Distinguishing Genuine Imperial Qing Dynasty Porcelain from Ancient Replicas by On-Site Non-Invasive XRF and Raman Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Artifacts
2.2. Methods
2.2.1. Portable X-ray Fluorescence Spectroscopy (pXRF)
2.2.2. Processing of XRF Data
2.2.3. Raman Microspectroscopy
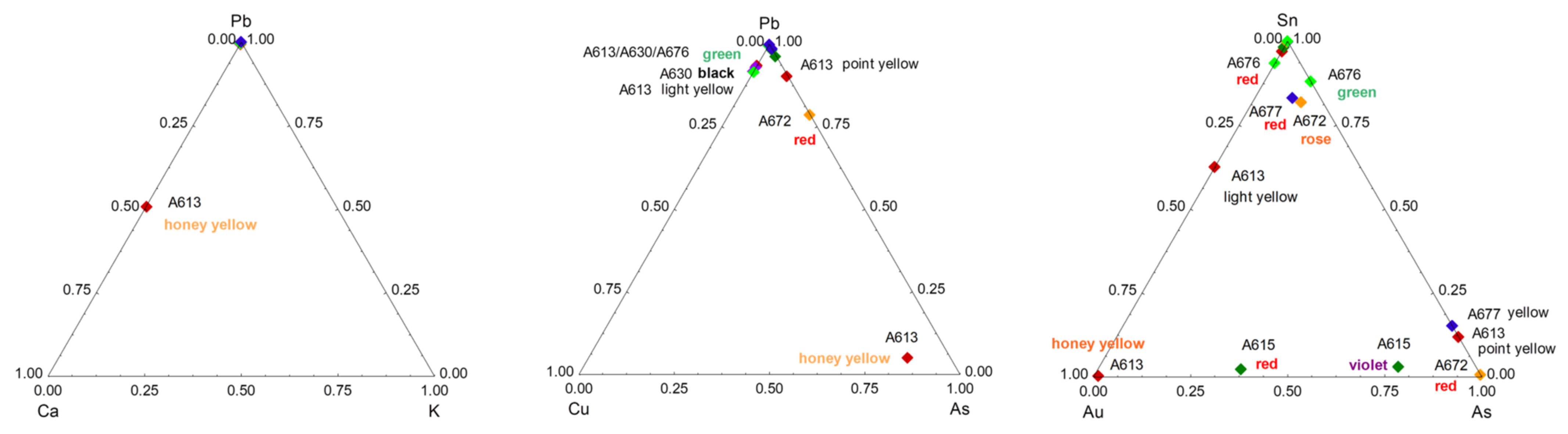
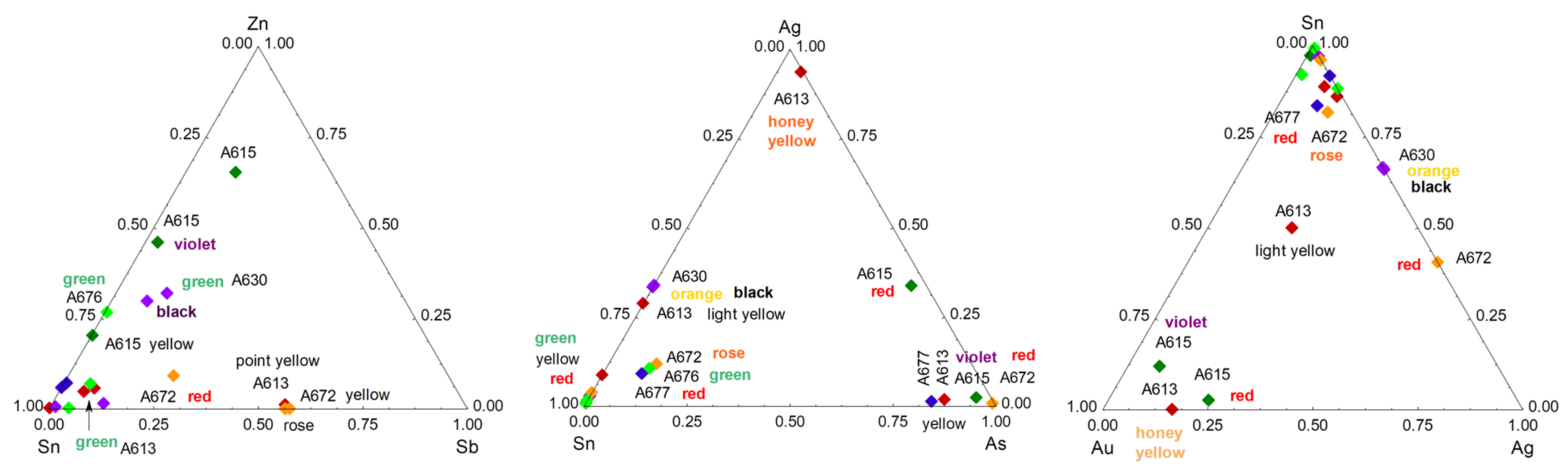
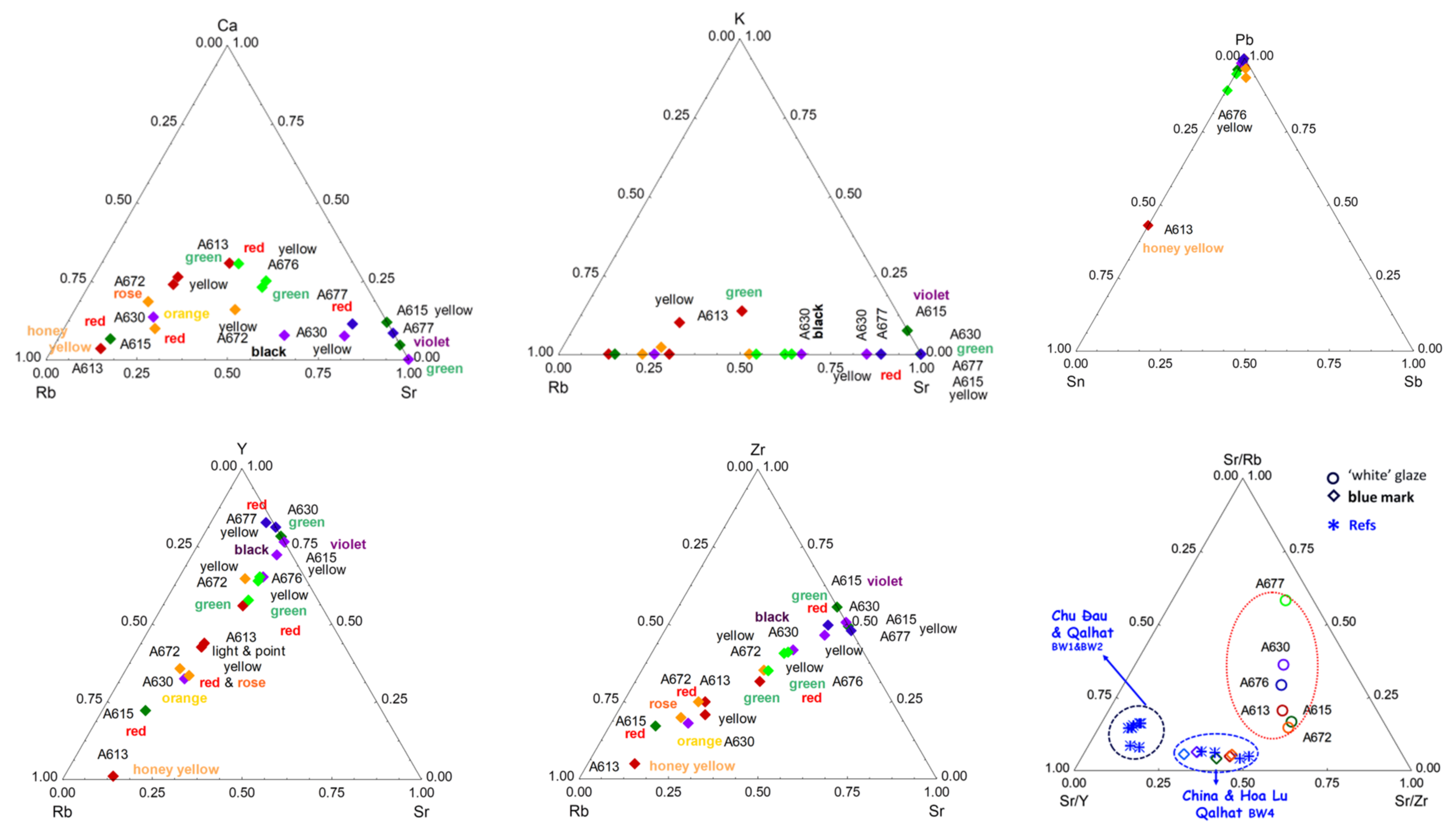
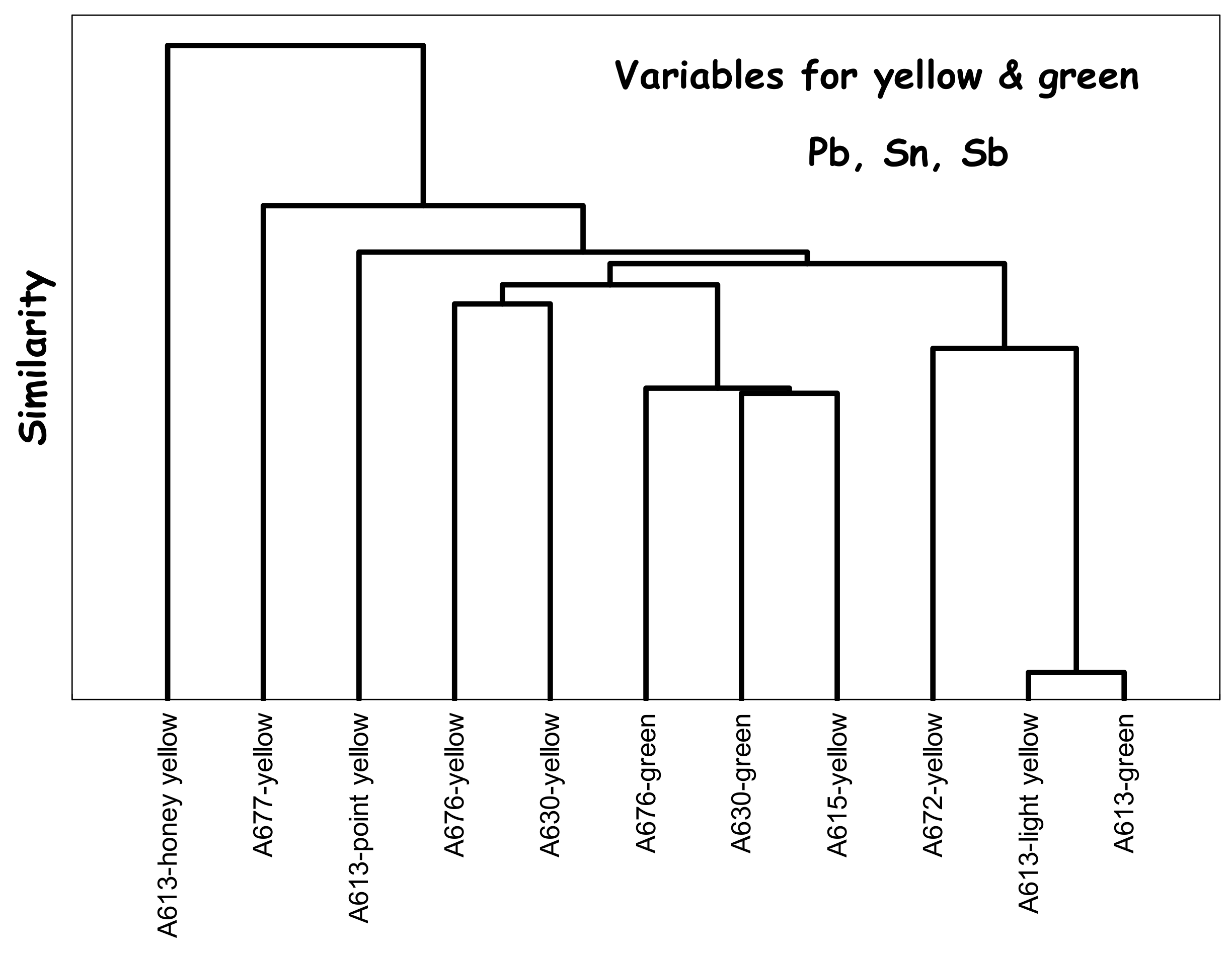
3. Results
3.1. Glazed Background
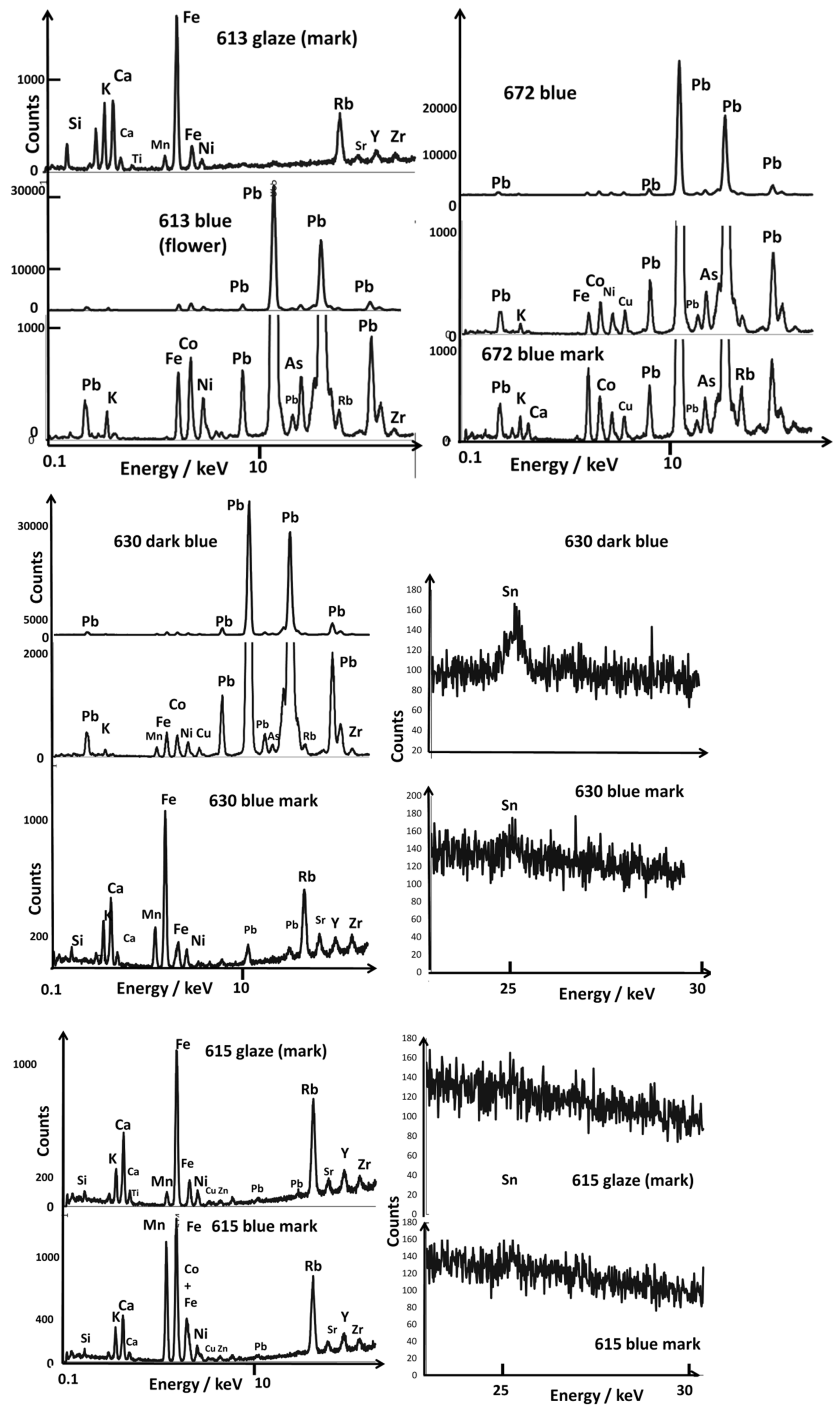
3.2. Painted Decor
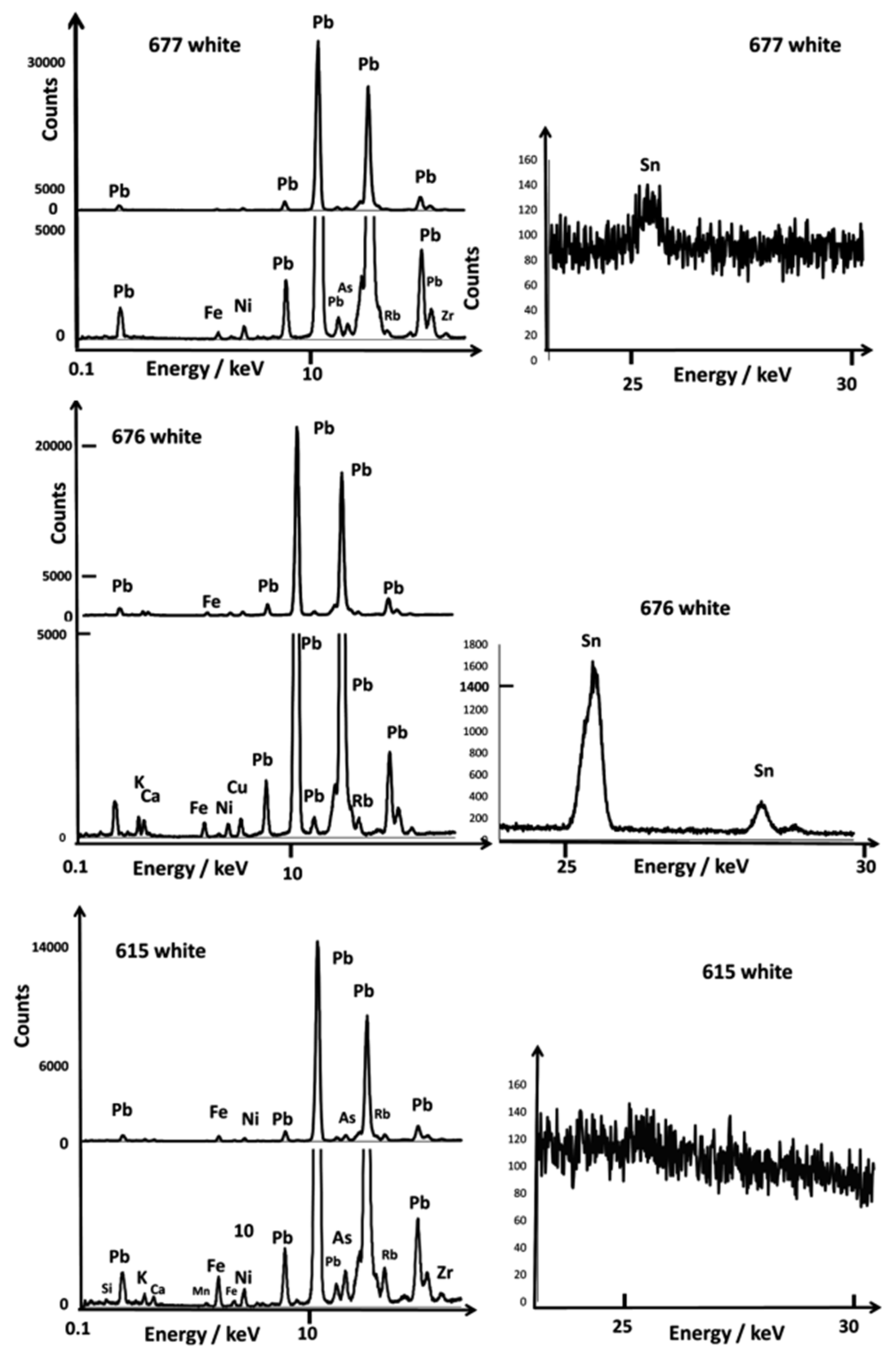

3.3. Reign Marks
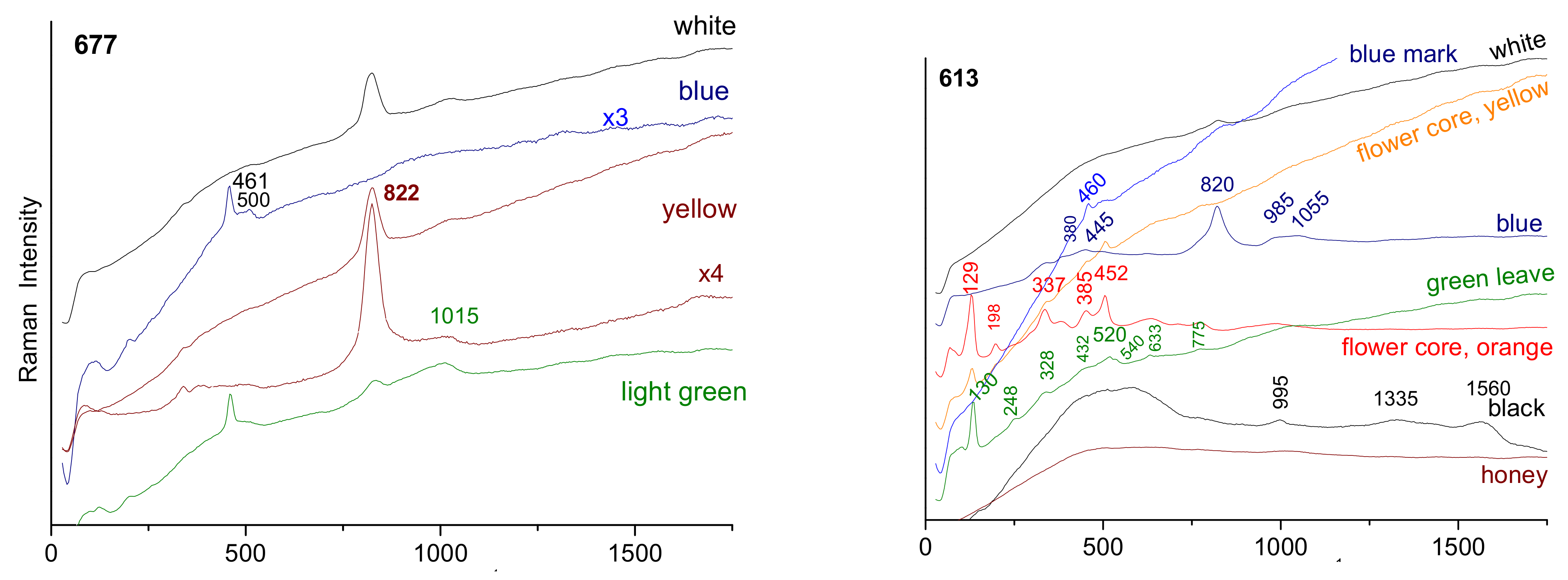
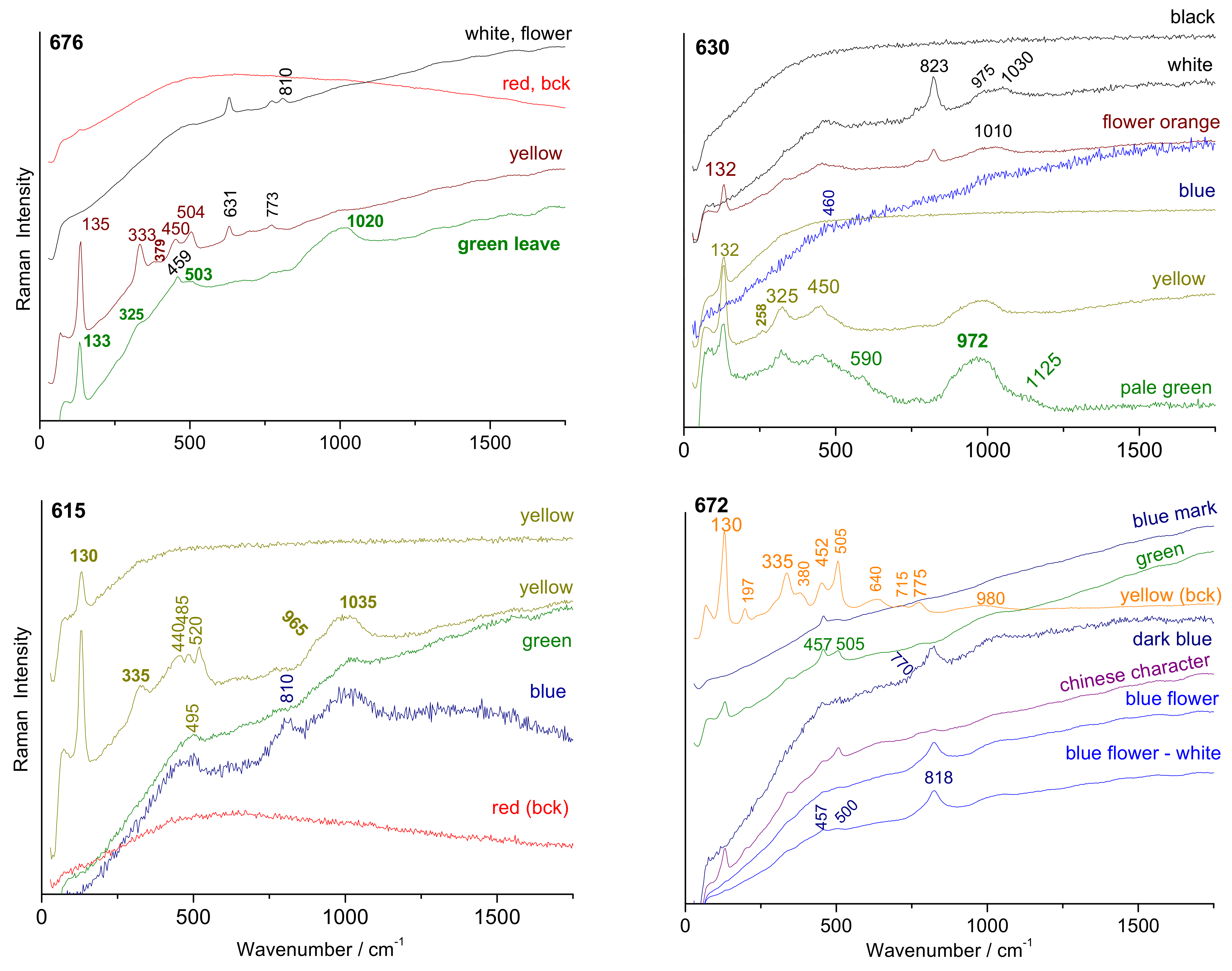
3.4. Phase Identification
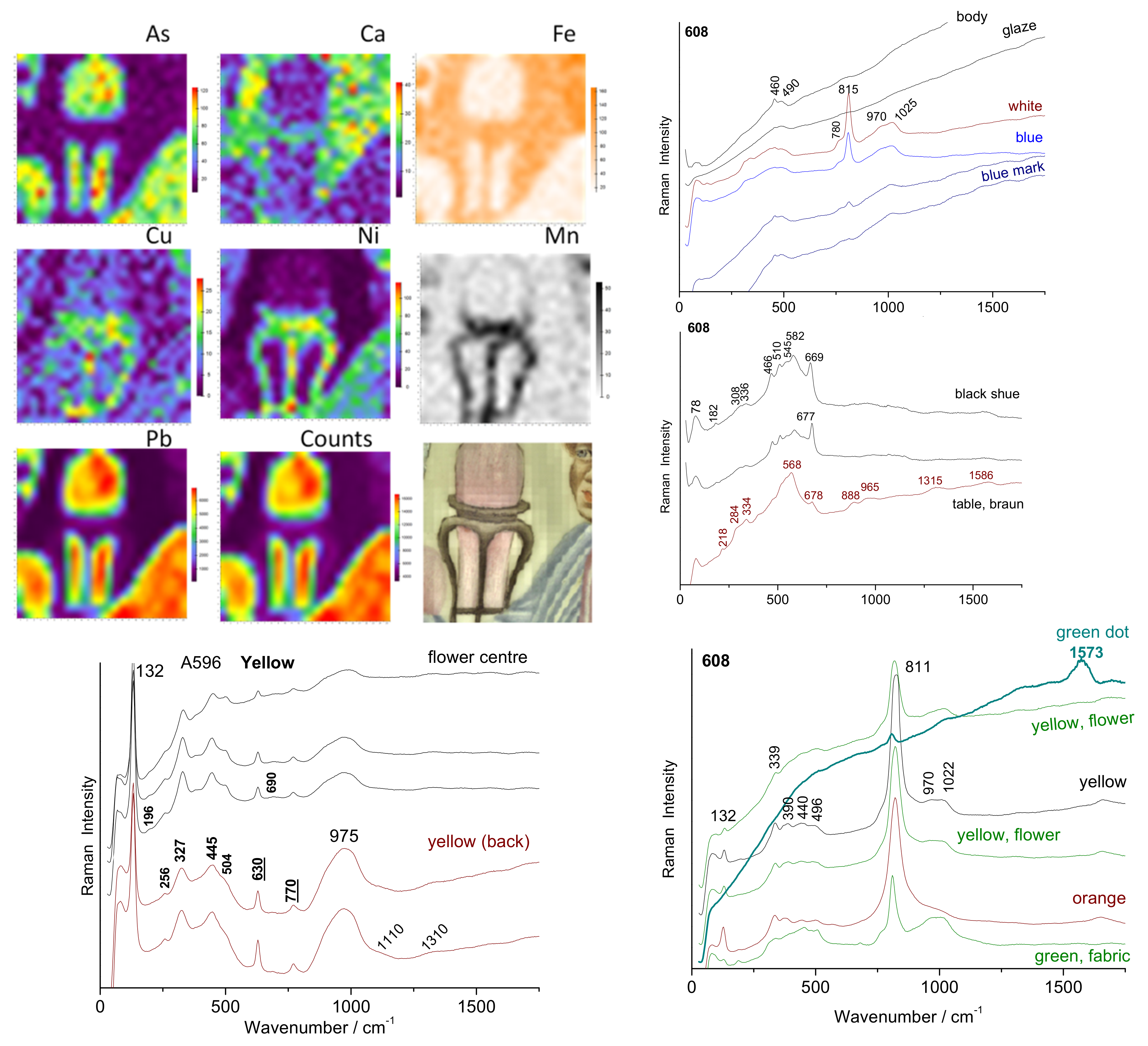
4. Discussion
4.1. Flux and Former
4.2. Gilding Technique
4.3. Yellow, Green, and Red to Pink Colors
4.4. Blue
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kerlan-Stephen, A.; Pirazzoli-t’Serstevens, M. Autour des Collections D’art en Chine au XVIIIe Siècle; Droz: Genève, Switzerland, 2008. [Google Scholar]
- Hsu, Y.-H. Antiquaries and Politics: Antiquarian Culture of The Northern Song 960-1127. In World Antiquarianism: Comparative Perspectives; Issues &Debates; Schnapp, A., Ed.; Getty Research Institute: Los Angeles, CA, USA, 2013; pp. 230–248. [Google Scholar]
- Li, S. Chinese Bronze Ware, 3rd ed.; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- Colomban, P.; Tournié, A.; Maucuer, M.; Meynard, P. On-site Raman and XRF analysis of Japanese/Chinese bronze/brass patina—The search of specific Raman signatures. J. Raman Spectrosc. 2012, 43, 799–808. [Google Scholar] [CrossRef]
