Numerical Modeling of Chloride Transport in Concrete under Cyclic Exposure to Chloride
Abstract
:1. Introduction
2. Modelling the Mass Transport in the Cement Matrix
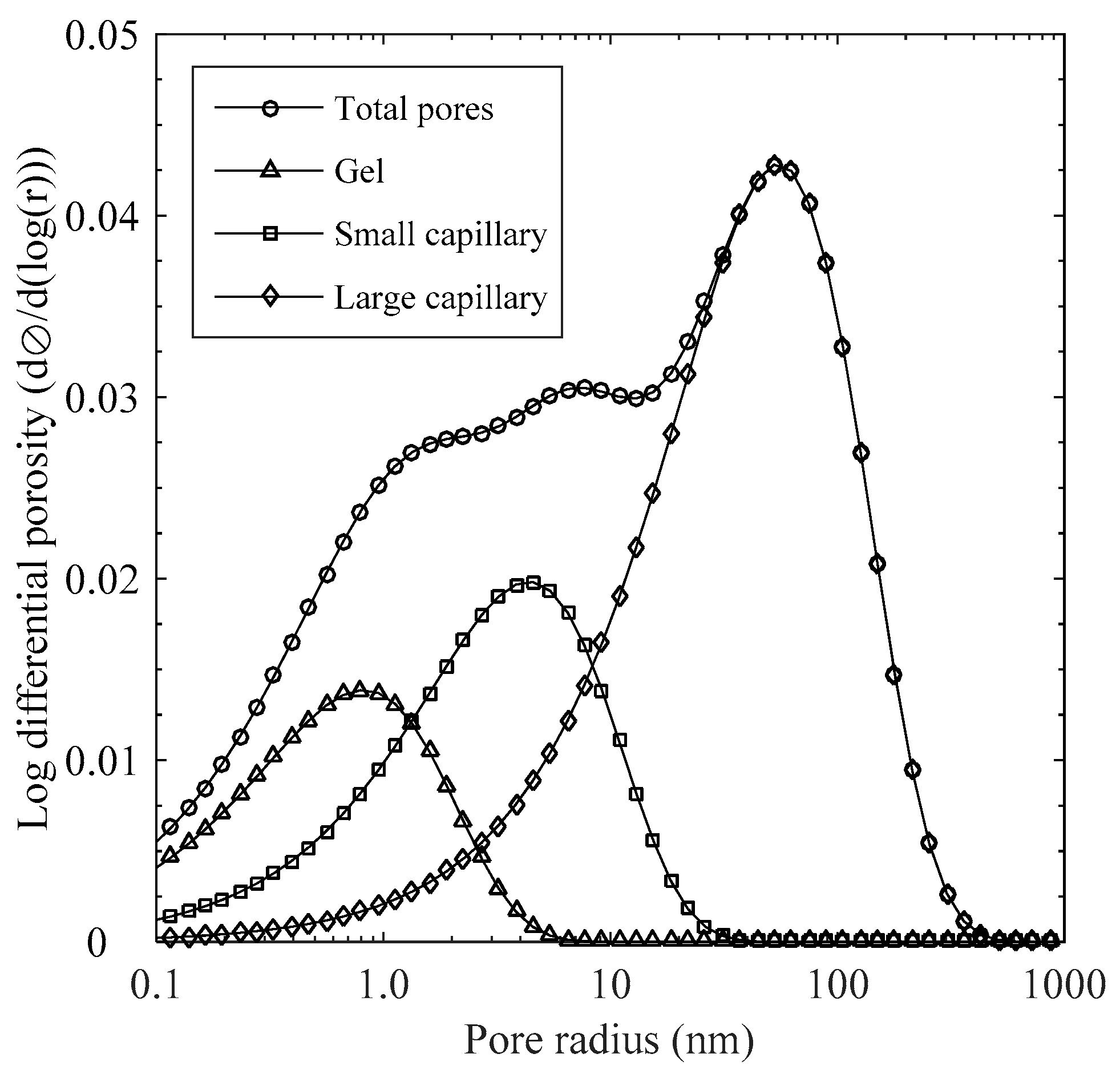
2.1. Pore Size Distribution
2.2. Tortuosity of Pores
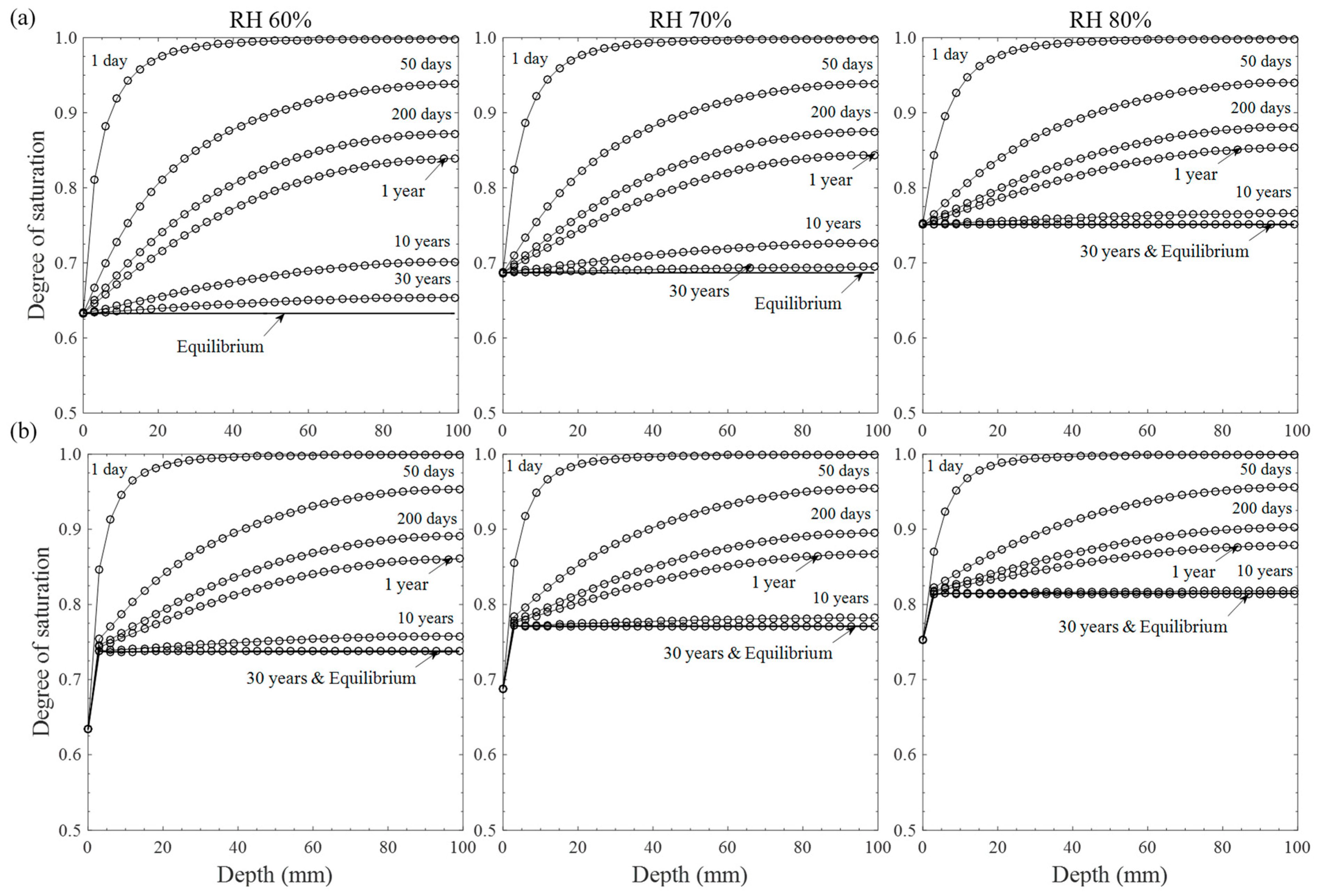
2.3. Moisture Distribution
2.4. Chloride Transport
