The Influence of Prolonged High-Concentration Ozone Exposure on Superhydrophobic Coatings in Static and High-Speed Flow Atmospheres
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Surface Characterization
2.3. Ozonation Protocols
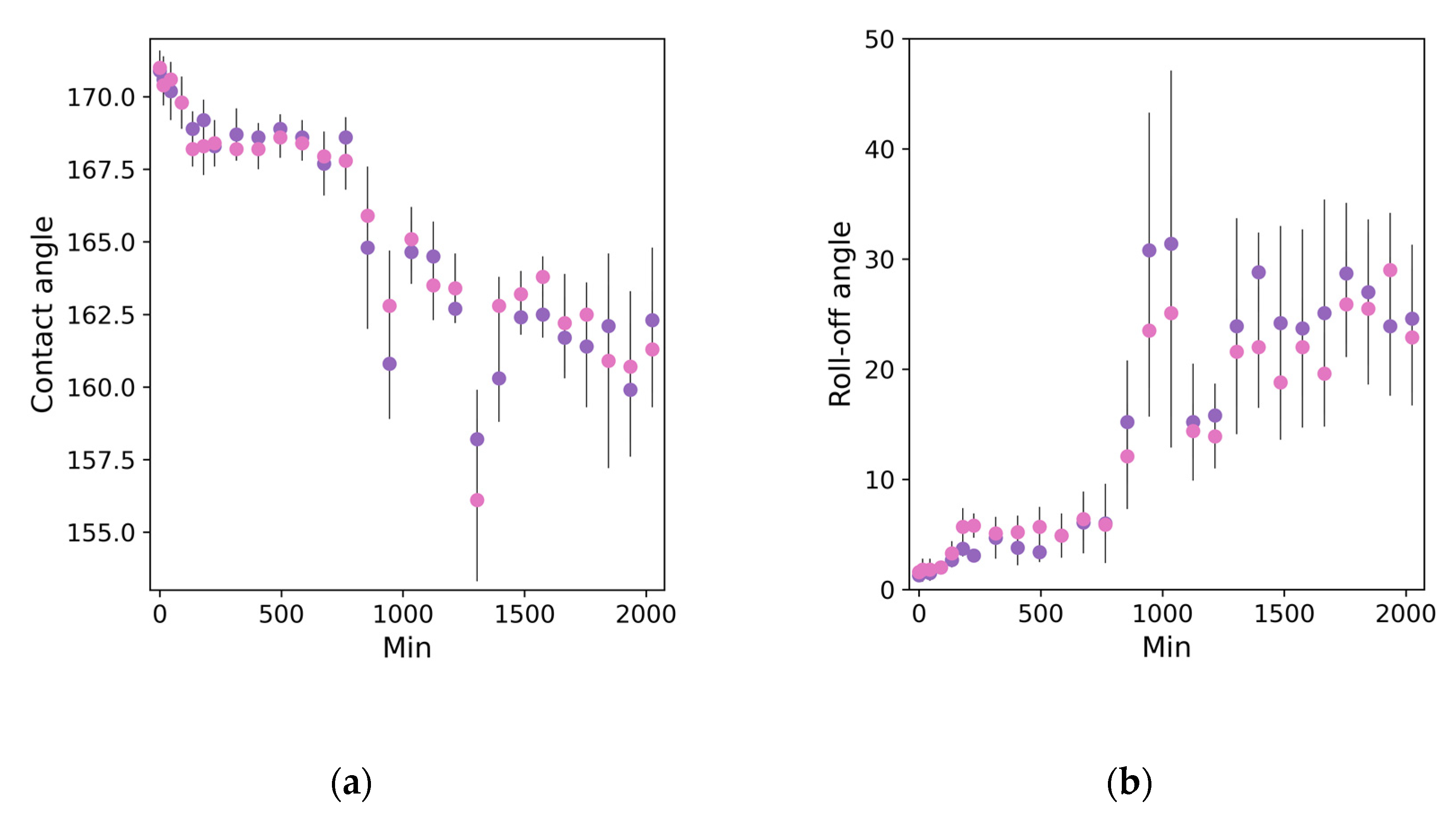
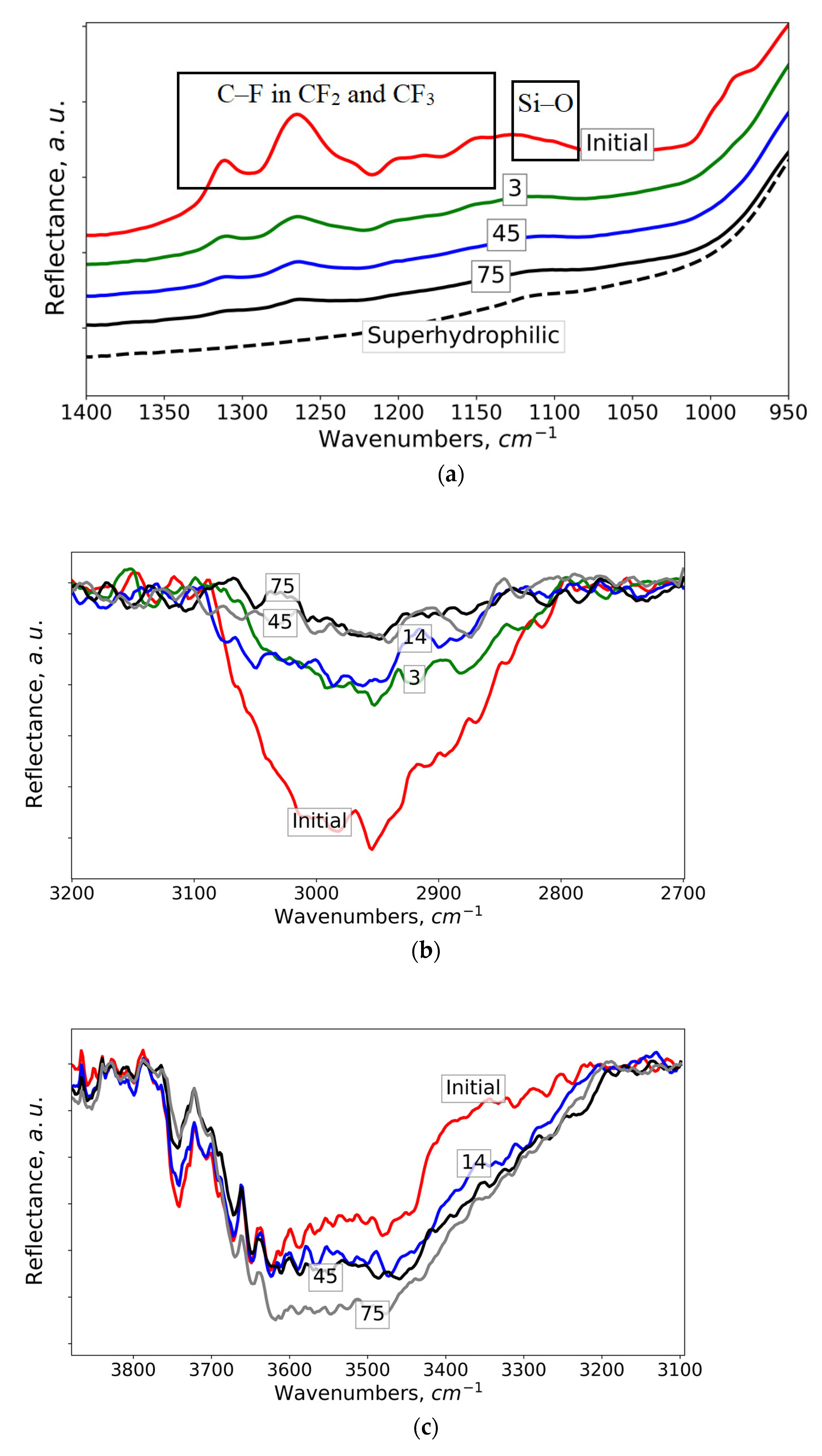
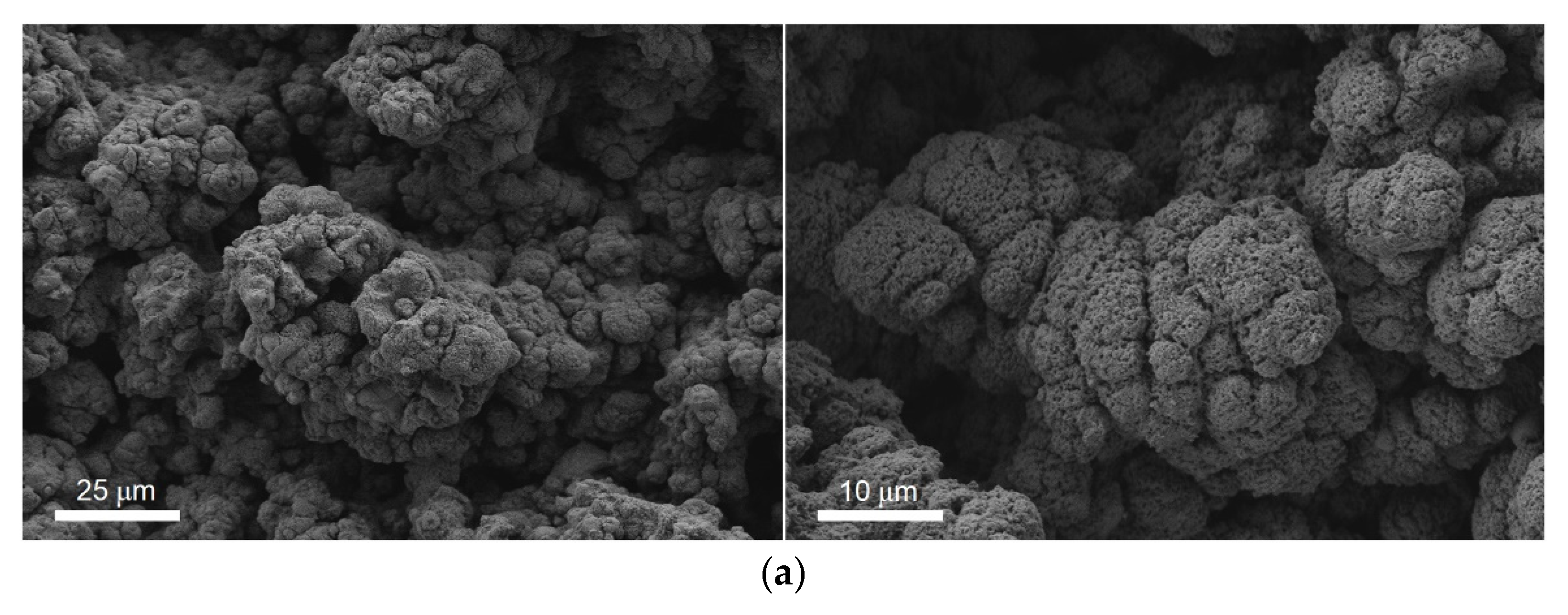
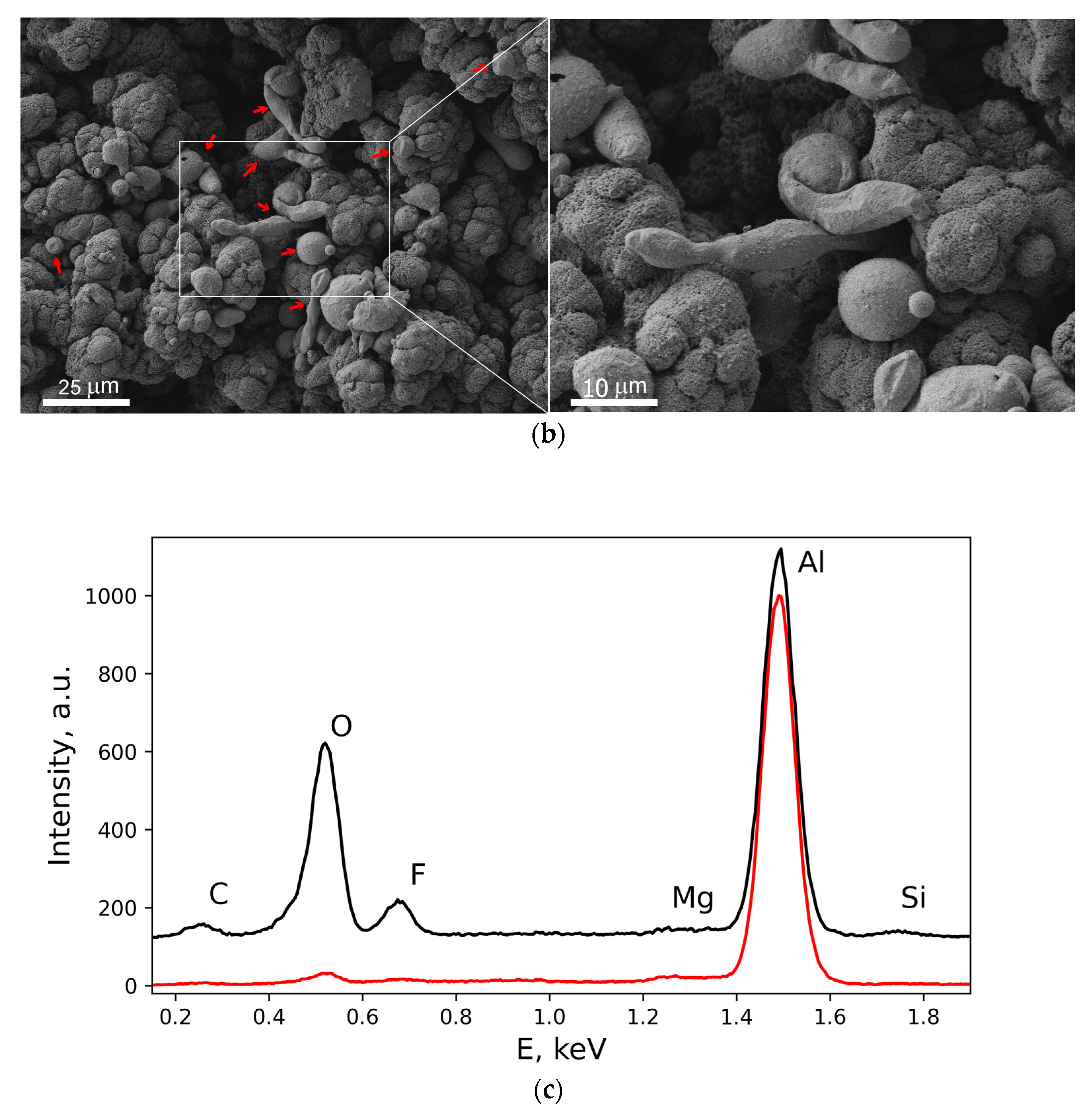
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dhyani, A.; Choi, W.; Golovin, K.; Tuteja, A. Surface design strategies for mitigating ice and snow accretion. Matter 2022, 5, 1423–1454. [Google Scholar] [CrossRef]
- Boinovich, L.B.; Emelyanenko, A.M.; Emelyanenko, K.A.; Modin, E.B. Modus operandi of protective and anti-icing mechanisms underlying the design of longstanding outdoor icephobic coatings. ACS Nano 2019, 13, 4335–4346. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, P.; Zhang, D.; Ou, J. Effect of surface nanostructure on enhanced atmospheric corrosion resistance of a superhydrophobic surface. Colloids Surf. A Physicochem. Eng. Asp. 2022, 647, 129058. [Google Scholar] [CrossRef]