- Zhang, F. The origin and development of traditional Chinese glazes and decorative ceramics color. In Ancient Technology to Modern Science; Kingery, W.D., Ed.; The American Ceramic Society: Colombus, OH, USA, 1985; Volume 1, pp. 163–180. [Google Scholar]
- Donnelly, P.J. Blanc de Chine; Faber and Faber: London, UK, 1969. [Google Scholar]
- Valenstein, S. A Handbook of Chinese Ceramics; Metropolitan Museum of Art: New York, NY, USA, 1998. [Google Scholar]
- Wood, N. Chinese Glazes: Their Origins, Chemistry and Recreation; A & C Black: London, UK, 1999. [Google Scholar]
- Ayers, J.; Bingling, Y. Blanc de Chine: Divine Images in Porcelain; China Institute: New York, NY, USA, 2002. [Google Scholar]
- Kerr, R.; Wood, N.; Needham, J.; Wood, N. Science and Civilisation in China, Volume 5, Part XII: Ceramic Technology; Cambridge University Press: Cambridge, UK, 2004. [Google Scholar]
- Finley, R. Porcelain City: Jingdezhen in the Eighteenth Century. In The Pilgrim Art: Cultures of Porcelain in World History, 1st ed.; University of California Press: Berkeley, CA, USA, 2010. [Google Scholar]
- Colomban, P. Grès et raku, in Grès et raku du Japon—Techniques décoratives et critères d’appréciation. Taoci 2003, 3, 3–10. [Google Scholar]
- Levy, E. Le Goût Chinois en Europe au XVIIIe Siècle: Catalogue du Musée des Arts Décoratifs, June–October 1910, Librairie Centrale des Beaux-Arts Paris 1910. Available online: https://gallica.bnf.fr/ark:/12148/bpt6k441547m/f1.texteImage (accessed on 28 November 2021).
- Castelluccio, S. Le Gout pour les Porcelaines de Chine et du Japon à Paris aux XVIIe et XVIIIe Siècles; Monelle Hayot Editions: Saint-Rémy-en-l’Eau, France, 2013. [Google Scholar]
- Wilson, G.; Watson, F.J.B. Mounted Oriental Porcelain in the J. Paul Getty Museum; J. Paul Getty Museum: Los Angeles, CA, USA, 1999. [Google Scholar]
