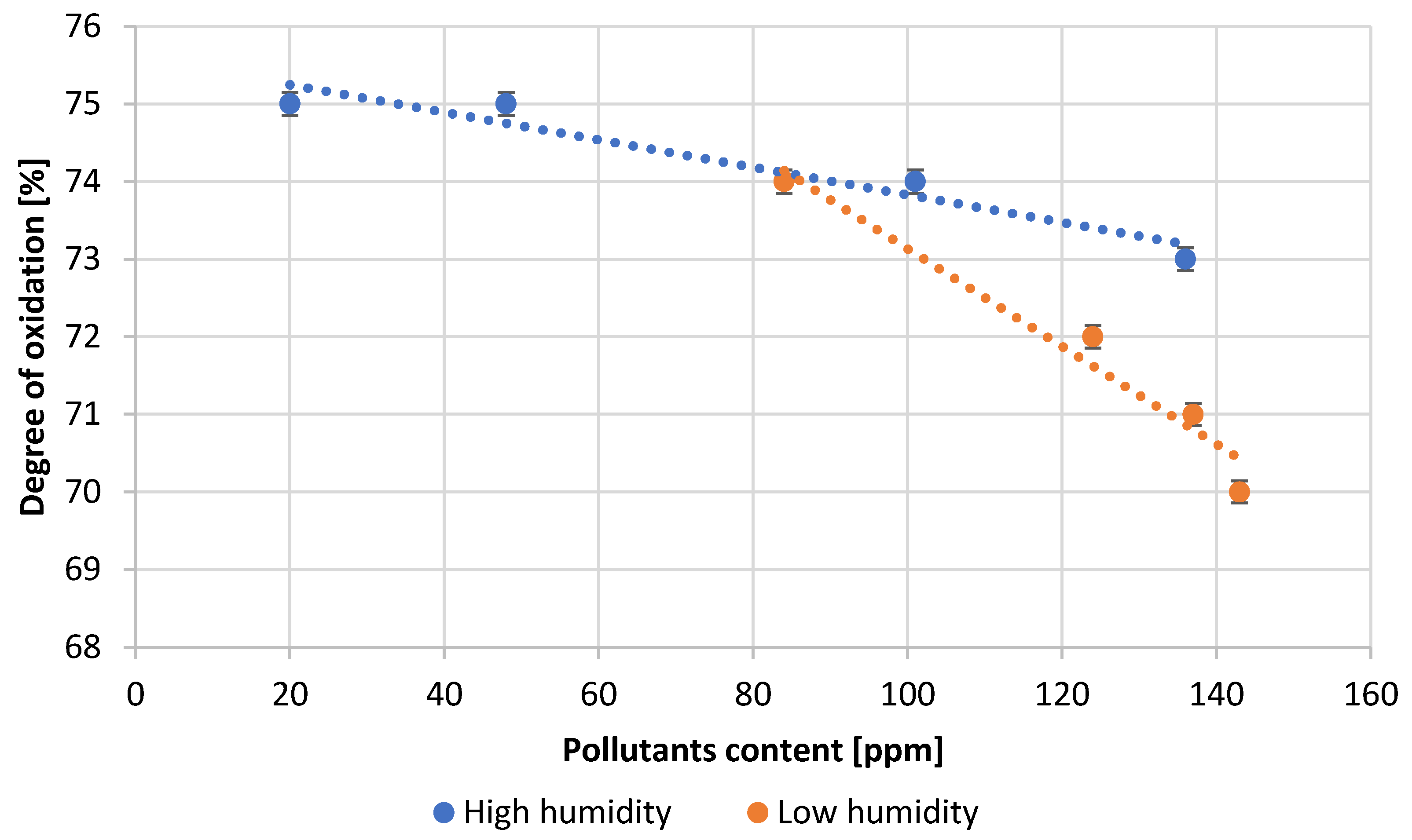
- Optimal lead oxide parameters for acid absorption expected to be above 16 g H2SO4/100 g PbO and an oxidation degree in the range of 75 ± 1% were obtained for an air humidity of about 60% at an impurity content below 100 ppm.
- Observation of the production process carried out under conditions of high humidity indicate a stable and predictable process in contrast to that carried out under low humidity.