2.5. Boundary Conditions
2.6. Numerical Strategy
3. Chloride Transport in Non-Saturated Concrete
3.1. Determination of Pore Structure
3.2. Moisture Distribution under Wet/Dry Cycle
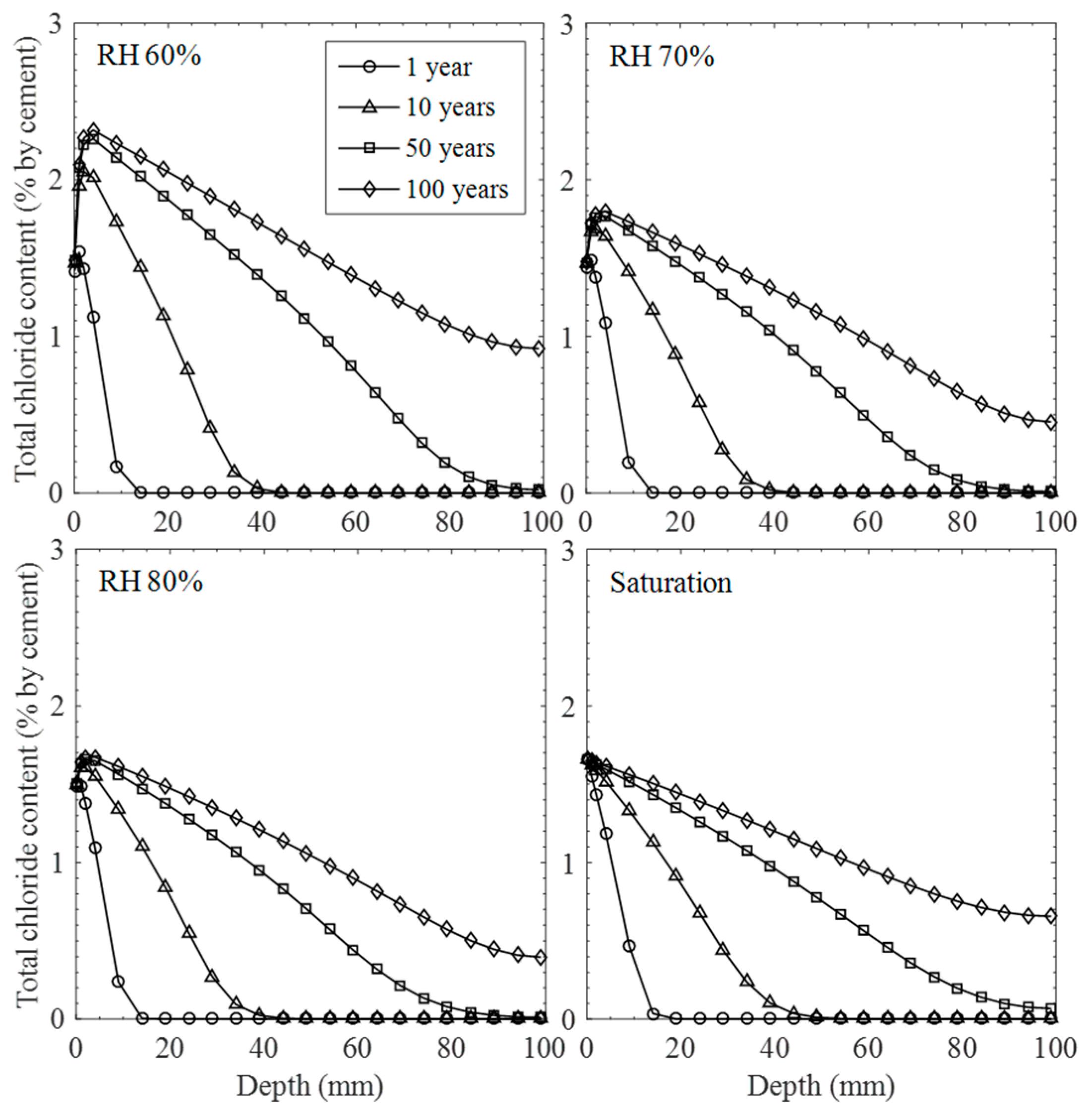
3.3. Influence of Moisture Distribution on Chloride Ingress
3.4. Expression for Chloride Diffusion in Non-Saturated Concrete
4. Validation of Transport Model