- Pinot de Villechenon, F. Les Expositions Universelles; Que Sais-je-Presse; Universitaire de France: Paris, France, 1992. [Google Scholar]
- Vasseur, E. Pourquoi organiser des Expositions universelles? Le «succès» de l’Exposition universelle de 1867. Hist. Econ. Soc. 2005, 4, 573–594. [Google Scholar]
- Maucuer, M. Grès et porcelaine: Distinction et appréciation par les amateurs occidentaux au XIXe siècle. Taoci 2003, 3, 10–20. [Google Scholar]
- D’Albis, T. Le grès japonais dans les collections françaises. Taoci 2003, 3, 20–29. [Google Scholar]
- Howald, C.; Saint-Raymond, L. Tracking dispersal: Auction sales from the Yuanmingyuan loot in Paris in the 1860s. J. Art Mark. Stud. 2018, 2, 1–23. [Google Scholar]
- Saint-Raymond, L. À la Conquête du Marché de L’art: Le Pari(s) des Enchères (1830–1939); Bibliothèque de L’économiste n°36; Classiques Garnier: Paris, France, 2021. [Google Scholar]
- Tythacott, L. Collecting and Displaying China’s Summer Palace in the West—The Yuanmingyuan in Britain and France; Routledge: London, UK, 2018. [Google Scholar]
- Huang, C.H. From the Imperial Court to the International Art Market: Jingdezhen Porcelain Production as Global Visual Culture. J. World Hist. 2012, 23, 115–145. [Google Scholar] [CrossRef]
- Chow, E.T.; Drake, F.S. Kuan-Yao and Min-Yao: A study on Imperial Porcelain and people’s Porcelain from K’ang-His to the End of the Ch’ing Dynasty. Arch. Chin. Art Soc. Am. 1959, 13, 54–74. [Google Scholar]
- Pierson, S. True Beauty of Form and Chaste Embellishment. Summer Palace Loot and Chinese Porcelain Collecting in Nineteenth-century Britain. In Collecting and Displaying China’s Summer Palace in the West—The Yuanmingyuan in Britain and France, The Histories of Material Culture and Collecting, 1700–1950; Tythacott, L., Ed.; Routledge: London, UK, 2018; pp. 72–86. [Google Scholar]