- The results obtained in this study can be used to develop a Barton reactor control system, e.g., by means of artificial neural networks.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pavlov, D. Lead-Acid Batteries: Science and Technology; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Garche, J.; Karden, E.; Moseley, P.T.; Rand, D.A.J. Lead-Acid Batteries for Future Automobiles; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Mingyang, L.; Jiakuan, Y.; Sha, L.; Huijie, H.; Jingping, H.; Bingchuan, L.; Kumar, R.V. Review on clean recovery of discarded/spent lead-acid battery and trends of recycled products. J. Power Sources 2019, 436, 226853. [Google Scholar]
- Wilberforce, T.; Thompson, J.; Olabi, A.G. Classification of energy storage materials. In Encyclopedia of Smart Materials; Elsevier: Amsterdam, The Netherlands, 2022; Volume 2, pp. 8–14. [Google Scholar]
- Chumchal, C.; Kurzweil, D. Lead-acid battery operation in micro-hybrid and electrified vehicles. In Lead-Acid Batteries for Future Automobiles; Elsevier: Amsterdam, The Netherlands, 2017; pp. 395–414. [Google Scholar]
- Rodby, K.E.; Carney, T.J.; Gandomi, Y.A.; Barton, J.L.; Darling, R.M.; Brushett, F.R. Assessing the levelized cost of vanadium redox flow batteries with capacity fade and rebalancing. J. Power Sources 2020, 460, 227958. [Google Scholar] [CrossRef]
