The Effect of Nucleating Agents on Enzyme-Induced Carbonate Precipitation and Corresponding Microscopic Mechanisms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Extraction of Urease
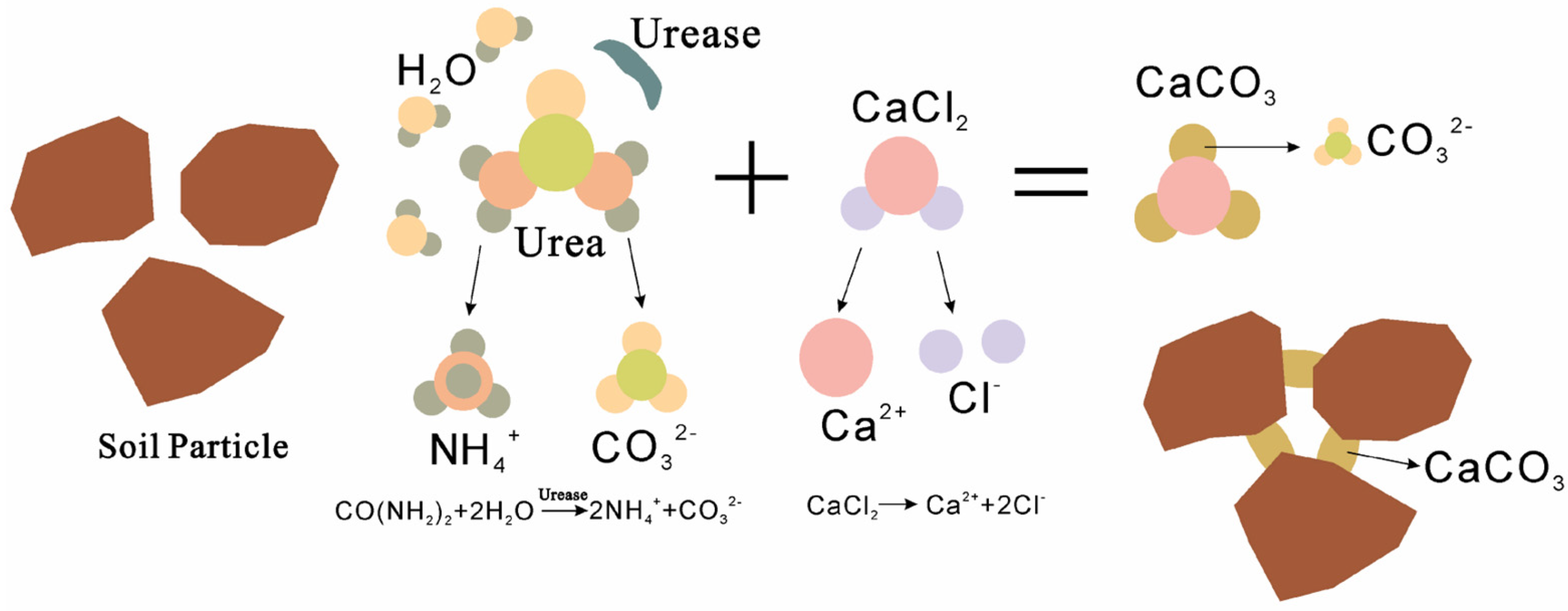
2.2.2. EICP
| Urease Source | Bean Powder Concentration (g/L) | Equimolar Concentration Urea–CaCl2 (mol/L) |
|---|---|---|
| Soybean | 40 | 0.25 |
| Soybean | 40 | 0.5 |
| Soybean | 40 | 0.75 |
| Soybean | 40 | 1 |
| Soybean | 40 | 1.25 |
| Black bean | 40 | 0.25 |
| Black bean | 40 | 0.5 |
| Black bean | 40 | 0.75 |
| Black bean | 40 | 1 |
| Black bean | 40 | 1.25 |
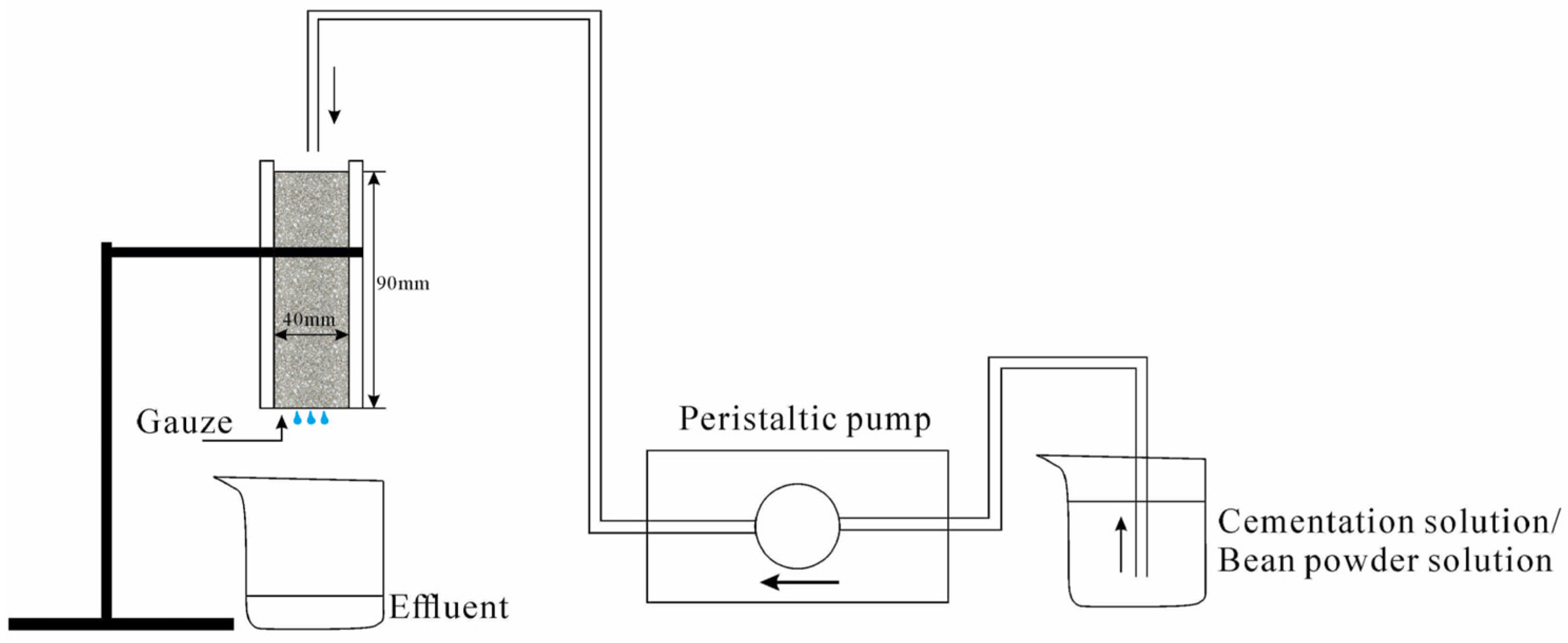
2.2.3. Sand Improvement
2.2.4. Unconfined Compressive Strength (UCS) Tests of Improved Sand
2.2.5. Determination of Calcium Carbonate Content
2.2.6. XRD and SEM
3. Results and Discussion
3.1. Preference of Cementation Solution
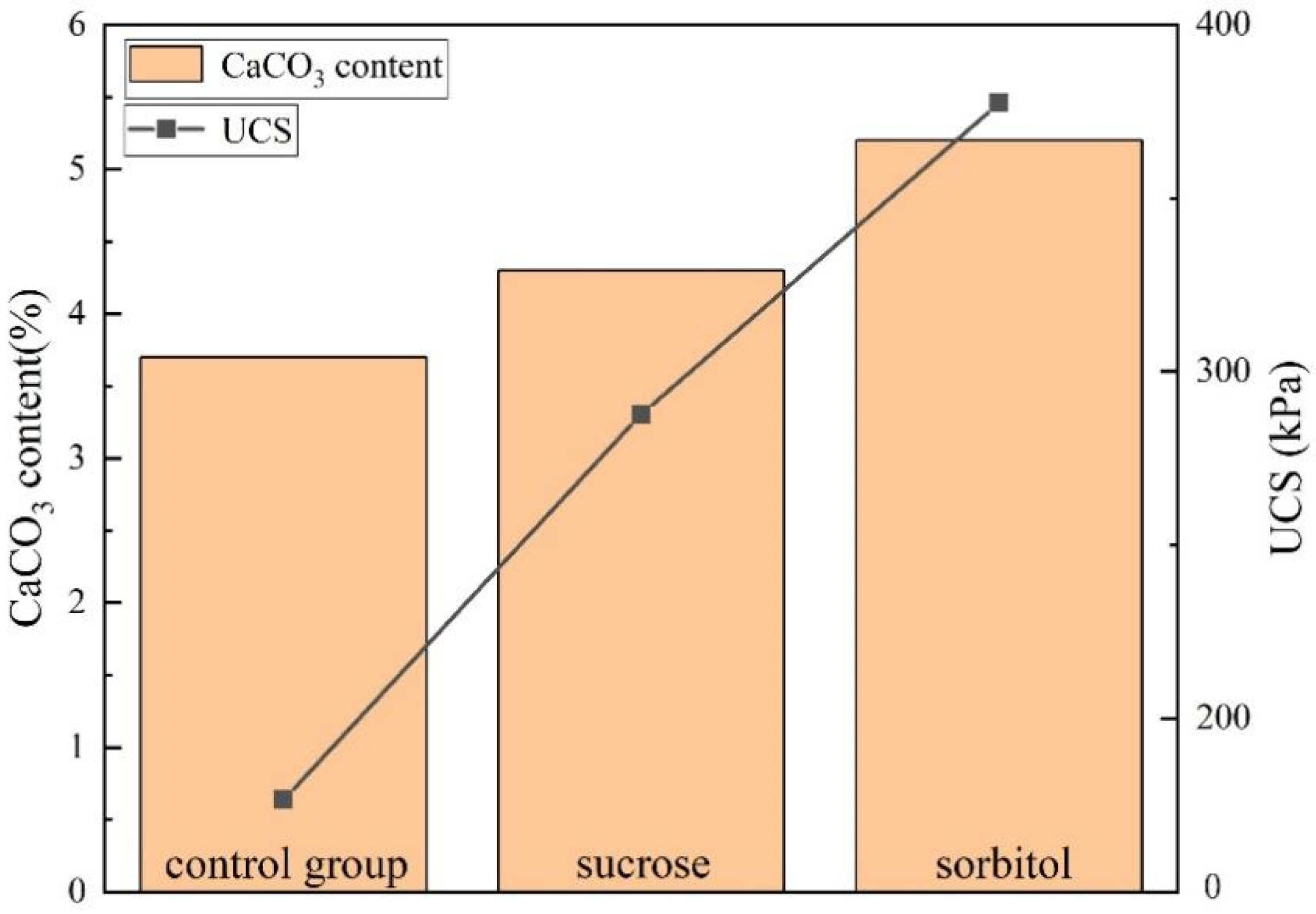
3.2. Effect of Nucleating Agent on Calcium Carbonate Productivity
3.3. Unconfined Compressive Strength
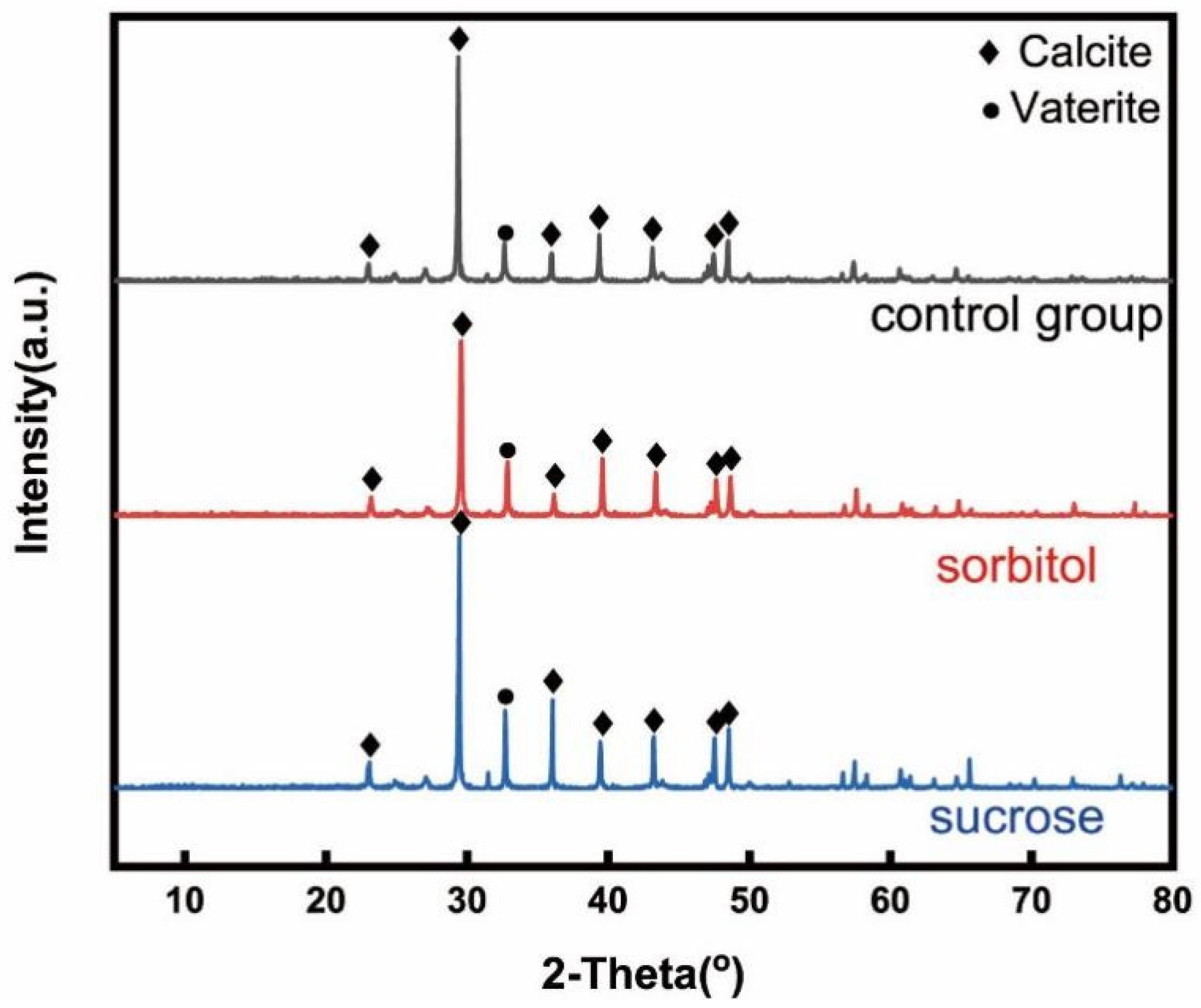
3.4. Microscopic Mechanisms
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kavazanjian, E.; Hamdan, N. Enzyme induced carbonate precipitation (EICP) columns for ground improvement. IFCEE2015 2015, 2252–2261. [Google Scholar] [CrossRef]
- Cheng, L.; Shahin, M.A. Microbially Induced Calcite Precipitation (MICP) for Soil Stabilization. In Ecological Wisdom Inspired Restoration Engineering; Springer: Berlin/Heidelberg, Germany, 2019; pp. 47–68. [Google Scholar]
- DeJong, J.T.; Mortensen, B.M.; Martinez, B.C.; Nelson, D.C. Bio-mediated soil improvement. Ecol. Eng. 2010, 36, 197–210. [Google Scholar] [CrossRef]