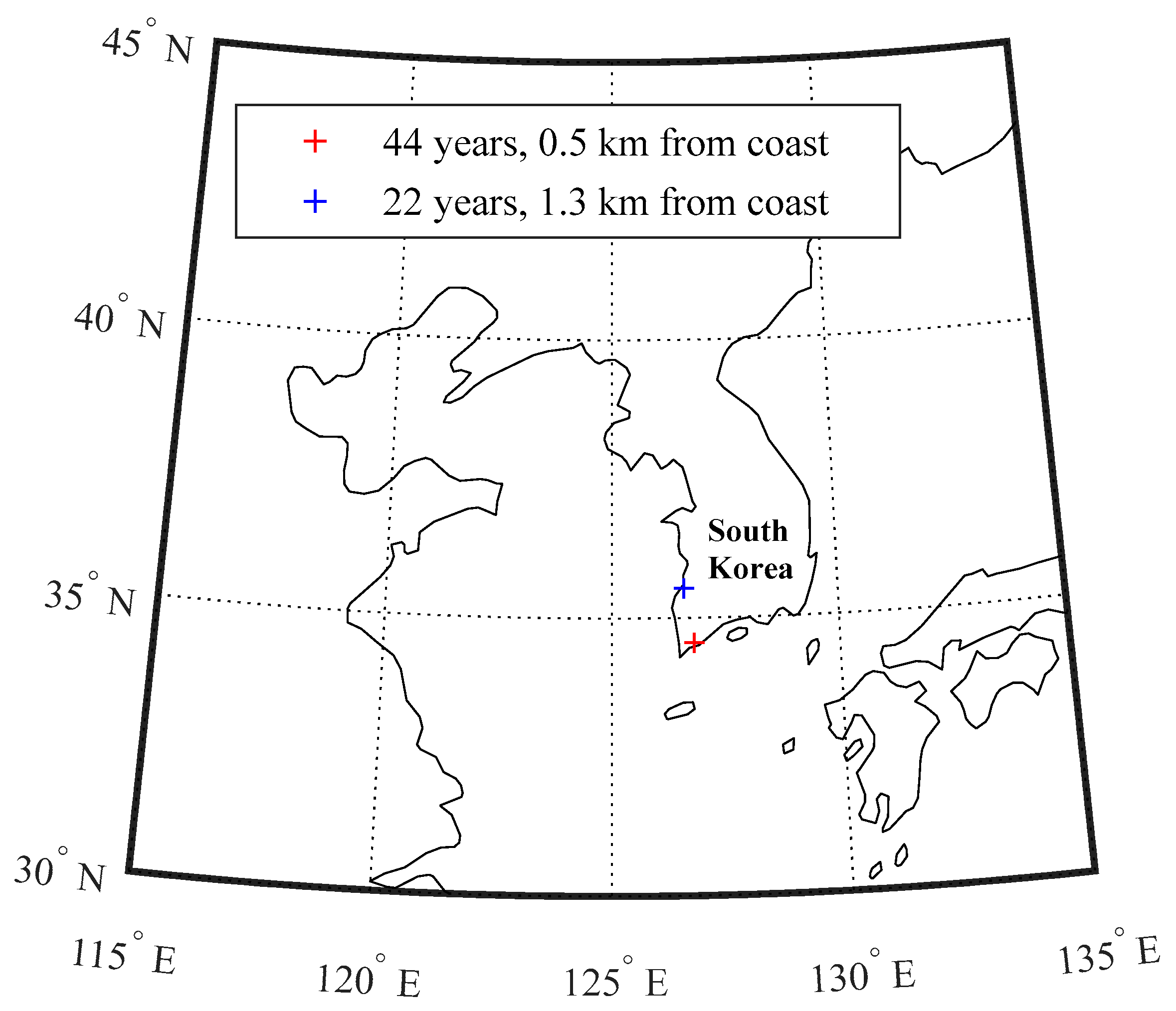
4.1. In Situ Investigation
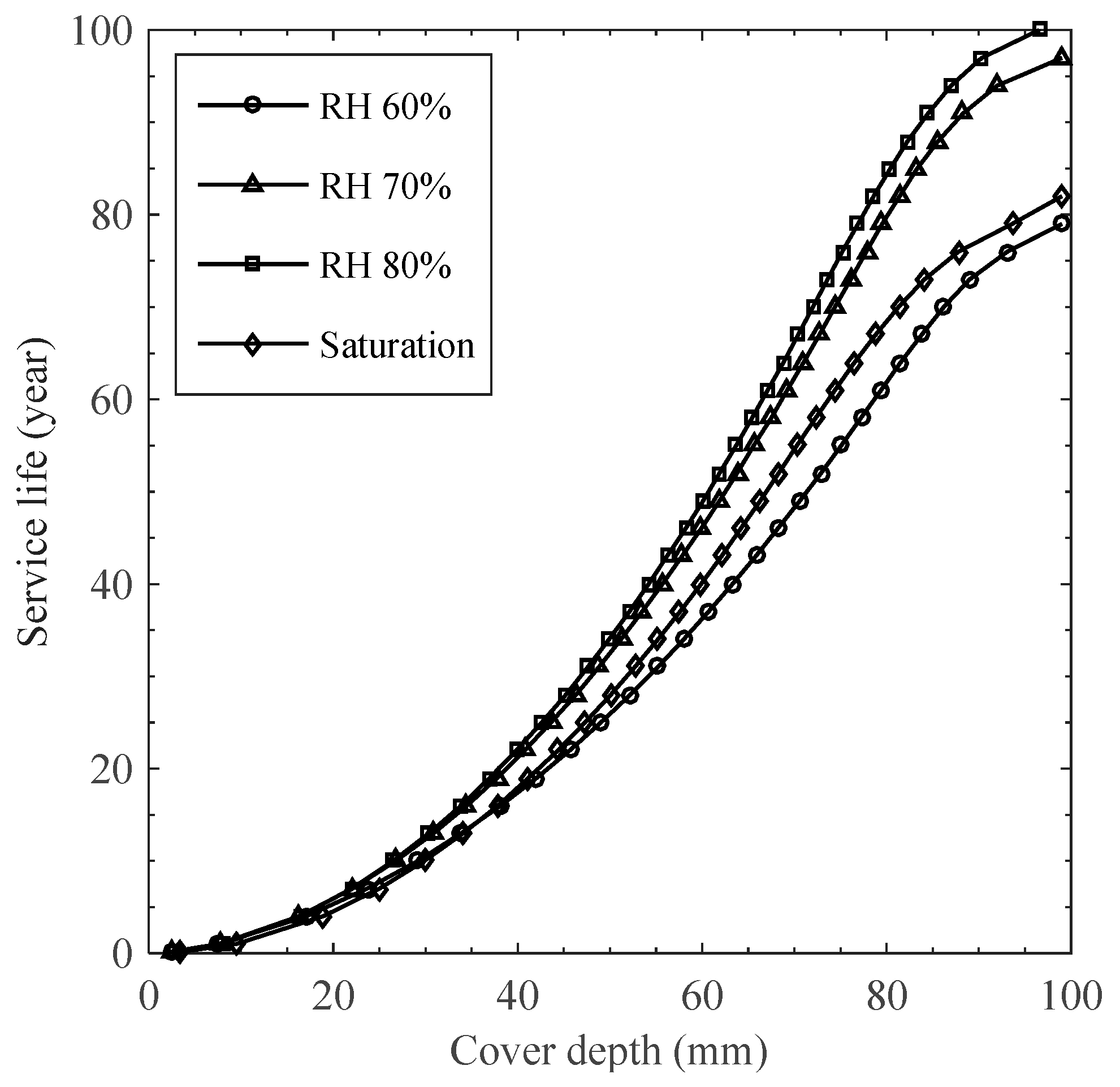
4.2. Simulation of Chloride Profile to In Situ Structure
5. Conclusions
- (1)
- Pore size distribution was determined to provide pore structure parameters for mass transport coefficients. By reconstructing pore size distribution with hysteric behavior between vapor adsorption and desorption, it was found that pore size with 4.0 nm in radius shows maximum connection probability to large pores. By applying the fraction of entrapped water content to the composite medium model, the effective chloride diffusivity covering gel to capillary pores can be derived.
- (2)
- Moisture distributions under constant and cyclic exposure conditions were calculated based on the proposed numerical solution. As a result, moisture tends to evaporate toward the drying surface to reach equilibrium through inner material depth, which is more quickly achieved by cyclic exposure conditions, and the inner moisture level at equilibrium increases with the ambient humidity level.