- Fusillo, G.; Rosestolato, D.; Scura, F.; Cattarin, S.; Mattarozzi, L.; Guerriero, P.; Gambirasi, A.; Brianese, N.; Staiti, P.; Guerriero, R.; et al. Lead paste recycling based on conversion into battery grade oxides. Electrochemical tests and industrial production of new batteries. J. Power Sources 2018, 381, 127–135. [Google Scholar] [CrossRef]
- Stevenson, M. Recycling lead-acid batteries: Overview. In Encyclopedia of Electrochemical Power Sources; Elsevier: Amsterdam, The Netherlands, 2009; pp. 165–178. [Google Scholar]
- Brahmesh, V.J.; Vipin, B.; Ramkumar, J.; Amit, R.K. Impact of policy instruments on lead-acid battery recycling: A system dynamics approach. Resour. Conserv. Recycl. 2021, 169, 105528. [Google Scholar]
- Meshram, S.; Raghwendra, S.T.; Jyoti, G.; Thakur, C.; Soni, A.B. Optimization of lead adsorption from lead-acid battery recycling unit wastewater using H2SO4 modified activated carbon. J. Indian Chem. Soc. 2022, 99, 100469. [Google Scholar] [CrossRef]
- Varshney, K.; Varshney, P.K.; Gautam, K.; Tanwar, M.; Chaudhary, M. Current trends and future perspectives in the recycling of spent lead acid batteries in India. Mater. Today Proc. 2020, 26, 592–602. [Google Scholar] [CrossRef]