- Mujah, D.; Cheng, L.; Shahin, M.A. Microstructural and geomechanical study on biocemented sand for optimization of MICP process. J. Mater.Civ. Eng. 2019, 31, 04019025. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Li, L.; Ogbonnaya, U.; Wen, K.; Tian, A.; Amini, F. Influence of fiber addition on mechanical properties of MICP-treated sand. J. Mater. Civ. Eng. 2016, 28, 04015166. [Google Scholar] [CrossRef]
- Li, M.; Wen, K.; Li, Y.; Zhu, L. Impact of oxygen availability on microbially induced calcite precipitation (MICP) treatment. Geomicrobiol. J. 2018, 35, 15–22. [Google Scholar] [CrossRef]
- Castanier, S.; Le Métayer-Levrel, G.; Perthuisot, J.P. Ca-carbonates precipitation and limestone genesis—The microbiogeologist point of view. Sediment. Geol. 1999, 126, 9–23. [Google Scholar] [CrossRef]
- Kavazanjian Jr, E.; Almajed, A.; Hamdan, N. Bio-inspired soil improvement using EICP soil columns and soil nails. Grouting 2017, 2017, 13–22. [Google Scholar]
- DeJong, J.T.; Soga, K.; Kavazanjian, E.; Burns, S.; Van, L.A. Biogeochemical processes and geotechnical applications progress, opportunities and challenges. In Proceedings of the Bio-and Chemo-Mechanical Processes in Geotechnical Engineering Géotechnique Symposium, London, UK, 3 June 2013. [Google Scholar]
- Harkes, M.P.; Van Paassen, L.A.; Booster, J.L.; Whiffin, V.S.; van Loosdrecht, M.C. Fixation and distribution of bacterial activity in sand to induce carbonate precipitation for ground reinforcement. Ecol. Eng. 2010, 36, 112–117. [Google Scholar] [CrossRef]
