Molecular Dynamics Study on the Welding Behavior in Dissimilar TC4-TA17 Titanium Alloys
Abstract
:1. Introduction
2. Computational Methods
2.1. Selection of Alloy Potential Function
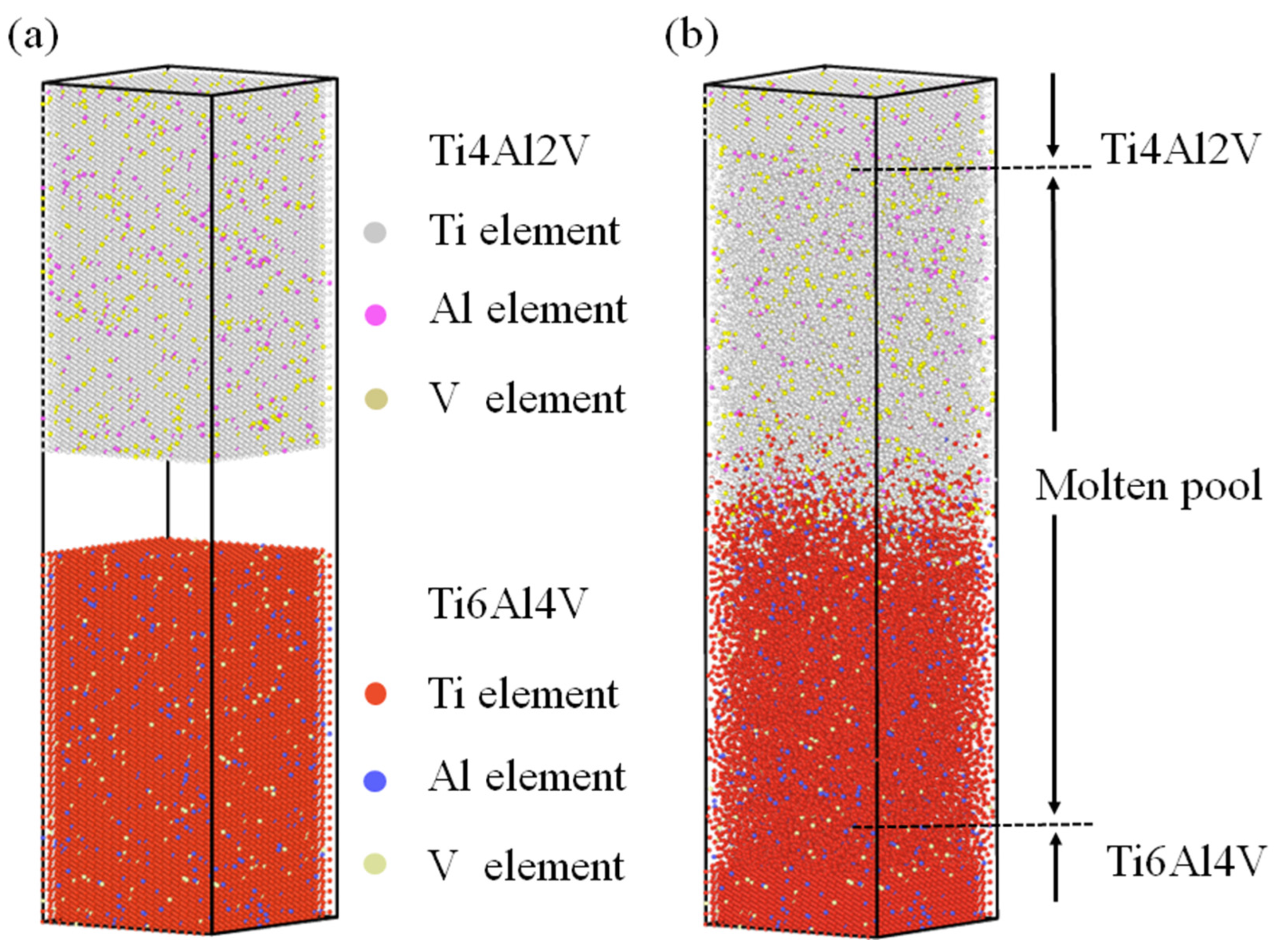
2.2. The Establishment of an Atomic Model