- Deck, T. La Faïence; Maison Quantin: Paris, France, 1887. [Google Scholar]
- Maucuer, M. La copie des objets asiatiques au XIX siècle: Emile Reiber, Théodore Deck et la collection Cernuschi. Rev. Louvre Mus. Fr. 2011, 3, 80–90. [Google Scholar]
- Simsek, G.; Colomban, P.; Milande, V. Tentative differentiation between Iznik tiles and copies with Raman spectroscopy using both laboratory and portable instruments. J. Raman Spectrosc. 2010, 41, 529–536. [Google Scholar] [CrossRef]
- Quéquet, S. Entre Beaux-Arts et Industrie: L’engagement des Peintres de Salon Dans les Manufactures Françaises de Céramique: 1848–1891. Ph.D. Thesis, Université de Picardie Jules Verne, Amiens, France, 2012. Available online: http://www.theses.fr/2012AMIE0014 (accessed on 18 July 2022).
- Preaud, T.; Ostergard, D.E. The Sevres Porcelain Manufactory: Alexandre Brongniart and the Triumph of Art and Industry, 1800–1847; Bard Graduate Center for Studies in the Decorative Arts: New York, NY, USA, 1997. [Google Scholar]
- Bongniart, A.; Riocreux, D. Description Méthodique du Musée de Céramique de la Manufacture Royale de porcelaine de Sèvres, A; Leleux Libraire-Editeur: Paris, France, 1845; Available online: http://gallica.bnf.fr/ark:/12148/bpt6k6430171d/f9.image (accessed on 21 December 2017).
- Brongniart, A. Traité des Arts Céramiques ou Des Poteries Considérées Dans Leur Histoire, Leur Pratique et Leur Théorie; Béchet Jeune—A. Mathias: Paris, France, 1844; Volume 3, Available online: http://catalogue.bnf.fr/ark:/12148/cb36023945s.public (accessed on 21 December 2017).