- Whiffin, V.S.; Van Paassen, L.A.; Harkes, M.P. Microbial carbonate precipitation as a soil improvement technique. Geomicrobiol. J. 2007, 24, 417–423. [Google Scholar] [CrossRef]
- Whiffin, V.S. Microbial CaCO3 Precipitation for the Production of Biocement. Ph.D. Thesis, Murdoch University, Perth, Australia, 2004. [Google Scholar]
- Blakeley, R.L.; Zerner, B. Jack bean urease the first nickel enzyme. J. Mol. Catal. 1984, 23, 263–292. [Google Scholar] [CrossRef]
- Mujah, D.; Shahin, M.A.; Cheng, L. State-of-the-art review of biocementation by microbially induced calcite precipitation (MICP) for soil stabilization. Geomicrobiol. J. 2017, 34, 524–537. [Google Scholar] [CrossRef]
- Shahin, M.A.; Jamieson, K.; Cheng, L. Microbial-induced carbonate precipitation for coastal erosion mitigation of sandy slopes. Géotechnique Lett. 2020, 10, 211–215. [Google Scholar] [CrossRef]
- Zhao, Q.; Li, L.; Li, C.; Li, M.; Amini, F.; Zhang, H. Factors affecting improvement of engineering properties of MICP-treated soil catalyzed by bacteria and urease. J. Mater. Civ. Eng. 2014, 26, 04014094. [Google Scholar] [CrossRef]
- Nafisi, A.; Safavizadeh, S.; Montoya, B.M. Influence of microbe and enzyme-induced treatments on cemented sand shear response. J. Geotech. Geoenviron. Eng. 2019, 145, 06019008. [Google Scholar] [CrossRef]
- Fragaszy, R.J.; Santamarina, J.C.; Amekudzi, A.; Assimaki, D.; Bachus, R.; Burns, S.E.; Tsouris, C. Sustainable development and energy geotechnology—Potential roles for geotechnical engineering. KSCE J. Civ. Eng. 2011, 15, 611–621. [Google Scholar] [CrossRef]