- (3)
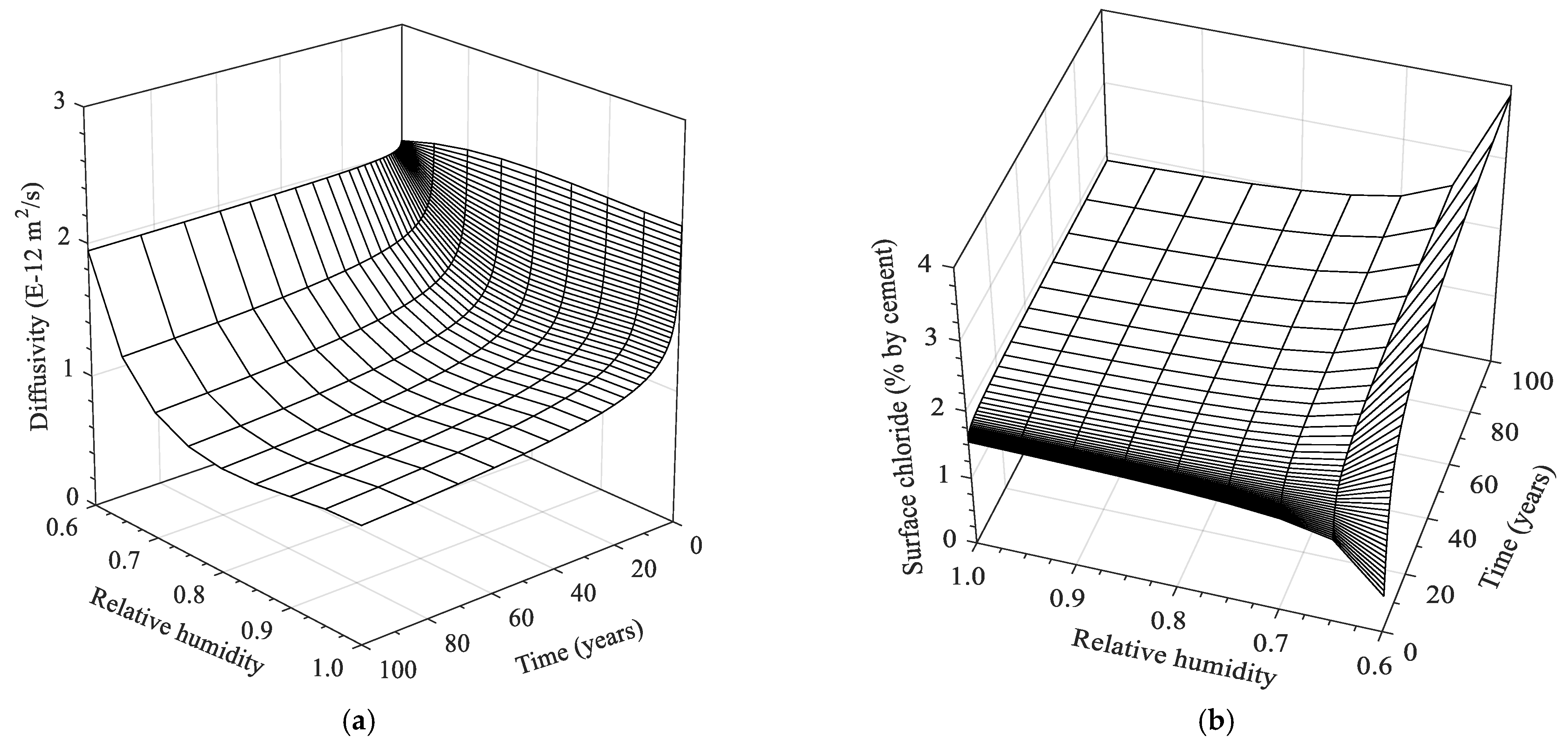
- Under the intermittent exposure of salt solution, the influence of moisture level on the behavior of chloride transport with concentration profiles varying with ambient humidity conditions was assessed. The chloride transport is particularly dominant at a 60% humidity level, showing a higher rate of chloride ingress with time, which also entails a higher risk of corrosion as critical concentration reaches the assumed steel depth. However, the rates of chloride transport at medium humidity levels (e.g., 70 and 80%) are relatively low compared to the saturation one due to the less-significant convection effects and reduced chloride diffusivity.
- (4)
- Analytical solutions of chloride diffusion parameters as functions of time and ambient humidity level were determined. As a result, both chloride diffusivity and surface chloride concentration are significantly dependent on the moisture level, implying that convection is a decisive factor in analyzing chloride diffusion under cyclic environments.