- Caggiani, M.C.; Valotteau, C.; Colomban, P. Inside the glassmaker technology: Search of Raman criteria to discriminate between Emile Gallé and Philippe-Joseph Brocard enamels and pigment signatures. J. Raman Spectrosc. 2014, 45, 456–464. [Google Scholar] [CrossRef]
- Millet, A. La manufacture de Sèvres ou les stratégies de l’imitation. Entre acquisition d’un savoir-faire et marqueur d’identité (XVIIIe–XIXe siècles). Entrep. Hist. 2015, 78, 36–48. [Google Scholar] [CrossRef]
- Mleziva, J. Iznik or Paris? Imitations of Ottoman pottery in the collection of the West Bohemian Museum in Pilsen. Ann. Naprstek Mus. 2016, 37, 33–40. [Google Scholar] [CrossRef]
- Slitine, F. Samson: Génie de L’imitation; Massin: Paris, France, 2002. [Google Scholar]
- Jouenne, C.-A. Traité de Céramique et Matériaux Minéraux; Editions Septima: Paris, France, 2001. [Google Scholar]
- Haussone, M. Technologie Générale: Faïences, Grès, Porcelaines; Bibliothèque Professionnelle, J.-B. Baillère & Fils: Paris, France, 1969. [Google Scholar]
- Colomban, P. Natural nanosized raw materials and Sol-Gel technology: The base of pottery since millenniums. In Nanosciences and Cultural Heritage; Dillmann, P., Bellot-Gurlet, L., Nenner, I., Eds.; Atlantis Press: Paris, France, 2016; pp. 59–74. [Google Scholar] [CrossRef]
- Colomban, P. Glass, Ceramics and Enamelled Objects. In Conservation Science: Heritage Materials, 2nd ed.; Garside, P., Richardson, E., Eds.; The Royal Society of Chemistry: London, UK, 2022; Chapter 7; pp. 200–247. [Google Scholar]
- Epler, R.A.; Epler, D.R. Glazes and Glass Coatings; The American Ceramic Society: Westerville, OH, USA, 2000. [Google Scholar]
- Fraser, H. Glazes for the Craft Potter, Revised Edition; A & C Black: London, UK; The American Ceramic Society: Westerville, OH, USA, 1998. [Google Scholar]
- Colomban, P. Glazes and Enamels. In Encyclopedia of Glass Science, Technology, History, and Culture; Richet, P., Ed.; John Wiley & Sons Inc.: New York, NY, USA, 2020; Chapter 10.6. [Google Scholar]
- Colomban, P.; Zhang, Y.; Zhao, B. Non-invasive Raman analyses of huafalang and related porcelain wares. Searching for evidence for innovative pigment technologies. Ceram. Int. 2017, 43, 12079–12088. [Google Scholar] [CrossRef] [Green Version]
- Colomban, P.; Ambrosi, F.; Ngo, A.-T.; Lu, T.-A.; Feng, X.-L.; Chen, S.; Choi, C.-L. Comparative analysis of wucai Chinese porcelains using mobile and fixed Raman microspectrometers. Ceram. Int. 2017, 43, 14244–14256. [Google Scholar] [CrossRef] [Green Version]
- Colomban, P.; Lu, T.-A.; Milande, V. Non-invasive on-site Raman study of blue-decorated early soft-paste porcelain: The use of arsenic-rich (European) cobalt ores—Comparison with huafalang Chinese porcelains. Ceram. Int. 2018, 44, 9018–9026. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, J.; Ji, L.; Shan, Y.; Jiang, S.; Chen, G.; Wang, C. Study of arsenic in Famille rose porcelain from the Imperial Palace of Qing Dynasty, Beijing, China. Ceram. Int. 2018, 44, 1627–1632. [Google Scholar] [CrossRef]
- Duan, H.; Zhang, X.; Kang, B.; Wang, G.; Qu, L.; Lei, Y. Non-destructive analysis and deterioration study of a decorated Famille Rose porcelain bowl of Qianlong Reign from the Forbidden City. Stud. Conserv. 2019, 64, 311–322. [Google Scholar] [CrossRef]
- Li, Y.; Sciau, P.; Zhu, J.; Ji, L.; Shan, Y.; Song, G. Microscopic analysis of overglaze green pigment on Chinese Famille rose porcelain from the Imperial Palace. Microsc. Res. Tech. 2020, 84, 1106–1114. [Google Scholar] [CrossRef]
- Colomban, P.; Simsek Franci, G.; Kırmızı, B. Cobalt and Associated Impurities in Blue (and Green) Glass, Glaze and Enamel: Relationships between Raw Materials, Processing, Composition, Phases and International Trade. Minerals 2021, 11, 633. [Google Scholar] [CrossRef]
- Colomban, P. Full spectral range Raman signatures related to changes in enameling technologies from the 18th to the 20th centuries: Guidelines, effectiveness and limitations of the Raman analysis. Materials 2022, 15, 3158. [Google Scholar] [CrossRef] [PubMed]
- Colomban, P.; Simsek Franci, G.; Gironda, M.; d’Abrigeon, P.; Schumacher, A.-C. pXRF Data Evaluation Methodology for On-site Analysis of Precious Artifacts: Cobalt used in the Blue Decoration of Qing Dynasty Overglazed Porcelain enameled at Custom District (Guangzhou), Jingdezhen and Zaobanchu (Beijing) workshops. Heritage 2022, 5, 1752–1778. [Google Scholar] [CrossRef]
- Pierson, S. True or False? Defining the Fake in Chinese porcelain. Cah. Fram. 2019, 31, 6168. [Google Scholar] [CrossRef]
- Chen, C.E. Fooling the eye: Trompe l’oeil porcelain in High Qing China. Cah. Fram. E-Stor. 2019, 31. [Google Scholar] [CrossRef]
- Colomban, P.; Gironda, M.; Vangu, D.; Kırmızı, B.; Zhao, B.; Cochet, V. The technology transfer from Europe to China in the 17th–18th centuries: Non-invasive on-site XRF and Raman analyses of Chinese Qing Dynasty enameled masterpieces made using European ingredients/recipes. Materials 2021, 14, 7434. [Google Scholar] [CrossRef] [PubMed]
- Colomban, P.; Kırmızı, B.; Gougeon, C.; Gironda, M.; Cardinal, C. Pigments and glassy matrix of the 17th–18th century enamelled French watches: A non-invasive on-site Raman and pXRF study. J. Cult. Herit. 2020, 44, 1–14. [Google Scholar] [CrossRef]
- Simsek, G.; Geckinli, A.E. An assessment study of tiles from Topkapı Palace Museum with energy-dispersive X-ray and Raman spectrometers. J. Raman Spectrosc. 2012, 43, 917–927. [Google Scholar] [CrossRef]
- Garner, H. The Origins of Famille Rose. Trans. Orient. Ceramic Soc. 1969, 37, 1–16. Available online: https://www.orientalceramicsociety.org.uk/publications/transactions/4 (accessed on 2 June 2022).
- Ayers, J. The Baur Collection, Geneva-Chinese Ceramics, Volume IV: Painted and Polychrome Porcelains of the Ch’ing Dynasty; Collection Baur: Geneva, Switzerland, 1974. [Google Scholar]
- Ayers, J. Chinese Ceramics in the Baur Collection; Collection Baur: Geneva, Switzerland, 1999; Volume 2. [Google Scholar]
- Available online: https://www.britishmuseum.org/collection/object/A_1936-0413-33 (accessed on 18 July 2022).
- Shih, C.-F.; Peng, Y.-C. On the Origin and Development of Three Terms for Qing Dynasty Overglazed Enamels: Falangcai, Yangcai, and Fencai. Nat. Palace Mus. Res. Q. 2012, 29, 1–74. [Google Scholar]
- Wang, C.M. Nomenclature for Painted Enamel on Porcelain in Qing Dynasty: A Scientific Viewpoint. Nat. Palace Mus. Res. Q. 2012, 29, 115–166. [Google Scholar]
- Hall, E.; Pollard, A.M. Analysis of Chinese Monochrome Glazes by X-Ray Fluorescence Spectrometry. In Scientific Insights on Ancient Chinese Pottery and Porcelain; Science Press: Beijing, China, 1986; pp. 382–386. [Google Scholar]
- Available online: https://www.britishmuseum.org/collection/object/A_Franks-577- (accessed on 22 July 2022).
- Colomban, P.; Gironda, M.; Edwards, H.G.M.; Mesqui, V. The enamels of the first (softpaste) European blue-and-white porcelains: Rouen, Saint-Cloud and Paris factories: Complementarity of Raman and X-ray fluorescence analyses with mobile instruments to identify the cobalt ore. J. Raman Spectrosc. 2021, 52, 2246–2261. [Google Scholar] [CrossRef]
- Colomban, P.; Kırmızı, B. Non-invasive on-site Raman study of polychrome and white enamelled glass artefacts in imitation of porcelain assigned to Bernard Perrot and his followers. J. Raman Spectrosc. 2020, 51, 133–146. [Google Scholar] [CrossRef]
- Sciau, P.; Noé, L.; Colomban, P. Metal nanoparticles in contemporary potters’ master pieces: Lustre and red “pigeon blood” potteries as models to understand the ancient pottery. Ceram. Int. 2016, 42, 15349–15357. [Google Scholar] [CrossRef]
- Loehr, G. Missionary-artists at the Manchu Court. Trans. Orient. Ceram. Soc. 1963, 34, 51–67. Available online: https://www.orientalceramicsociety.org.uk/publications/transactions/4 (accessed on 2 June 2022).