- Fragaszy, R.J.; Santamarina, J.C.; Amekudzi, A.; Assimaki, D.; Bachus, R.; Burns, S.E.; Tsouris, C. Strengthening of soft marine clay using bioencapsulation. Mar. GeoresourcesGeotechnol. 2015, 33, 320–324. [Google Scholar]
- Nemati, M.; Voordouw, G. Modification of porous media permeability, using calcium carbonate produced enzymatically in situ. Enzym. Microb. Technol. 2003, 33, 635–642. [Google Scholar] [CrossRef]
- Al-Thawadi, S. High strength in-situ biocementation of soil by calcite precipitating locally isolated ureolytic bacteria. Ph.D. Thesis, Murdoch University, Perth, Australia, 2008. [Google Scholar]
- Yasuhara, H.; Hayashi, K.; Okamura, M. Evolution in mechanical and hydraulic properties of calcite-cemented sand mediated by biocatalyst. Geo-Front. 2011 Adv. Geotech. Eng. 2011, 3984–3992. [Google Scholar] [CrossRef]
- Putra, H.; Yasuhara, H.; Fauzan, M. Review of enzyme-induced calcite precipitation as a ground-improvement technique. Infrastructures 2020, 5, 66. [Google Scholar] [CrossRef]
- Song, J.Y.; Sim, Y.; Jang, J.; Hong, W.T.; Yun, T.S. Near-surface soil stabilization by enzyme-induced carbonate precipitation for fugitive dust suppression. Acta Geotech. 2019, 1–14. [Google Scholar] [CrossRef]
- Neupane, D.; Yasuhara, H.; Kinoshita, N.; Unno, T. Applicability of enzymatic calcium carbonate precipitation as a soil-strengthening technique. J. Geotech. Geoenviron. Eng. 2013, 139, 2201–2211. [Google Scholar] [CrossRef]
- Zhang, J.; Li, M.; Ke, L.; Yi, J. Distributions of lateral earth pressure behind rock-socketed circular diaphragm walls considering radial deflection. Comput. Geotech. 2022, 143, 104604. [Google Scholar] [CrossRef]