- Boinovich, L.B.; Emelyanenko, A.M.; Modestov, A.D.; Domantovsky, A.G.; Emelyanenko, K.A. Not simply repel water: The diversified nature of corrosion protection by superhydrophobic coatings. Mendeleev Commun. 2017, 27, 254–256. [Google Scholar] [CrossRef]
- George, J.S.; Hoang, A.T.; Kalarikkal, N.; Nguyen-Tri, P.; Thomas, S. Recent advances in bio-inspired multifunctional coatings for corrosion protection. Prog. Org. Coat. 2022, 168, 106858. [Google Scholar] [CrossRef]
- Genser, J.; Efimenko, K. Recent developments in superhydrophobic surfaces and their relevance to marine fouling: A review. Biofouling 2006, 22, 339–360. [Google Scholar] [CrossRef]
- Mishra, V.K.; Saini, R.; Kumar, N. A review on applications of superhydrophobic coatings. Int. Res. J. Adv. Sci. Hub 2021, 3, 43–55. [Google Scholar] [CrossRef]
- Stratakis, E.; Bonse, J.; Heitz, J.; Siegel, J.; Tsibidis, G.D.; Skoulas, E.; Papadopoulos, A.; Mimidis, A.; Joel, A.-C.; Comanns, P.; et al. Laser engineering of biomimetic surfaces. Mater. Sci. Eng. R. 2020, 141, 100562. [Google Scholar] [CrossRef]
- Saji, V.S. Recent progress in superhydrophobic and superamphiphobic coatings for magnesium and its alloys. J. Magnes. Alloy. 2021, 9, 748–778. [Google Scholar] [CrossRef]