- Ma, Y.; Huang, P.; Cao, J.; Zhang, J.; Huang, Y.; Chen, B. Analysis of a more sustainable method for recycling waste lead batteries: Surface renewal promotes desulfurization agent regeneration. Waste Manag. 2022, 137, 319–328. [Google Scholar] [CrossRef]
- Kucharski, M. Recycling of Non-Ferrous Metals; Akademii Górniczo-Hutniczej im. Stanisława Staszica: Krakow, Poland, 2010; pp. 349–392. [Google Scholar]
- Dobrzyński, L.A. Podstawy Nauki o Materiałach i Metaloznawstwo, Materiały Inżynierskie z Podstawami Projektowania Materiałowego; Wydawnictwa Naukowo-Techniczne: Warsaw, Poland, 2002; p. 789. [Google Scholar]
- Boden, P.D. Improved oxides for production of lead/acid battery plates. J. Power Sources 1998, 73, 56–59. [Google Scholar] [CrossRef]
- Krishna, M.; Fraser, E.J.; Wills, R.G.A.; Walsh, F.C. Developments in soluble lead flow batteries and remaining challenges: An illustrated review. J. Energy Storage 2018, 15, 69–90. [Google Scholar] [CrossRef] [Green Version]
- Hardy, D.; Marx, R. New developments in battery oxide production. J. Power Sources 1992, 38, 75–85. [Google Scholar] [CrossRef]
- Brockmann, K.H. Innovations and developments in oxide production for lead/acid batteries. J. Power Sources 1988, 23, 87–91. [Google Scholar] [CrossRef]