2.3. Phase Change, Welding and Stretching Process Simulation
3. Results and Discussion
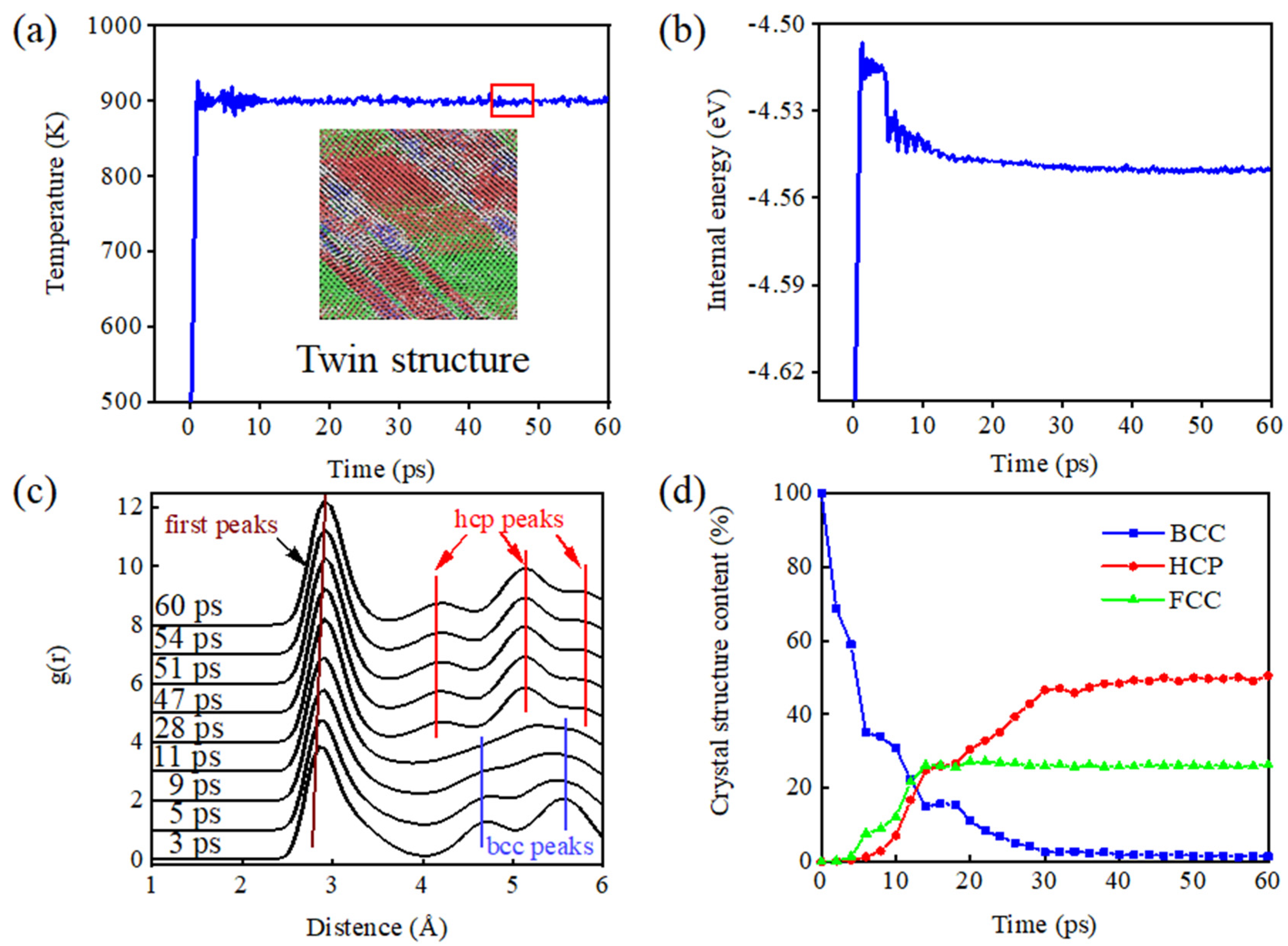
3.1. Simulation of the Aging Phase Transition
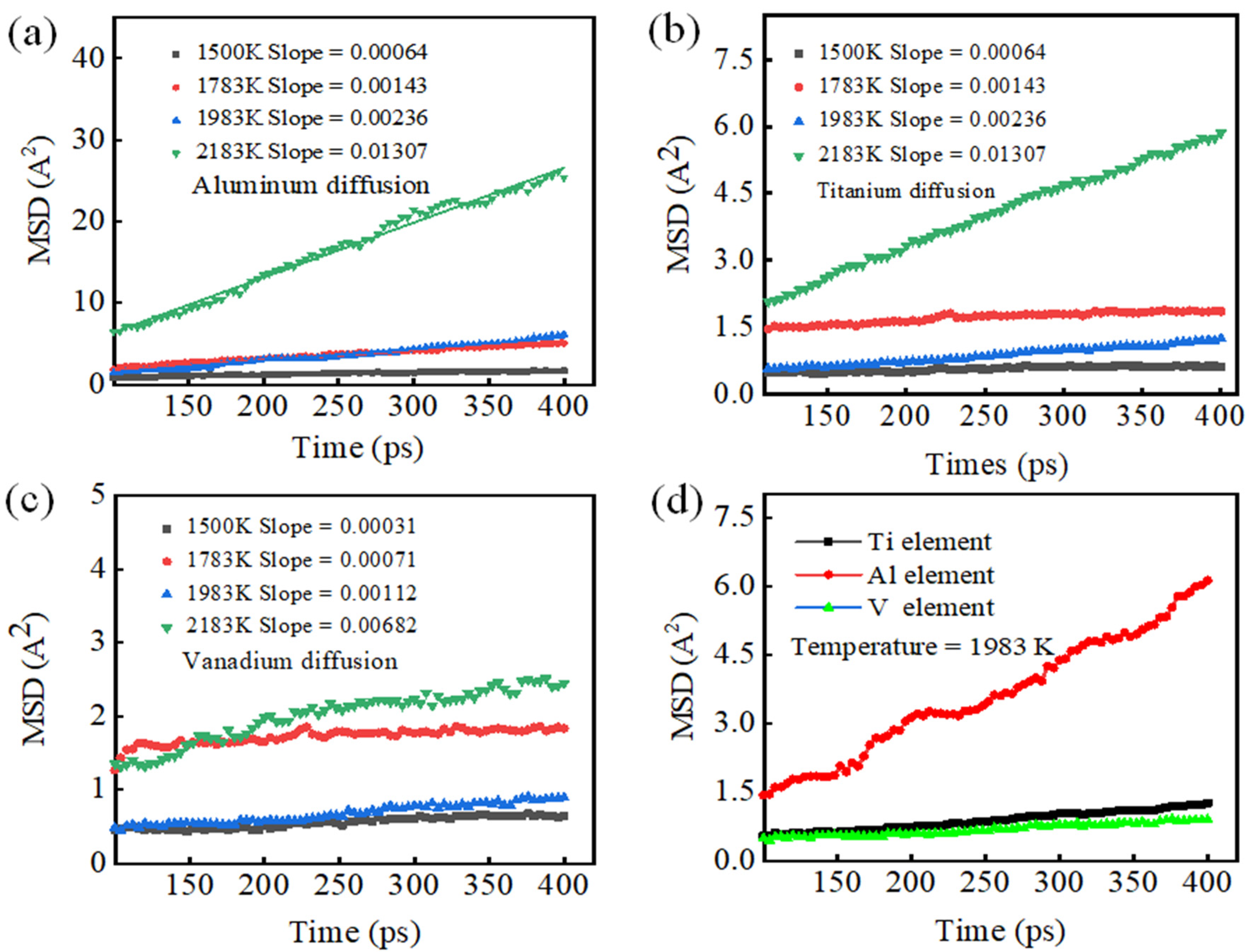