- Boinovich, L.; Domantovskiy, A.; Emelyanenko, A.; Pashinin, A.; Ionin, A.; Kudryashov, S.; Saltuganov, P. Femtosecond laser treatment for the design of electro-insulating superhydrophobic coatings with enhanced wear resistance on glass. ACS Appl. Mater. Interfaces 2014, 6, 2080–2085. [Google Scholar] [CrossRef]
- Zhi, D.; Lu, Y.; Sathasivam, S.; Parkin, I.P.; Zhang, X. Large-scale fabrication of translucent and repairable superhydrophobic spray coatings with remarkable mechanical, chemical durability and UV resistance. J. Mater. Chem. A 2017, 5, 10622–10631. [Google Scholar] [CrossRef]
- Domantovsky, A.G.; Emel’yanenko, A.M.; Emel’yanenko, K.A.; Boinovich, L.B. The threshold effect in ozone-induced degradation of superhydrophobic coatings. Tech. Phys. 2022, 66, 1100–1105. [Google Scholar] [CrossRef]
- Gleissner, C.; Landsiedel, J.; Bechtold, T.; Pham, T. Surface activation of high performance polymer fibers: A review. Polym. Rev. 2022, 1–32. [Google Scholar] [CrossRef]
- Lunin, V.V.; Popovich, M.P.; Tkachenko, S.N. Fizicheskaya Khimiya Ozona (Physical Chemistry of Ozone); MSU Publishing House: Moscow, Russia, 1998. (In Russian) [Google Scholar]