- Dix, J.E. A comparison of barton-pot and ball-mill processes for making leady oxide. J. Power Sources 1987, 19, 157–161. [Google Scholar] [CrossRef]
- Prout, L. Aspects of lead/acid battery technology 8. battery oxide. J. Power Sources 1994, 47, 197–217. [Google Scholar] [CrossRef]
- Lam, L.T.; Mawston, I.G.; Pavlov, D.; Rand, D.A.J. Aspects of lead/acid battery manufacture and performance. J. Power Sources 1994, 48, 257–268. [Google Scholar] [CrossRef]
- Mayer, M.G.; Rand, D.A.J. Leady oxide for lead/acid battery positive plates: Scope for improvement? J. Power Sources 1996, 59, 17–24. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. Lead oxide and pigment production. In AP 42, 5th ed.; Chapter 12: Metallurgical Industry; United States Environmental Protection Agency: Washington, DC, USA, 1995; Volume I. [Google Scholar]
- Corino, G.L.; Hill, R.J.; Jessel, A.M.; Rand, D.A.J.; Wanderlich, J.A. A Study of the phase composition, crystallinity, morphology, porosity and surface area of leady oxides used in lead/acid battery plates. J. Power Sources 1985, 16, 141–168. [Google Scholar] [CrossRef]
- Brockmann, K.H. Modern Technology for Leady Oxide Production. J. Power Sources 1989, 28, 121–125. [Google Scholar] [CrossRef]
- Gillian, W.F.; Hardman, A.M.; Kiessling, R.; Lambert, D.W.H.; Manders, J.E.; Rand, D.A.J. Technical and research aspects of lead/acid battery production. J. Power Sources 1989, 28, 217–235. [Google Scholar] [CrossRef]