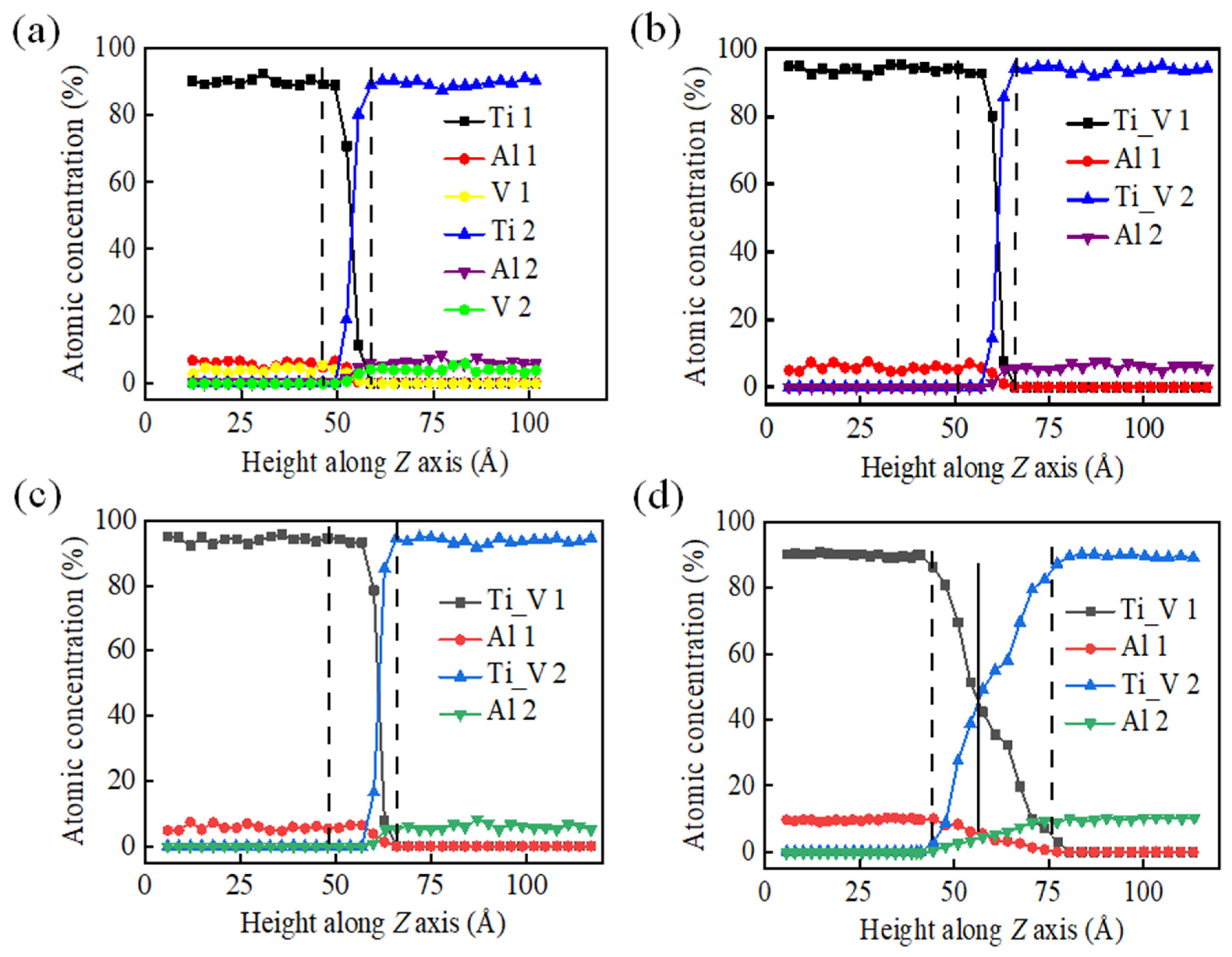
3.2. Simulation of K-TIG Welding of TC4-TA17 Alloy
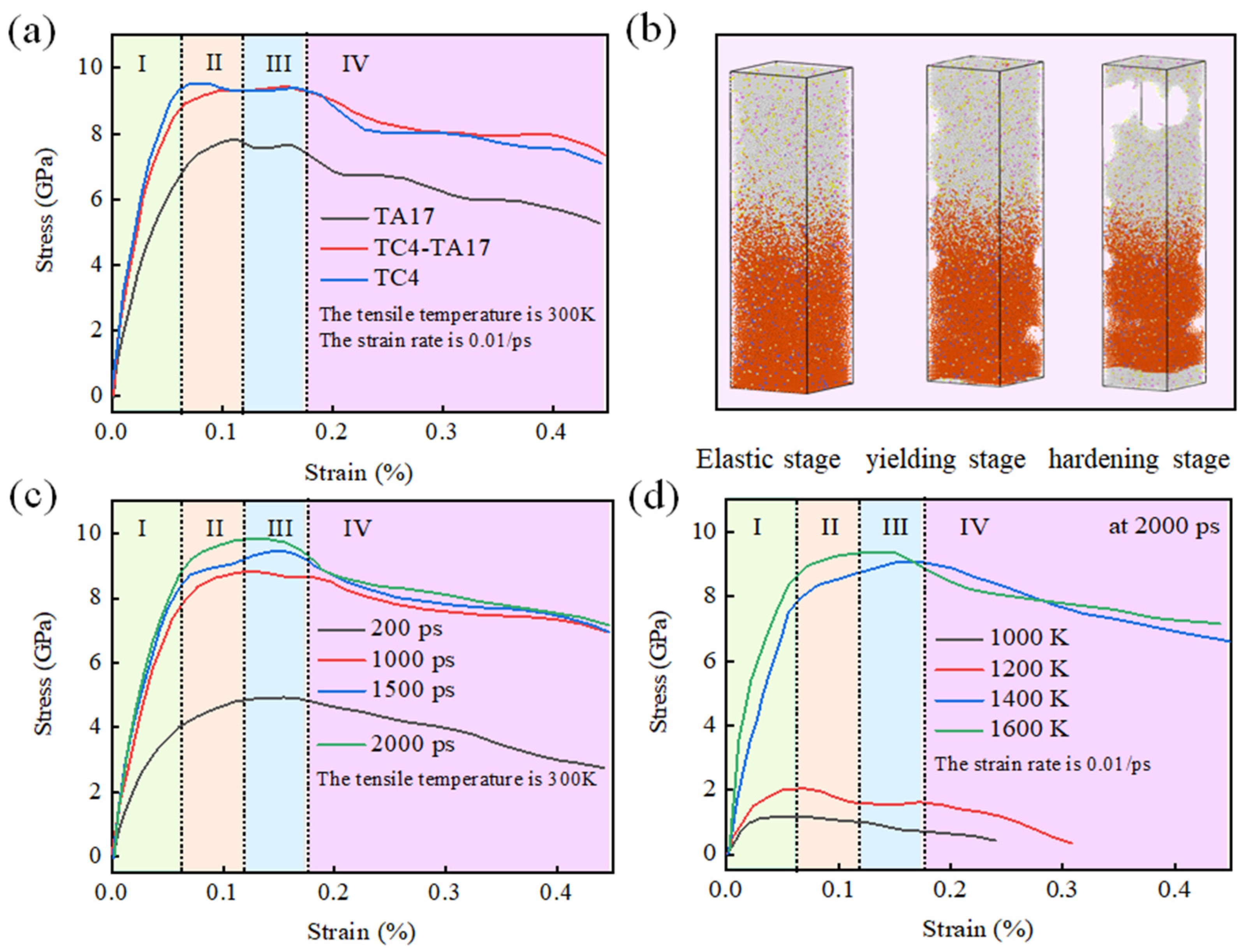
3.3. Simulation of Tensile Mechanical Properties
4. Conclusions
- (1)
- During the phase transformation process, crystal defects such as lamination and twin boundary are prone to occurring at the junction of grain boundaries, so as to alleviate stress caused by the distortion. Increasing the content of Al, as an α-phase-stabilizing element, facilitates nucleation and precipitation of this phase and facilitates the phase change.
- (2)
- A higher holding temperature promotes the plastic yield of the diffusion connection interface, thus enlarging the contact area and reducing the probability of void formation.