- Shih, C.F. Wenhua jingzhi: Chaoyue shidai pimei xiyang de Kangxi chao qinggong huafalang Cultural Contending: Kangxi Painted Enamelware as Global Competitor. Minsu quyi 2013, 182, 149–219. [Google Scholar]
- Colomban, P.; Treppoz, F. Identification and Differentiation of Ancient and Modern European Porcelains by Raman Macro- and Microspectroscopy. J. Raman Spectrosc. 2001, 32, 93–102. [Google Scholar] [CrossRef]
- Colomban, P.; Sagon, G.; Faurel, X. Differentiation of antique ceramics from the Raman spectra of their coloured glazes and paintings. J. Raman Spectrosc. 2001, 32, 351–360. [Google Scholar] [CrossRef]
- Colomban, P.; Maggetti, M.; d’Albis, A. Non-invasive Raman identification of crystalline and glassy phases in a 1781 Sèvres Royal Factory soft paste porcelain plate. J. Eur. Ceram. Soc. 2018, 38, 5228–5233. [Google Scholar] [CrossRef]
- Van Pevenage, J.; Lauwers, D.; Herremans, D.; Verhaeven, E.; Vekemans, B.; De Clercq, W.; Vincze, L.; Moens, L.; Vandenabeele, P. A Combined Spectroscopic Study on Chinese Porcelain Containing Ruan-Cai Colours. Anal. Methods 2014, 6, 387–394. [Google Scholar] [CrossRef]
- Giannini, R.; Freestone, I.; Shortland, A.J. European cobalt sources identified in the production of Chinese Famille rose porcelain. J. Archaeol. Sci. 2017, 80, 27–36. [Google Scholar] [CrossRef] [Green Version]
- Colomban, P.; Ngo, A.-T.; Fournery, N. Non-invasive Raman Analysis of 18th Century Chinese Export/Armorial Overglazed Porcelain: Identification of the Different Enameling Technology. Heritage 2022, 5, 233–259. [Google Scholar] [CrossRef]
- Colomban, P.; Kırmızı, B.; Zhao, B.; Clais, J.-B.; Yang, Y.; Droguet, V. Investigation of the Pigments and Glassy Matrix of Painted Enamelled Qing Dynasty Chinese Porcelains by Noninvasive On-site Raman Microspectrometry. Heritage 2020, 3, 915–940. [Google Scholar] [CrossRef]
- Colomban, P.; Kırmızı, B.; Zhao, B.; Clais, J.-B.; Yang, Y.; Droguet, V. Non-invasive on-site Raman study of pigments and glassy matrix of the 17th–18th century painted enamelled Chinese metal wares: Comparison with French enamelling technology. Coatings 2020, 10, 471. [Google Scholar] [CrossRef]
- Sakellariou, K.; Miliani, C.; Morresi, A.; Ombelli, M. Spectroscopic investigation of yellow majolica glazes. J. Raman Spectrosc. 2004, 35, 61–67. [Google Scholar] [CrossRef]
- Sandalinas, C.; Ruiz-Moreno, S.; Lopez-Gil, A.; Miralles, J. Experimental confirmation by Raman spectroscopy of a Pb-Sn-Sb triple oxide yellow pigment in sixteenth-century Italian pottery. J. Raman Spectrosc. 2006, 37, 1146–1153. [Google Scholar] [CrossRef]
- Rosi, F.; Manuali, V.; Miliani, C.; Brunetti, B.G.; Sgamellotti, A.; Grygar, T.; Hradil, D. Raman scattering features of lead pyroantimonate compounds. Part I: XRD and Raman characterization of Pb2Sb2O7 doped with tin and zinc. J. Raman Spectrosc. 2009, 40, 107–111. [Google Scholar] [CrossRef]
- Pereira, M.; de Lacerda-Aroso, T.; Gomes, M.J.M.; Mata, A.; Alves, L.C.; Colomban, P. Ancient Portuguese ceramic wall tiles (“Azulejos”): Characterization of the glaze and ceramic pigments. J. Nano Res. 2009, 8, 79–88. [Google Scholar] [CrossRef]
- Pelosi, C.; Agresti, G.; Santamaria, U.; Mattei, E. Artificial yellow pigments: Production and characterization through spectroscopic methods of analysis. e-Preserv. Sci. 2010, 7, 108–115. [Google Scholar]
- Rosi, F.; Manuali, V.; Grygar, T.; Bezdicka, P.; Brunetti, B.G.; Sgamellotti, A.; Burgio, L.; Seccaroni, C.; Miliani, C. Raman scattering features of lead pyroantimonate compounds: Implication for the non-invasive identification of yellow pigments on ancient ceramics. Part II. In situ characterisation of Renaissance plates by portable micro-Raman and XRF studies. J. Raman Spectrosc. 2011, 42, 407–414. [Google Scholar] [CrossRef]
- Cartechini, L.; Rosi, F.; Miliani, C.; d’Acapito, F.; Brunetti, G.; Sgamellotti, A.A. Modified Naples yellow in Renaissance majolica: Study of Pb-Sb-Zn and Pb-Sb-Fe ternary pyroantimonates by X-ray absorption spectroscopy. J. Anal. At. Spectrom. 2011, 26, 2500–2507. [Google Scholar] [CrossRef]
- Colomban, P. Polymerization degree and Raman identification of ancient glasses used for jewellery, ceramic enamels and mosaics. J. Non-Crystall. Solids 2003, 323, 180–187. [Google Scholar] [CrossRef]
- Colomban, P.; Tournié, A.; Bellot-Gurlet, L. Raman identification of glassy silicates used in ceramic, glass and jewellery: A tentative differentiation guide. J. Raman Spectrosc. 2006, 37, 841–852. [Google Scholar] [CrossRef] [Green Version]
- Colomban, P.; Robert, I.; Roche, C.; Sagon, G.; Milande, V. Raman Identification of 18th century soft-paste porcelain: Saint-Cloud, Chantilly, Mennecy et Vincennes/Sèvres. ArchéoSci. Rev. Archéom. 2004, 28, 153–167. [Google Scholar] [CrossRef]
- Colomban, P.; Jullian, S.; Parlier, M.; Monge-Cadet, P. Identification of the high-temperature impact/friction of aeroengine lades and cases y micro Raman spectroscopy. Aerosp. Sci. Technol. 1999, 3, 447–459. [Google Scholar]
- Froment, F.; Tournié, A.; Colomban, P. Raman identification of natural red to yellow pigments: Ochre and iron-containing ores. J. Raman Spectrosc. 2008, 39, 560–568. [Google Scholar] [CrossRef]
- Available online: https://xrfcheck.bruker.com/InfoDepth (accessed on 6 July 2022).