- Peeling, J.; Clark, D.T. Surface ozonation and photooxidation of polyethylene film. J. Polym. Sci. Polym. Chem. Ed. 1983, 21, 2047–2055. [Google Scholar] [CrossRef]
- Emelyanenko, K.A.; Domantovsky, A.G.; Platonov, P.S.; Kochenkov, P.S.; Emelyanenko, A.M.; Boinovich, L.B. The durability of superhydrophobic and slippery liquid infused porous surface coatings under corona discharge characteristic of the operation of high voltage power transmission lines. Energy Rep. 2022, 8, 6837–6844. [Google Scholar] [CrossRef]
- Sataeva, N.E.; Boinovich, L.B.; Emelyanenko, K.A.; Domantovsky, A.G.; Emelyanenko, A.M. Laser-assisted processing of aluminum alloy for the fabrication of superhydrophobic coatings withstanding multiple degradation factors. Surf. Coat. Technol. 2020, 397, 125993. [Google Scholar] [CrossRef]
- Emelyanenko, A.M.; Boinovich, L.B. Analysis of wetting as an efficient method for studying the characteristics of coatings and surfaces and the processes that occur on them: A review. Inorg. Mater. 2011, 47, 1667–1675. [Google Scholar] [CrossRef]
- Roy, S.; Coyne, J.M.; Novak, J.A.; Edwards, M.A. Flow-induced failure mechanisms of copper pipe in potable water systems. Corros. Rev. 2018, 36, 449–481. [Google Scholar] [CrossRef]
- Kuruvila, R.; Kumaran, S.T.; Khan, M.A.; Uthayakumar, M. A brief review on the erosion-corrosion behavior of engineering materials. Corros. Rev. 2018, 36, 435–447. [Google Scholar] [CrossRef]
- Aribo, S.; Fakorede, A.; Ige, O.; Olubambi, P. Erosion-corrosion behaviour of aluminum alloy 6063 hybrid composite. Wear 2017, 376, 608–614. [Google Scholar] [CrossRef]
- Zvyagintsev, A.M.; Belikov, I.B.; Elanskii, N.F.; Kuznetsova, I.N.; Romanyuk, Y.O.; Sosonkin, M.G.; Tarasova, O.A. Surface ozone concentration variability in Moscow and Kiev. Russ. Meteorol. Hydrol. 2010, 35, 806–812. [Google Scholar] [CrossRef]
- Zvyagintsev, A.M.; Kruchenitskii, G.M.; Belikov, I.B.; Elansky, N.F.; Skorokhod, A.I.; Egorov, V.I.; Nikolaev, A.N.; Kuznetsova, I.N.; Obukhova, Z.B. Positive anomalies in the surface ozone concentration in July–August 2002 over Moscow and its suburbs, Izvestiya. Atmos. Ocean. Phys. 2004, 40, 68–79. [Google Scholar]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D.J.; Bryce, D.L. Spectrometric Identification of Organic Compounds, 8th ed.; John Wiley & Sons: New York, NY, USA, 2014. [Google Scholar]
- Smith, A.L. Applied Infrared Spectroscopy; John Wiley: New York, NY, USA, 1979. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domantovsky, A.G.; Emelyanenko, K.A.; Emelyanenko, A.M.; Boinovich, L.B. The Influence of Prolonged High-Concentration Ozone Exposure on Superhydrophobic Coatings in Static and High-Speed Flow Atmospheres. Materials 2022, 15, 5725. https://doi.org/10.3390/ma15165725
Domantovsky AG, Emelyanenko KA, Emelyanenko AM, Boinovich LB. The Influence of Prolonged High-Concentration Ozone Exposure on Superhydrophobic Coatings in Static and High-Speed Flow Atmospheres. Materials. 2022; 15(16):5725. https://doi.org/10.3390/ma15165725
Chicago/Turabian StyleDomantovsky, Alexander G., Kirill A. Emelyanenko, Alexandre M. Emelyanenko, and Ludmila B. Boinovich. 2022. "The Influence of Prolonged High-Concentration Ozone Exposure on Superhydrophobic Coatings in Static and High-Speed Flow Atmospheres" Materials 15, no. 16: 5725. https://doi.org/10.3390/ma15165725