- Blair, T.L. Lead oxide technology—Past, present, and future. J. Power Sources 1998, 73, 47–55. [Google Scholar] [CrossRef]
- Yang, F.; Zhou, H.; Hu, J.; Ji, S.; Lai, C.; Wang, H.; Sun, J.; Lei, L. Thorn-like and dendrite lead sulfate as negative electrode materials for enhancing the cycle performance of lead-acid batteries. J. Energy Storage 2022, 49, 104112. [Google Scholar] [CrossRef]
- Pierson, J.R. Control of vital chemical processes in the preparation of lead-acid battery active materials. J. Power Sources 2006, 158, 868–873. [Google Scholar] [CrossRef]
- Ferg, E.E.; Billing, D.G.; Venter, A.M. Thermal characterization of tetrabasic lead sulfate used in the lead acid battery technology. Solid State Sci. 2017, 64, 13–22. [Google Scholar] [CrossRef]
- De Guibert, A.; Chaumont, B.; Albert, L.; Caillerie, J.L.; Ueberschaer, A.; Höhn, R.; Davis, W.; Weighall, M.J. Use of secondary lead for new generations of lead/acid batteries. J. Power Sources 1993, 42, 11–24. [Google Scholar] [CrossRef]

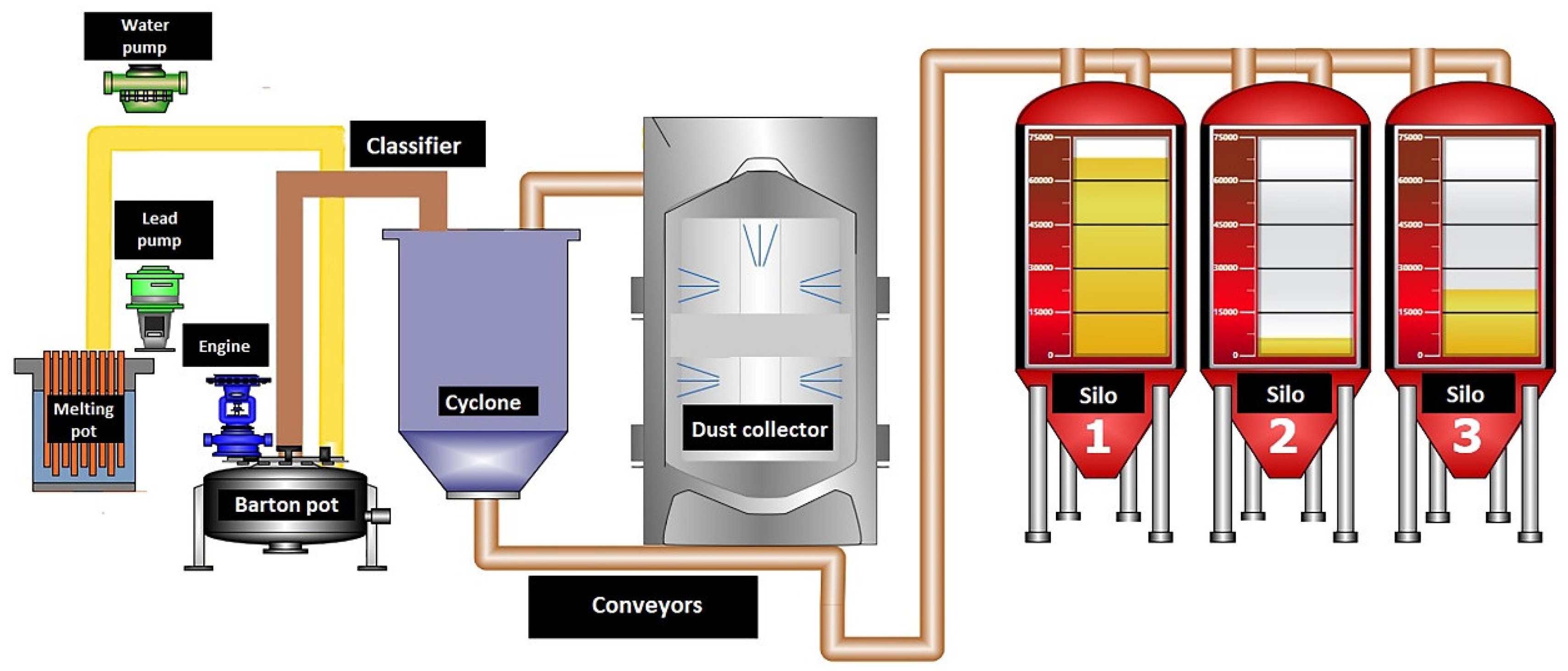

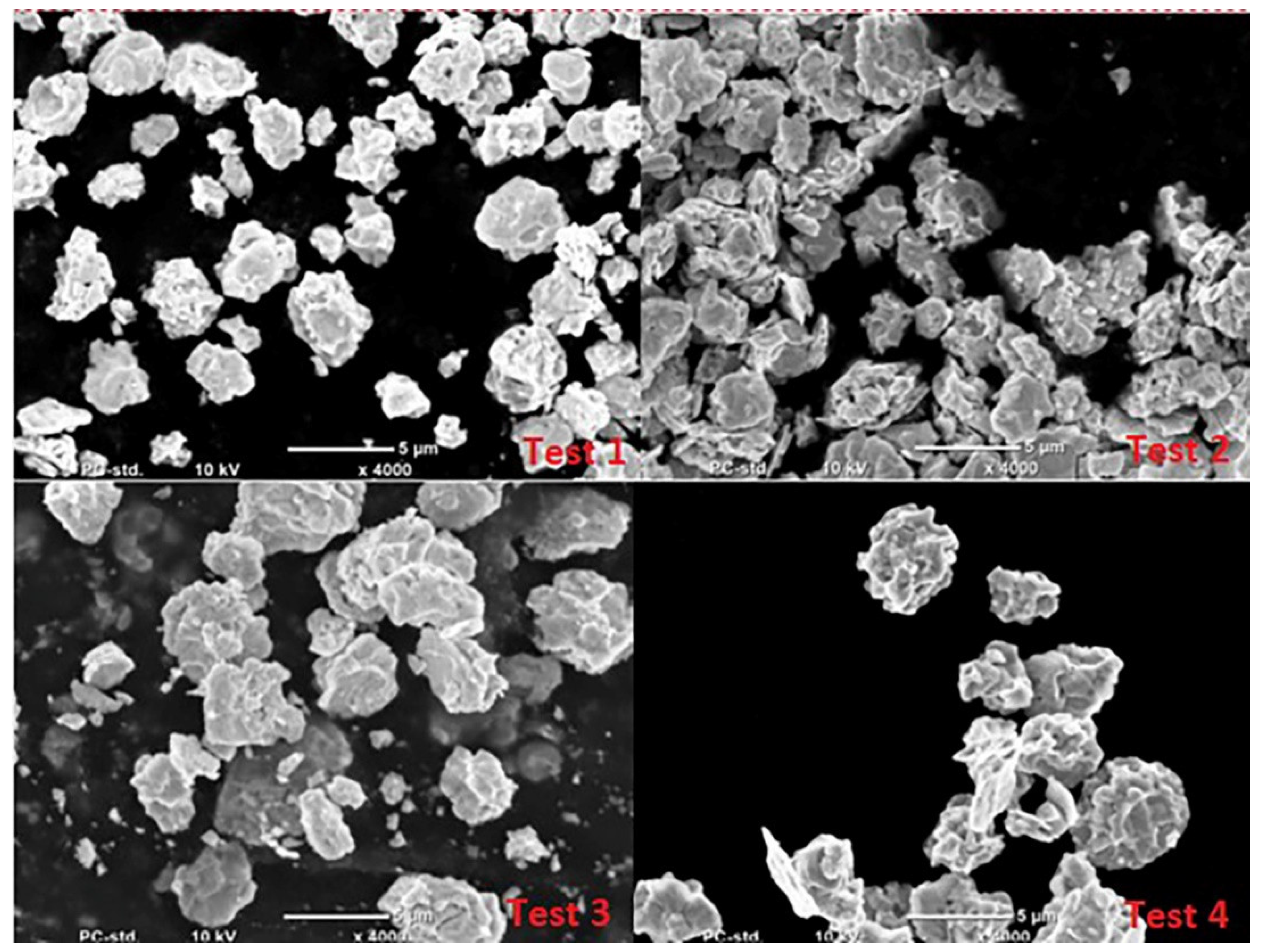
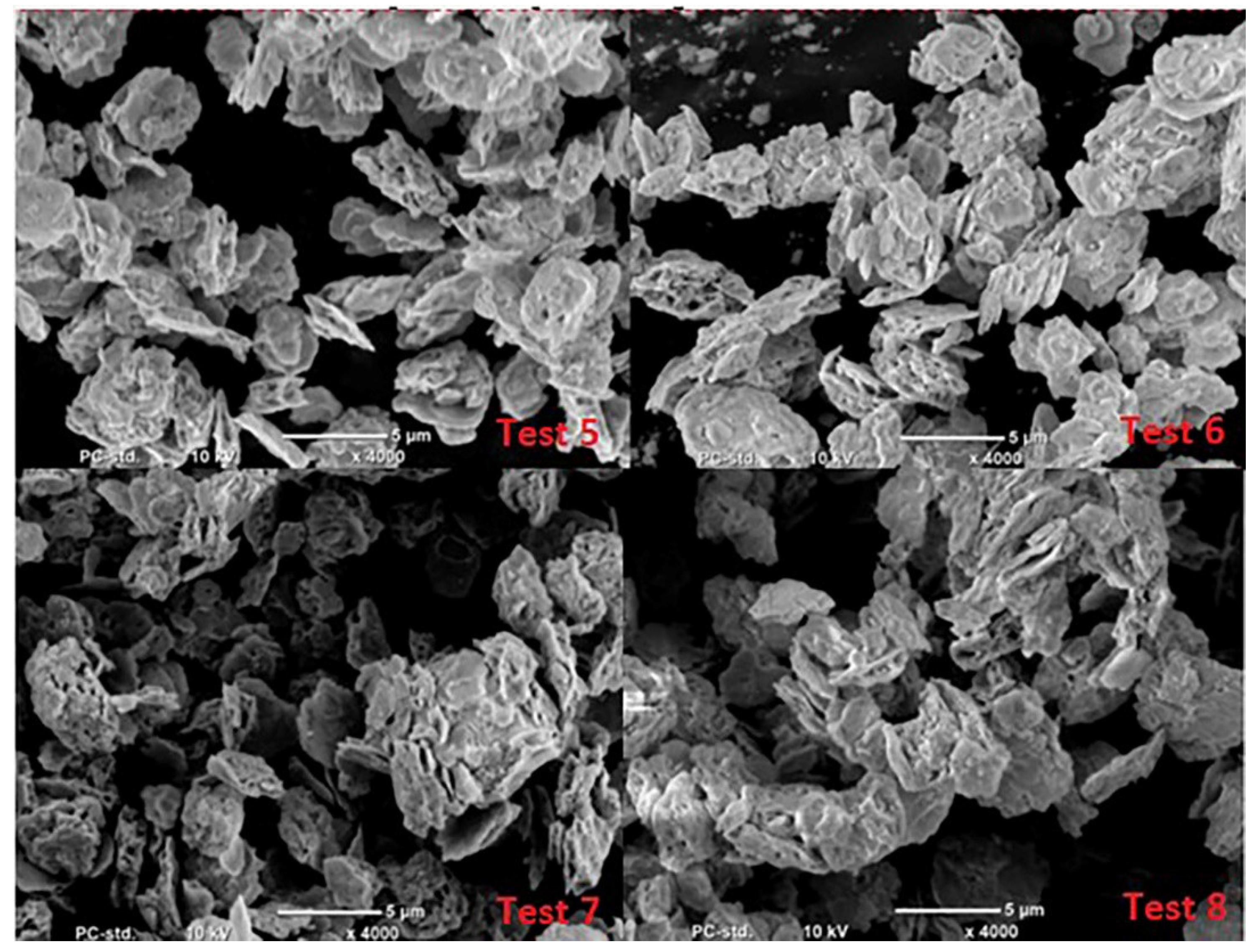
| Test No | Admixture Content, ppm | |||||||