- (3)
- The tensile process in TC4-TA17 simulation is similar to those of macroscopic materials, involving elastic deformation and a plastic deformation stage, and showing a necking phenomenon. When the system enters plastic deformation, the stress–strain curve shows a spike, and high yield strength is acquired.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Malahy, K.A.; Hodgkiess, T. Comparative studies of the seawater corrosion behaviour of a range of materials. Desalination 2003, 158, 35–42. [Google Scholar] [CrossRef]
- Fu, Y.Q.; Zhou, F.; Wang, Q.Z.; Zhang, M.D.; Zhou, Z.F. Electrochemical and tribocorrosion performances of CrMoSiCN coating on Ti-6Al-4V titanium alloy in artificial seawater. Corros. Sci. 2020, 165, 108385. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, M.; Cui, W.C.; Tang, W.X.; Wang, F.; Pan, B.B. Elastic-plastic buckling of deep sea spherical pressure hulls. Mar. Struct. 2018, 57, 38–51. [Google Scholar] [CrossRef]
- Wagner, H.N.R.; Hühne, C.; Niemann, S. Robust knockdown factors for the design of spherical shells under external pressure: Development and validation. Int. J. Mech. Sci. 2018, 141, 58–77. [Google Scholar] [CrossRef]
- Li, L.Z.; Wang, S.G.; Huang, W.; Jin, Y. Microstructure and mechanical properties of electron beam welded TC4/TA7 dissimilar titanium alloy joint. J. Manuf. Process. 2020, 50, 295–304. [Google Scholar] [CrossRef]
- Liu, H.; Dai, X.; Shi, M.H. EBSD Analysis on Tube-Plate Assembly Welding Line of TA 16 and TA17 Alloys. Rare Metal Mat. Eng. 2012, 41, 1756–1760. [Google Scholar]
- Luo, Q.; Wang, L.; Chen, X.; Liu, S.W. Studies on the corrosion behavior of TA16 and TA17 titanium alloys in high temperature and high pressure water. Light Met. 2012, 2, 56–59. [Google Scholar]
- Wang, C.M.; Guo, Q.L.; Shao, M.H.; Zhang, H.; Wang, F.F.; Song, B.Y.; Ji, T.J.; Li, H.X. Microstructure and corrosion behavior of linear friction welded TA15 and TC17 dissimilar joint. Mater. Charact. 2022, 187, 111871. [Google Scholar] [CrossRef]
- Liu, Z.Y.; He, B.; Lyu, T.Y.; Zou, Y. A review on additive manufacturing of titanium alloys for aerospace applications: Directed energy deposition and beyond Ti-6Al-4V. JOM 2021, 73, 1804–1818. [Google Scholar] [CrossRef]
- Dong, L.L.; Lu, J.W.; Fu, Y.Q.; Huo, W.T.; Liu, Y.; Li, D.D.; Zhang, Y.S. Carbonaceous nanomaterial reinforced Ti-6Al-4V matrix composites: Properties, interfacial structures and strengthening mechanisms. Carbon 2020, 164, 272–286. [Google Scholar] [CrossRef]
- Li, Z.; Wang, J.; Dong, Y.Z.; Xu, D.K.; Zhang, X.H.; Wu, J.H.; Gu, T.Y.; Wang, F.H. Synergistic effect of chloride ion and Shewanella algae accelerates the corrosion of Ti-6Al-4V alloy. J. Mater. Sci. Technol. 2021, 71, 177–185. [Google Scholar] [CrossRef]
- Yan, B.; Kong, N.; Zhang, J.; Li, H.B. High temperature formability of Ti–4Al–2V titanium alloy under hot press forming process. Mater. Res. Express 2019, 6, 126501. [Google Scholar] [CrossRef]
- Fu, C.; Wang, Y.Q.; He, S.L.; Zhang, C.L.; Jing, X. Microstructural characterization and mechanical properties of TIG weld joint made by forged Ti–4Al–2V alloy. Mater. Sci. Eng. A 2021, 821, 141604. [Google Scholar] [CrossRef]
- Huang, D.M.; Yang, X.F.; Wei, Q.F.; Chen, Y.; Guo, H.; Liang, S.L.; Wang, Y. Causes of surface stripe cracks of Ti–4Al–2V alloy cold-rolled sheet. Rare Met. 2014, 33, 522–526. [Google Scholar] [CrossRef]