- Wu, L.Y.; MIAOL, C.; Sun, X.H.; Chen, R.F.; Wang, C.C. Experimental study on solidification of sand by calcium carbonate induced by plant urease. Chin. J. Geotech. Eng. 2020, 42, 714–720. [Google Scholar]
- Khodadadi, T.H.; Kavazanjian, E.; Bilsel, H. Mineralogy of calcium carbonate in MICP-Treated soil using soaking and injection treatment methods. Geotech. Front. 2017, 2017, 195–201. [Google Scholar]
- Hoang, T.; Alleman, J.; Cetin, B.; Ikuma, K.; Choi, S.-G. Sand and silty-sand soil stabilization using bacterial enzyme–Induced calcite precipitation (BEICP). Can. Geotech. J. 2019, 56, 808–822. [Google Scholar] [CrossRef]
- Lang, C.; Li, M.; Qiu, L.; Zuo, Z. Effect of different legume resource and technical parameters on urea hydrolysis. Glob. J. Eng. Technol. Adv. 2022, 11, 055–062. [Google Scholar] [CrossRef]
- Farrell, H.M.; Kumosinski, T.F.; Malin, E.L.; Brown, E.M. The caseins of milk as calcium-Binding proteins. Calcium-Bind. Protein Protoc. 2002, 97–140. [Google Scholar]
- Almajed, A.; Khodadadi Tirkolaei, H.; Kavazanjian, E., Jr. Baseline investigation on enzyme-Induced calcium carbonate precipitation. J. Geotech. Geoenviron. Eng. 2018, 144, 04018081. [Google Scholar] [CrossRef]
- Yuan, H.; Ren, G.; Liu, K.; Zheng, W.; Zhao, Z. Experimental study of EICP combined with organic materials for silt improvement in the yellow river flood area. Appl. Sci. 2020, 10, 7678. [Google Scholar] [CrossRef]
- Rohy, H.; Arab, M.; Zeiada, W.; Omar, M.; Tahmaz, A. One phase soil bio-Cementation with EICP-Soil mixing. In Proceedings of the 4th World Congress on Civil, Structural, and Environmental Engineering (CSEE’19), Rome, Italy, 7–9 April 2019; pp. 1–8. [Google Scholar]