- Colomban, P.; Calligaro, T.; Vibert-Guigue, C.; Nguyen, Q.L.; Edwards, H.G.M. Dorures des céramiques et tesselles anciennes: Technologies et accrochage. ArchéoSciences 2005, 29, 7–20. [Google Scholar] [CrossRef] [Green Version]
- Colomban, P.; Kirmizi, B.; Clais, J.-B.; Gironda, M. An on-site Raman and pXRF study of Joseph Coteau and Philippe Parpette’s jewelled porcelain: A summit of ceramic art. J. Cult. Herit. 2020, 46, 82–94. [Google Scholar] [CrossRef]
- Geyssant, J. Secret du verre rouge transparent de Bernard Perrot et comparaison avec celui de Johann Kunckel. In Bernard Perrot (1640–1709), Secrets et Chefs-D’œuvre des Verreries Royales d’Orléans, Catalogue; SOMOGY Editions, d’Arts; Klinka Ballesteros, I., de Valence, C., Maitte, C., Ricke, H., Eds.; Musée des Beaux-Arts d’Orléans: Paris, France, 2013; pp. 51–54. [Google Scholar]
- Bandiera, M.; Verita, M.; Léhuédé, P.; Vilariguès, M. The Technology of Copper-Based Red Glass Sectilia from the 2nd Century AD Lucius Verus Villa in Rome. Minerals 2020, 10, 875. [Google Scholar] [CrossRef]
- Simsek, G.; Colomban, P.; Wong, S.; Zhao, B.; Rougeulle, A.; Liem, N.Q. Toward a fast non-destructive identification of pottery: The sourcing of 14th–16th century Vietnamese and Chinese ceramic shards. J. Cult. Herit. 2015, 16, 159–172. [Google Scholar] [CrossRef]
- Simsek Franci, G. Blue Print: Archaeometric Studies of Colored Glazed Chinese Ceramics and Production of Replica, Final Report; The Scientific and Research Council of Turkey, The Scientific and Technological Projects Funding Program: Ankara, Turkey, 2021; unpublished report.
- Simsek Franci, G. Handheld X-ray Fluorescence (XRF) versus wavelength dispersive XRF: Characterization of Chinese blue and white porcelain sherds using handheld and laboratory-type XRF instruments. Appl. Spectrosc. 2020, 74, 314–322. Available online: https://opg.optica.org/as/abstract.cfm?uri=as-74-3-314 (accessed on 22 July 2022). [CrossRef] [PubMed]
- Colomban, P.; Sagon, G.; Huy, L.Q.; Liem, N.Q.; Mazerolles, L. Vietnamese (15th Century) blue-and-white tam thai and luster porcelains/stonwares: Glaze composition and decoration techniques. Archaeometry 2004, 46, 125–136. [Google Scholar] [CrossRef]
- Yap, C.T.; Tang, S.M. X-ray fluorescence analysis of modern and recent Chinese porcelains. Archaeometry 1984, 26, 78–81. [Google Scholar] [CrossRef]
- Yap, C.T. A quantitative spectrometric analysis of trace concentrations of manganese and cobalt in ceramics and the significance of As/Co and Mn/Co ratios. J. Archaeol. Sci. 1988, 15, 173–177. [Google Scholar] [CrossRef]
- Yu, K.N.; Miao, J.M. Locating the origins of blue and white porcelains using EDXRF. Appl. Radiat. Isot. 1997, 48, 953–959. [Google Scholar] [CrossRef]
- Yu, K.N.; Miao, J.M. Multivariate analysis of the energy dispersive X-ray fluorescence results from blue and white Chinese porcelains. Archaeometry 1998, 40, 331–339. [Google Scholar] [CrossRef]
- Yu, K.N.; Miao, J.M. Characterization of blue and white porcelains using Mn/Fe ratio from EDXRF, with particular reference to porcelains of the Xuande period (1426 to 1435 A.D.). Appl. Radiat. Isot. 1999, 51, 279–283. [Google Scholar] [CrossRef]
- Morimoto, A.; Yamasaki, K. Technical Studies on Ancient Ceramics Found in North and Central Vietnam; Fukuoka Museum: Fukuoka, Japan, 2001. [Google Scholar]
- Cheng, H.S.; Zhang, B.; Xia, H.N.; Jiang, J.C.; Yang, F.J. Non-destructive analysis and appraisal of ancient Chinese porcelain by PIXE. Nucl. Instrum. Methods Phys. Res. Sect. B 2002, 190, 488–491. [Google Scholar] [CrossRef]
- Wen, J.X.; Chen, Z.K.; Zeng, Q.G.; Hu, L.S.; Wang, B.; Shi, J.P.; Zhang, G.X. Multi-micro analytical studies of blue-and-white porcelain (Ming dynasty) excavated from Shuangchuan island. Ceram. Int. 2019, 45, 13362–13368. [Google Scholar] [CrossRef]
- Jiang, X.; Ma, Y.; Chen, Y.; Li, Y.; Ma, Q.; Zhang, Z.; Wang, C.; Yang, Y. Raman analysis of cobalt blue pigment in blue and white porcelain: A reassessment. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 190, 61–67. [Google Scholar] [CrossRef]
- Wang, T.; Zhu, T.Q.; Feng, Z.Y.; Fayard, B.; Pouyet, E.; Cotte, M.; De Nolf, W.; Sciau, P. Synchrotron radiation-based multi-analytical approach for studying underglaze color: The microstructure of Chinese Qinghua blue decors (Ming dynasty). Anal. Chim. Acta 2016, 928, 20–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, R.; Wang, C.S.; Mao, Z.W.; Huang, Y.Y.; Pollard, A.M. The chemical composition of blue pigment on Chinese blue-and-white porcelain of the Yuan and Ming Dynasties (AD 1271–1644). Archaeometry 2007, 49, 101–115. [Google Scholar] [CrossRef]
- Zhou, Y.H.; Hu, Y.J.; Tao, Y.; Sun, J.; Cui, Y.; Wang, K.; Hu, D.B. Study on the microstructure of the multilayer glaze of the 16th–17th century export blue-and-white porcelain excavated from Nan’ao Shipwreck. Ceram. Int. 2016, 42, 17456–17465. [Google Scholar] [CrossRef]
- Zhang, R.; Garachon, I.; Gethin, P.; van Campen, J. Double layers glaze analysis of the Fujian export blue-and-white porcelain from the Witte Leeuw shipwreck (1613). Ceram. Int. 2020, 46, 13474–13481. [Google Scholar] [CrossRef]
- De Pauw, E.; Track, P.; Verhaeven, E.; Bauters, S.; Acke, L.; Vekemans, B.; Vincze, L. Microbeam X-ray fluorescence and X-ray absorption spectroscopic analysis of Chinese blue-and-white porcelain dating from the Ming dynasty. Spectrochim. Acta Part B 2018, 149, 190–196. [Google Scholar] [CrossRef]