- Du, S.; Wang, S.; Ding, K. A novel method of friction-diffusion welding between TiAl alloy and GH3039 high temperature alloy. J. Manuf. Process. 2020, 56, 688–696. [Google Scholar] [CrossRef]
- Karfoul, M.K.; Tatlock, G.J. Interfacial processes during diffusion welding of titanium alloy/aluminium couples under ambient atmosphere. Weld. World 2019, 63, 841–849. [Google Scholar] [CrossRef]
- Zhu, L.L.; Zhang, Q.F.; Chen, Z.Q.; Wei, C.D.; Cai, G.M.; Jiang, L.; Jin, Z.P.; Zhao, J.C. Measurement of interdiffusion and impurity diffusion coefficients in the bcc phase of the Ti–X (X= Cr, Hf, Mo, Nb, V, Zr) binary systems using diffusion multiples. J. Mater. Sci. 2017, 52, 3255–3268. [Google Scholar] [CrossRef]
- Shimono, M.; Onodera, H. Molecular dynamics study on formation and crystallization of Ti–Al amorphous alloys. Mater. Sci. Eng. A 2001, 304, 515–519. [Google Scholar] [CrossRef]
- Semiatin, S.L.; Knisley, S.L.; Fagin, P.N.; Barker, D.R.; Zhang, F. Microstructure evolution during alpha-beta heat treatment of Ti-6Al-4V. Metall. Mater. Trans. A 2003, 34, 2377–2386. [Google Scholar] [CrossRef]
- Plimpton, S. Fast parallel algorithms for short-range molecular dynamics. J. Comput. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef]
- Harish, M.S.; Patra, P.K. Temperature and its control in molecular dynamics simulations. Mol. Simulat. 2021, 47, 701–729. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, L.F.; Han, J.Q.; Dee, W.N.E. PMD-kit: A deep learning package for many-body potential energy representation and molecular dynamics. Comput. Phys. Commun. 2018, 228, 178–184. [Google Scholar] [CrossRef]
- Daw, M.S.; Baskes, M.I. Semiempirical, quantum mechanical calculation of hydrogen embrittlement in metals. Phys. Rev. Lett. 1983, 50, 1285. [Google Scholar] [CrossRef]
- Baskes, M.I. Modified embedded-atom potentials for cubic materials and impurities. Phys. Rev. B 1992, 46, 2727–2742. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.W.; Johnson, R.A.; Wadley, H.N.G. Misfit-energy-increasing dislocations in vapor-deposited CoFe/NiFe multilayers. Phys. Rev. B 2004, 69, 144113. [Google Scholar] [CrossRef]
- Zhao, S.J.; Xiong, Y.X.; Ma, S.H.; Zhang, J.; Xu, B.; Kai, J.J. Defect accumulation and evolution in refractory multi-principal element alloys. Acta Mater. 2021, 219, 117233. [Google Scholar] [CrossRef]
- Pinsook, U. Molecular dynamics study of vibrational entropy in bcc and hcp zirconium. Phys. Rev. B 2002, 66, 024109. [Google Scholar] [CrossRef]
- Ackland, G.J.; Jones, A.P. Applications of local crystal structure measures in experiment and simulation. Phys. Rev. B 2006, 73, 4104. [Google Scholar] [CrossRef]
- Zhao, F.; Zhang, J.; He, C.W.; Zhang, Y.; Gao, X.L.; Xie, L. Molecular dynamics simulation on creep behavior of nanocrystalline TiAl alloy. Nanomaterials 2020, 10, 1693. [Google Scholar] [CrossRef]
- Song, C.B.; Lin, T.S.; He, P.; Jiao, Z.; Tao, J.; Ji, Y.J. Molecular dynamics simulation of linear friction welding between dissimilar Ti-based alloys. Comp. Mater. Sci. 2014, 83, 35–38. [Google Scholar] [CrossRef]
- Stukowski, A. Structure identification methods for atomistic simulations of crystalline materials. Model. Simul. Mater. Sci. Eng. 2012, 20, 045021. [Google Scholar] [CrossRef]
- Falconi, S.; Ackland, G.J. Ab initio simulations in liquid caesium at high pressure and temperature. Phys. Rev. B 2006, 73, 184204. [Google Scholar] [CrossRef]