- Carmona, J.P.S.F.; Oliveira, P.J.V.; Lemos, L.J.L. Biostabilization of a sandy soil using enzymatic calcium carbonate precipitation. Procedia Eng. 2016, 143, 1301–1308. [Google Scholar] [CrossRef] [Green Version]
- Chandra, A.; Ravi, K. Application of Enzyme-Induced Carbonate Precipitation (EICP) to Improve the Shear Strength of Different Type of Soils. In Problematic Soils and Geoenvironmental Concerns; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Chandra, A.; Ravi, K. Effect of magnesium incorporation in Enzyme-Induced Carbonate Precipitation (EICP) to improve shear strength of soil. In Advances in Computer Methods and Geomechanics; Springer: Berlin/Heidelberg, Germany, 2020; pp. 333–346. [Google Scholar]
- Al-Thawadi, S.M. Ureolytic bacteria and calcium carbonate formation as a mechanism of strength enhancement of sand. J. Adv. Sci. Eng. Res. 2011, 1, 98–114. [Google Scholar]
- Refaei, M.; Arab, M.G.; Omar, M. Sandy Soil Improvement through Biopolymer Assisted EICP//Geo-Congress 2020: Foundations, Soil Improvement, and Erosion; American Society of Civil Engineers: Reston, VA, USA, 2020; pp. 612–619. [Google Scholar]
- Almajed, A.; Lemboye, K.; Arab, M.G.; Alnuaim, A. Mitigating wind erosion of sand using biopolymer-assisted EICP technique. Soils Found. 2020, 60, 356–371. [Google Scholar] [CrossRef]
- Gowthaman, S.; Nakashima, K.; Kawasaki, S. Freeze-thaw durability and shear responses of cemented slope soil treated by microbial induced carbonate precipitation. Soils Found. 2020, 60, 840–855. [Google Scholar] [CrossRef]