|---|---|---|---|---|---|---|---|---|
| Sn | Sb | Bi | Cu | As | Ni | Ag | Other | |
| 1 | 0 | 0.2 | 18 | 0.3 | 0.1 | 0.1 | 1.7 | 0 |
| 2 | 0 | 0.2 | 42 | 0.6 | 0.1 | 0.4 | 2.6 | 2.1 |
| 3 | 0.2 | 0.4 | 88 | 0.1 | 0.2 | 0.5 | 10.4 | 1.2 |
| 4 | 0.1 | 2.9 | 109 | 1.1 | 0.8 | 0.6 | 20 | 1.5 |
| 5 | 0 | 0 | 75.2 | 0.1 | 0 | 0.3 | 7.7 | 0.5 |
| 6 | 1.5 | 1.1 | 115.2 | 2.3 | 0.4 | 0.8 | 2.4 | 0.3 |
| 7 | 1.1 | 2.4 | 88.9 | 0.9 | 1.4 | 0.8 | 19 | 22.5 |
| 8 | 0 | 4.3 | 110.1 | 1.9 | 0 | 0.4 | 19.6 | 6.7 |
| Test Number | Type of Lead | Moisture Content, [%] | Pollutants Content, [ppm] |
|---|---|---|---|
| 1 | Original | 61 | 20 |
| 2 | Original | 59 | 48 |
| 3 | Secondary | 60 | 101 |
| 4 | Secondary | 59 | 136 |
| 5 | Original | 20 | 84 |
| 6 | Secondary | 21 | 124 |
| 7 | Secondary | 19 | 137 |
| 8 | Secondary | 19 | 143 |
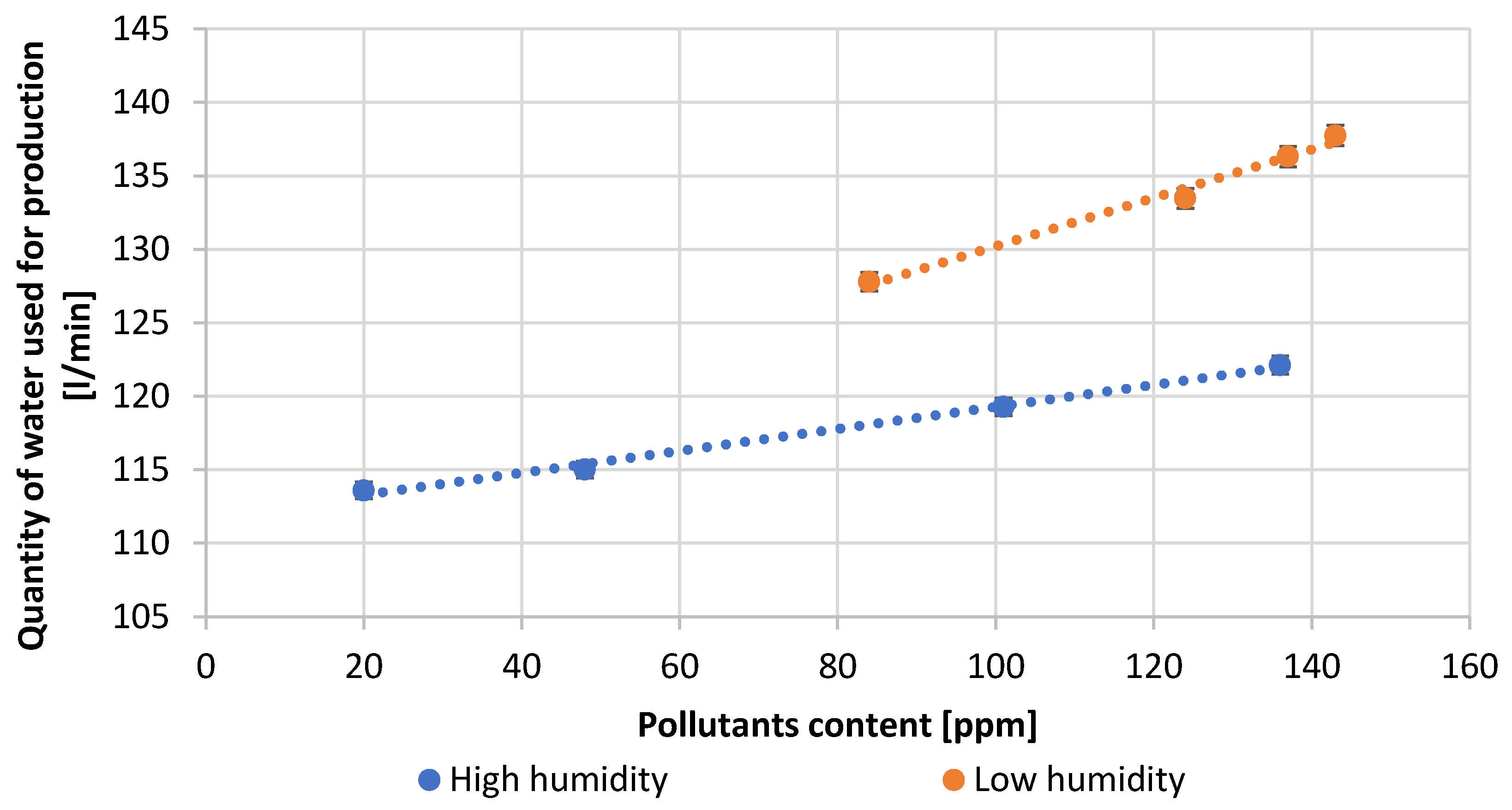
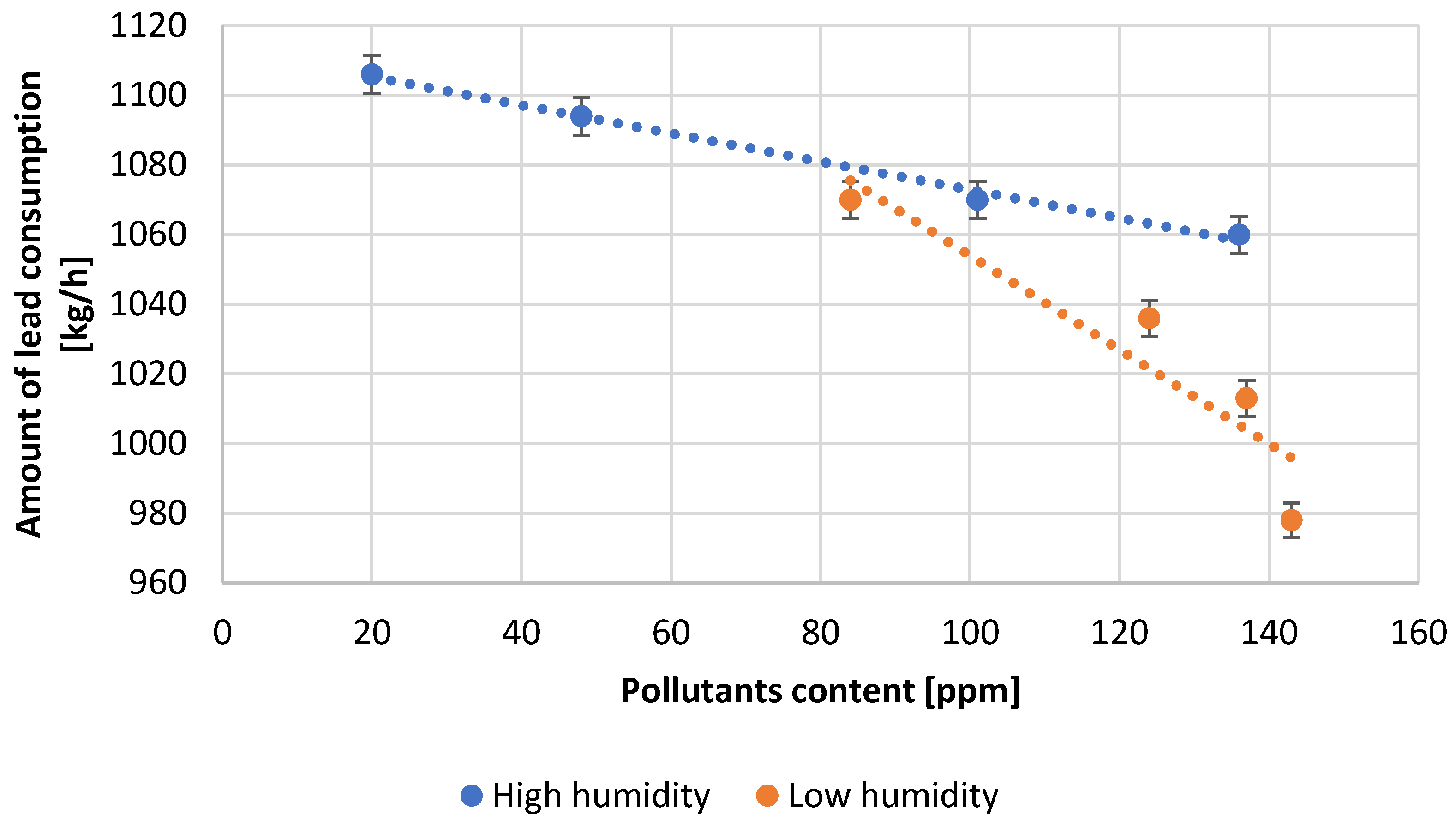
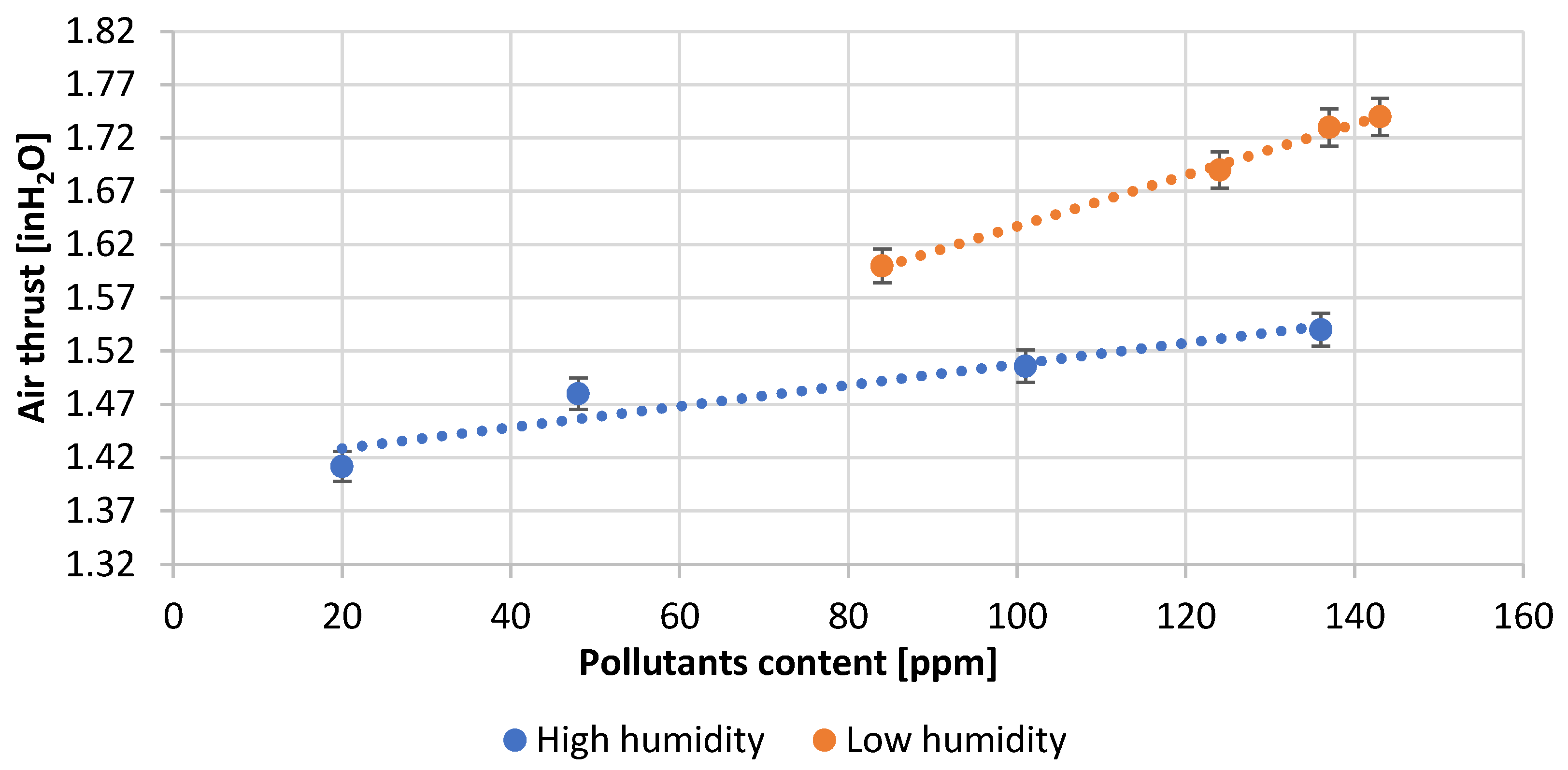
| Test Number | Reactor Load, [A] | Quantity H2O, [L/min] | Amount of Pb Consumed, [kg/h] | Air Thrust, [inH2O] | Efficiency, [%] | Amount of Scrapes, [%] |
|---|---|---|---|---|---|---|
| 1 | 91.3 | 113.6 | 1106.0 | 1.41 | 95.0 | 0.18 |
| 2 | 94.5 | 115.1 | 1094.0 | 1.48 | 94.0 | 0.21 |
| 3 | 110.1 | 119.3 | 1070.0 | 1.51 | 92.0 | 0.25 |