- Cui, Z.Q.; Zhou, X.L.; Meng, Q.B. Atomic-scale mechanism investigation of mass transfer in laser fabrication process of Ti-Al alloy via molecular dynamics simulation. Metals 2020, 10, 1660. [Google Scholar] [CrossRef]
- Bai, D.S.; Sun, J.F.; Chen, W.Y.; Du, D.X. Molecular dynamics simulation of the diffusion behaviour between Co and Ti and its effect on the wear of WC/Co tools when titanium alloy is machined. Ceram. Int. 2016, 42, 17754–17763. [Google Scholar] [CrossRef]
- Li, P.; Wang, L.S.; Yan, S.L.; Meng, M.; Xue, K.M. Temperature effect on the diffusion welding process and mechanism of B2–O interface in the Ti2AlNb-based alloy: A molecular dynamics simulation. Vacuum 2020, 173, 109118. [Google Scholar] [CrossRef]
- Liu, C.H.; Zhu, X.J.; Li, X.M.; Shi, Q.S.J. Investigation on sintering processes and mechanical properties of Ti–Ta alloys by molecular dynamics simulation. Powder Technol. 2021, 11, 117069. [Google Scholar] [CrossRef]
- Xu, Z.F.; Rong, J.; Yu, X.H.; Song, Y.M.; Zhan, Z.L.; Liu, J.X.; Meng, K. Preparation of Al-based coatings of titanium ingot and its high temperature oxidation resistance. Rare Met. Mat. Eng. 2017, 46, 1961–1965. [Google Scholar]
- Rong, J.; Wang, Y.; Li, R.Y.; Yu, X.H.; Meng, K. Interface structure between titanium substrate and high temperature oxidation resistant aluminum-base coatings. Rare Met. Mat. Eng. 2018, 47, 682–686. [Google Scholar]
- Zhang, B.; Zhang, X.Y.; Li, C.; Zhou, K.C. Molecular dynamics simulation on phase transformation of Ti-Al alloy with low Al content. Rare Met. Mat. Eng. 2012, 41, 1010–1015. [Google Scholar] [CrossRef]
- Wang, S.Q.; Li, W.Y.; Jing, K.; Zhang, X.Y.; Chen, D.L. Microstructural evolution and mechanical properties of electron beam welded dissimilar titanium alloy joints. Mat. Sci. Eng. A 2017, 697, 224–232. [Google Scholar] [CrossRef]
- Wang, S.Q.; Liu, J.H.; Chen, D.L. Effect of strain rate and temperature on strain hardening behavior of a dissimilar joint between Ti–6Al–4V and Ti17 alloys. Mater. Des. 2014, 56, 174–184. [Google Scholar] [CrossRef]
- Wang, S.Q.; Liu, J.H.; Lu, Z.X.; Chen, D.L. Cyclic deformation of dissimilar welded joints between Ti–6Al–4V and Ti17alloys: Effect of strain ratio. Mat. Sci. Eng. A 2014, 598, 122–134. [Google Scholar] [CrossRef]
- Xu, W.F.; Ma, J.; Luo, Y.X.; Fang, Y.X. Microstructure and high-temperature mechanical properties of laser beam welded TC4/TA15 dissimilar titanium alloy joints. Trans. Nonferrous Met. Soc. China 2020, 30, 160–170. [Google Scholar] [CrossRef]
- Zhao, P.K.; Fu, L. Strain hardening behavior of linear friction welded joints between TC11 andTC17 dissimilar titanium alloys. Mat. Sci. Eng. A 2015, 621, 149–156. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ou, P.; Cao, Z.; Rong, J.; Yu, X. Molecular Dynamics Study on the Welding Behavior in Dissimilar TC4-TA17 Titanium Alloys. Materials 2022, 15, 5606. https://doi.org/10.3390/ma15165606
Ou P, Cao Z, Rong J, Yu X. Molecular Dynamics Study on the Welding Behavior in Dissimilar TC4-TA17 Titanium Alloys. Materials. 2022; 15(16):5606. https://doi.org/10.3390/ma15165606
Chicago/Turabian StyleOu, Peng, Zengqiang Cao, Ju Rong, and Xiaohua Yu. 2022. "Molecular Dynamics Study on the Welding Behavior in Dissimilar TC4-TA17 Titanium Alloys" Materials 15, no. 16: 5606. https://doi.org/10.3390/ma15165606
APA StyleOu, P., Cao, Z., Rong, J., & Yu, X. (2022). Molecular Dynamics Study on the Welding Behavior in Dissimilar TC4-TA17 Titanium Alloys. Materials, 15(16), 5606. https://doi.org/10.3390/ma15165606