- Arab, M.G.; Rohy, H.; Zeiada, W.; Arab, M.G.; Rohy, H.; Zeiada, W.; Almajed, A.; Omar, M. One-Phase EICP Biotreatment of Sand Exposed to Various Environmental Conditions. J. Mater. Civ. Eng. 2021, 33, 04020489. [Google Scholar] [CrossRef]
- Dilrukshi, R.A.N.; Watanabe, J.; Kawasaki, S. Sand cementation test using plant-derived urease and calcium phosphate compound. Mater. Trans. 2015, 56, 1565–1572. [Google Scholar] [CrossRef] [Green Version]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Li, M.; Tao, X.; Zhang, S.; He, J.; Zhu, L.; Wen, K. The Effect of Nucleating Agents on Enzyme-Induced Carbonate Precipitation and Corresponding Microscopic Mechanisms. Materials 2022, 15, 5814. https://doi.org/10.3390/ma15175814
Yang Y, Li M, Tao X, Zhang S, He J, Zhu L, Wen K. The Effect of Nucleating Agents on Enzyme-Induced Carbonate Precipitation and Corresponding Microscopic Mechanisms. Materials. 2022; 15(17):5814. https://doi.org/10.3390/ma15175814
Chicago/Turabian StyleYang, Yuanjiang, Mingdong Li, Xueqing Tao, Shiai Zhang, Jia He, Liping Zhu, and Kejun Wen. 2022. "The Effect of Nucleating Agents on Enzyme-Induced Carbonate Precipitation and Corresponding Microscopic Mechanisms" Materials 15, no. 17: 5814. https://doi.org/10.3390/ma15175814
APA StyleYang, Y., Li, M., Tao, X., Zhang, S., He, J., Zhu, L., & Wen, K. (2022). The Effect of Nucleating Agents on Enzyme-Induced Carbonate Precipitation and Corresponding Microscopic Mechanisms. Materials, 15(17), 5814. https://doi.org/10.3390/ma15175814