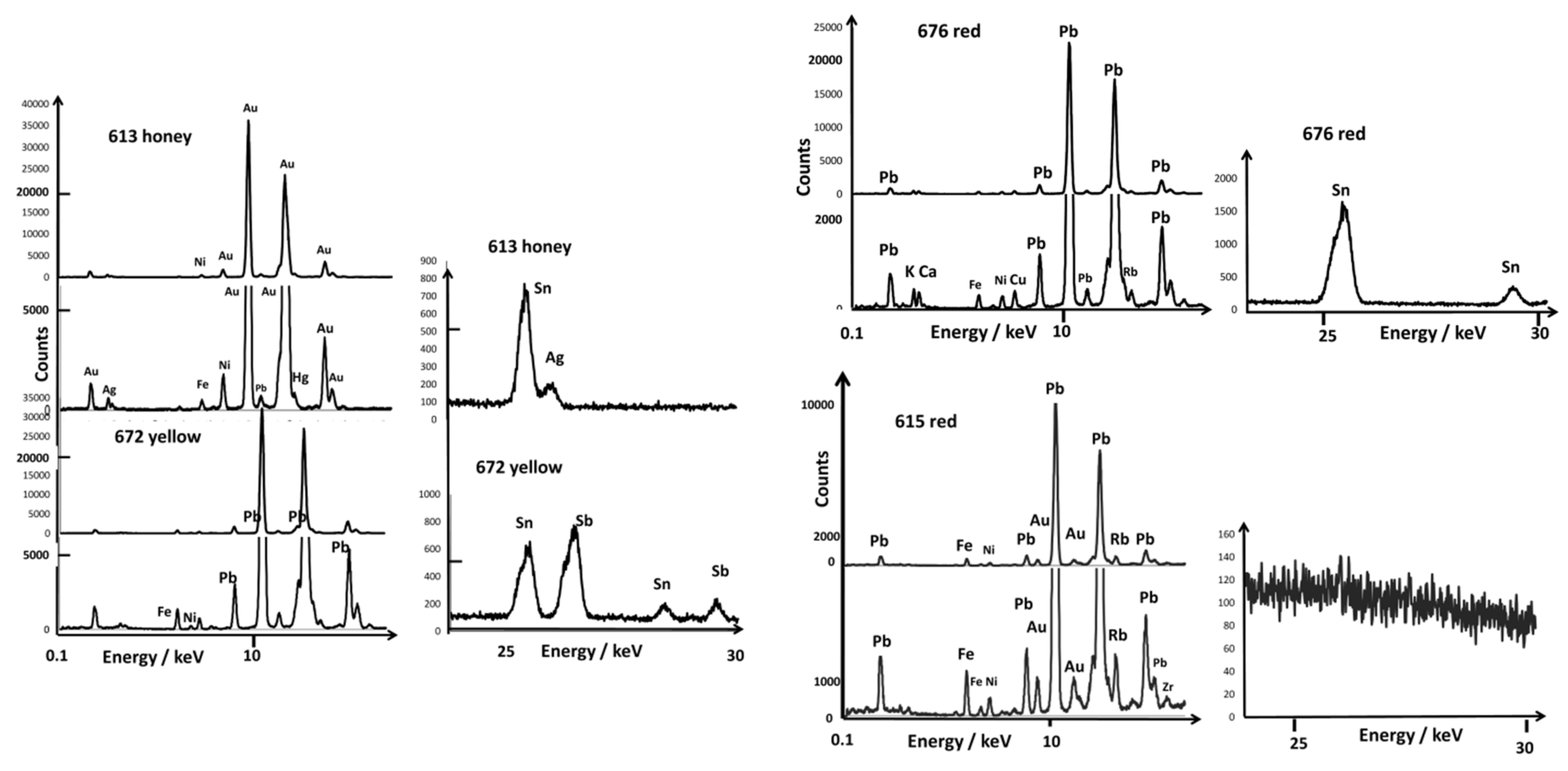
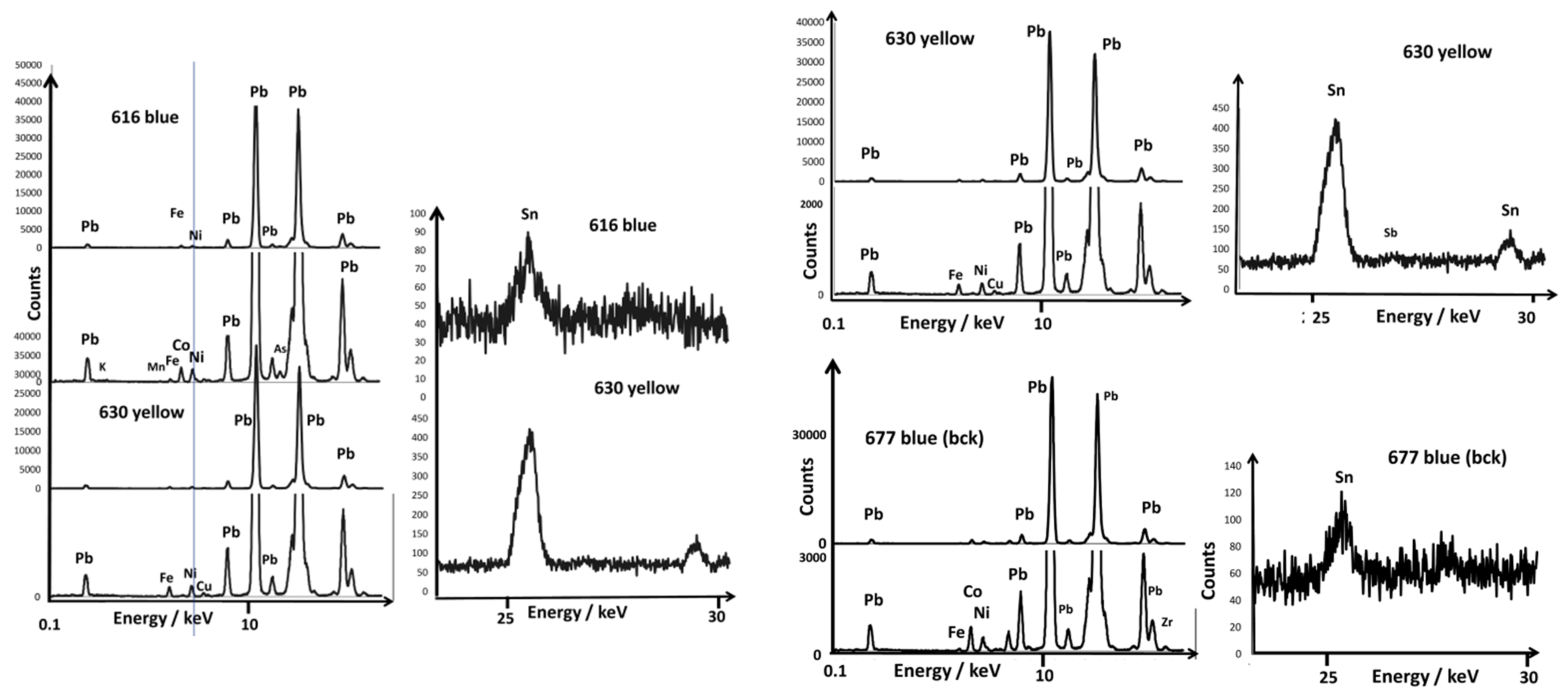
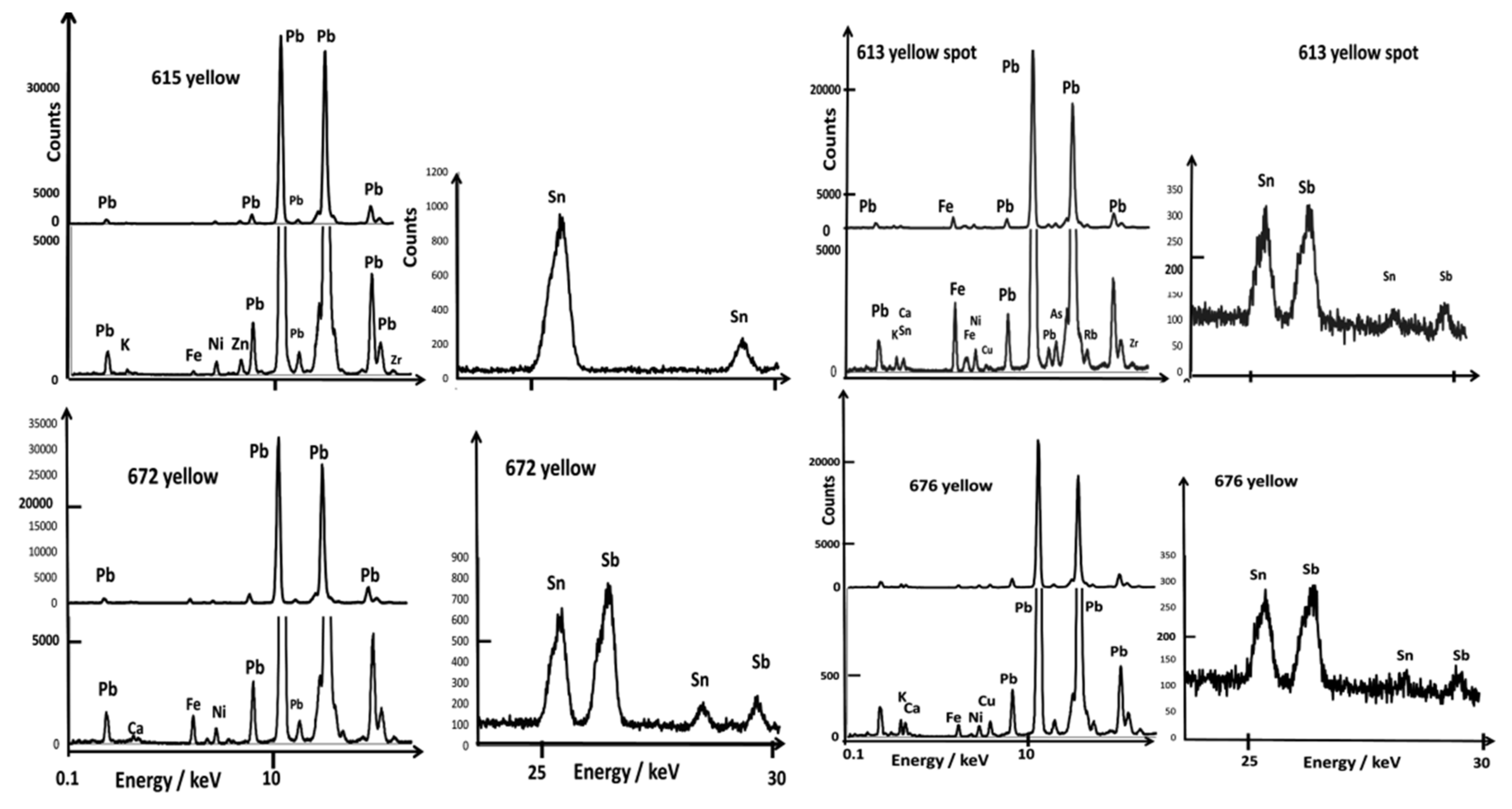


| Artifact | Inventory Number | Reign Mark | Dimension/cm and Weight/g | Spots Analyzed by XRF and Raman | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Body | Glaze | Yellow (Honey) | Blue | Green | Red-Orange | White | Black | ||||
| bowl | CB.CC.1936.677 | Kangxi reign mark in red but probably later period | D. 14.5; H. 7.4; 297 | yes | yellow | bck | red | white | |||
| bowl | CB.CC.1931.676 | Kangxi mark in colloidal red | D. 14.5 H. 6, 1; 127 | yellow | green | red | white | ||||
| bowl | CB.CC.1932.613b (from a pair) | Kangxi mark in cobalt blue | D. 12.5; 151 | yes | close to flower close to mark | light-yellow honey-yellow | flower light mark | green | white | ||
| bowl | CB.CC.1950.672 | Kangxi reign mark in cobalt blue | D. 12.5; H. 6.5; 156 | yes | close to mark | yellow | flower mark | rose scale line | white | ||
| bowl | CB.CC.1937.615 | Yongzheng reign mark in underglaze cobalt blue | D. 11; 108 | yes | close to mark | yellow | flower mark | red(bck) violet(scale) | white | ||
| bowl | CB.CC.1930.616 | Yongzheng reign mark but maybe later date | D. 9.3; 74 | close to mark | -blue (bck) -mark | ||||||
| dish | CB.CC.1936.596 | Yongzheng reign mark in overglaze cobalt blue | D. 20 | (yellow) | |||||||
| dish | CB.CC.1935.608 | Qianlong reign mark in cobalt blue | D. 17.4; 169 | men coat (mapping) | |||||||
| bowl | CB.CC.1930.630 | Daoguang reign mark in cobalt blue | D.18.5; 489 | close to mark | yellow | flower mark | green | orange | white | black | |
| Artifact | Reign Mark | Phases and Characteristic Elements (Major, Minor/Traces; Main Raman Peaks (cm−1)) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Background (Color) | Mark | Yellow | Blue | Green | Violet, Red to Orange | White | Black | Matrix | ||
| Bowl A677 | Kangxi reign mark in red but probably later period | (blue: Co2+) Quartz, glassy phase. Co | Arsenate (822) Pb,As,Sn | Bck Mn,Fe,Co, Ni,Cu,Zn | Arsenate (822) Quartz Glassy phase NY | Fe,Ni,Sn | Arsenate (822) Pb,As,Sn,Fe | (1015) | ||
| Bowl A676 | Kangxi mark in colloidal red | (red: Cu) Pb, Sn,Cu,Ni Fluorescence | Cassiterite NY (135,333, 505) Pb,Sn,Sb As,Cu,Ni, Fe | NY (133,325,459) | Red: Pb,Sn Fe,Ni,Cu, | Cassiterite Arsenate (810) Pb, Sn, As,Cu,Ni, Fe | ||||
| Bowl A613 | Kangxi reign mark in cobalt blue | (honey:Au) Au,Ag,Hg, Pb,Sn,As Fluorescence | Quartz, glassy phase Mn,Fe,Ni, Co | Light yellow: Sn,Cu NY (129,198,452) Pb,As,Fe, Ni,Cu,Sn, Sb | Arsenate (820) Pb,As Co, Fe,Ni | NY (130,328,520) Pb,Cu,As, Ni, Sn (trace of cassiterite) | Violet: Pb,Au,Fe, Ni,Co,As, Sn | Arsenate (820) Pb, Au,Cu,As, Fe,Ni, Sn | 580 | 1020 |
| Bowl A672 | Kangxi reign mark in cobalt blue | (yellow) Pb, Sn,Sb | Quartz, glassy phase | NY (130,197, 335,505) Pb,Fe,Ni, Sn,Sb | Arsenate (818) Pb,As,Fe, Co,Ni | Cassiterite trace? NY (130,197,335, 505) | Rose: Pb,As,Au, Sn,Fe,Ni Red: Pb,Fe,Ni | Pb,As,Fe,Ni, Sn,Sb | ||
| Bowl A615 | Yongzheng reign mark in cobalt blue | (red) Pb,Au, Sn,Cu,Ni,As Fluorescence | Fe,Mn,Co, Ni, | NY Arsenate (810) Pb,Sn,Ni,Zn | Arsenate (810) Pb,As,Fe, Co,Ni, Cu,Mn,Sn | Glassy phase Pb,Sn,Cu, Ni,Zn,Mn | Violet (scale) As,Au,Zn | Pb, As,Fe, Ni | 1035 | |
| Bowl A616 | Yongzheng reign mark but maybe later date | (blue) Pb,Sn,Co, Mn, Fe,Ni,As | ||||||||
| Dish A596 | Yongzheng mark and period | (yellow) a Cassiterite NY (132,327,445) | Cassiterite NY (132,325,445) | 975 (lead-rich) | ||||||
| Dish A608 | Qianlong reign mark in overglaze cobalt blue | Quartz, Glassy phase Arsenate (780–815) | NY Arsenate (780–815) | Arsenate (780–815) Co,Ni,As | Arsenate (780–815) | Arsenate (780–815) NY(132) | Arsenate (780–815) | Spinel MnO2 Pb,Cu,Mn, Ni,Fe,As | 970–1020 (lead-rich) | |
| Bowl A630 | Daoguang 1825–1850 Fe,Mn, Ni,Co underglaze | (yellow) Pb,Sn, Fe,Ni | Fe,Mn,Ni, (Co) | NY (132,325,450) Pb,Sn,Ni, Fe,Cu | Quartz, Glassy phase Pb,As Co,Cu,Mn, Fe,Ni,Sn | NY (132,325,450) Pb,Sn,Ni, Fe,Cu,Sb | (Orange) Arsenate (823) NY (132) Pb,Fe,Ni, Sn | Arsenate (823) | Fluorescence Pb,Cu,Fe, Ni,Mn,Sn, Sb | 975–1030 (lead-rich) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colomban, P.; Gironda, M.; Simsek Franci, G.; d’Abrigeon, P. Distinguishing Genuine Imperial Qing Dynasty Porcelain from Ancient Replicas by On-Site Non-Invasive XRF and Raman Spectroscopy. Materials 2022, 15, 5747. https://doi.org/10.3390/ma15165747
Colomban P, Gironda M, Simsek Franci G, d’Abrigeon P. Distinguishing Genuine Imperial Qing Dynasty Porcelain from Ancient Replicas by On-Site Non-Invasive XRF and Raman Spectroscopy. Materials. 2022; 15(16):5747. https://doi.org/10.3390/ma15165747
Chicago/Turabian StyleColomban, Philippe, Michele Gironda, Gulsu Simsek Franci, and Pauline d’Abrigeon. 2022. "Distinguishing Genuine Imperial Qing Dynasty Porcelain from Ancient Replicas by On-Site Non-Invasive XRF and Raman Spectroscopy" Materials 15, no. 16: 5747. https://doi.org/10.3390/ma15165747
APA StyleColomban, P., Gironda, M., Simsek Franci, G., & d’Abrigeon, P. (2022). Distinguishing Genuine Imperial Qing Dynasty Porcelain from Ancient Replicas by On-Site Non-Invasive XRF and Raman Spectroscopy. Materials, 15(16), 5747. https://doi.org/10.3390/ma15165747