| 4 | 115.0 | 122.1 | 1060.0 | 1.54 | 91.0 | 0.27 |
| 5 | 97.7 | 127.8 | 1070.0 | 1.60 | 90.0 | 0.20 |
| 6 | 118.0 | 133.5 | 1036.0 | 1.69 | 89.0 | 0.28 |
| 7 | 119.0 | 136.3 | 1013.0 | 1.73 | 87.0 | 0.30 |
| 8 | 120.0 | 137.7 | 978.0 | 1.74 | 84.0 | 0.31 |
| Test Number | Absorption, [g H2SO4/100 g PbO] | Degree of Oxidation, [%] | Bulk Density of PbO, [g/cm3] |
|---|---|---|---|
| 1 | 18.1 | 75.0 | 1.30 |
| 2 | 18.0 | 75.0 | 1.31 |
| 3 | 17.8 | 74.0 | 1.33 |
| 4 | 17.3 | 73.0 | 1.34 |
| 5 | 13.6 | 74.0 | 1.35 |
| 6 | 13.5 | 72.0 | 1.37 |
| 7 | 13.4 | 71.0 | 1.38 |
| 8 | 13.1 | 70.0 | 1.39 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szela, R.; Małecki, S.; Gargul, K. Lead Oxide Production in Barton Reactor—Effect of Increased Air Humidity on Lead Oxide Production Parameters. Materials 2022, 15, 4941. https://doi.org/10.3390/ma15144941
Szela R, Małecki S, Gargul K. Lead Oxide Production in Barton Reactor—Effect of Increased Air Humidity on Lead Oxide Production Parameters. Materials. 2022; 15(14):4941. https://doi.org/10.3390/ma15144941
Chicago/Turabian StyleSzela, Rafał, Stanisław Małecki, and Krzysztof Gargul. 2022. "Lead Oxide Production in Barton Reactor—Effect of Increased Air Humidity on Lead Oxide Production Parameters" Materials 15, no. 14: 4941. https://doi.org/10.3390/ma15144941