- (5)
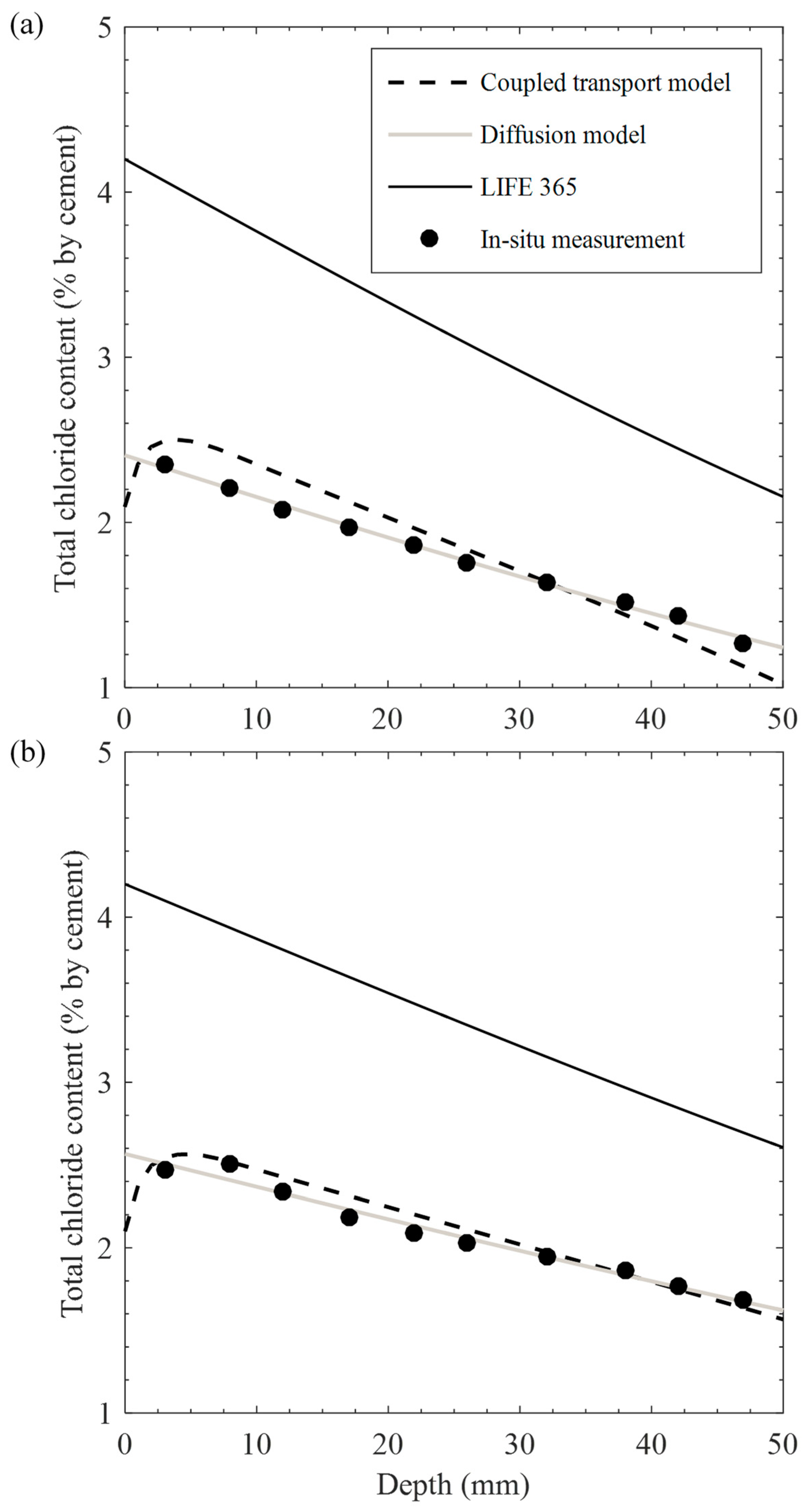
- Chloride profiles obtained from the proposed transport model, analytical solution, and LIFE 365 were compared, indicating that correlations with the in situ data were satisfactory in using the proposed model and highest for the analytical solution with optimized diffusion parameters. The results are far from those for LIFE 365, giving poor correlations. This implies that convection effects on ambient conditions and diffusion properties on concrete pore structure are essential in predicting the service life under cyclic environments.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ann, K.Y.; Song, H.-W. Chloride threshold level for corrosion of steel in concrete. Corros. Sci. 2007, 49, 4113–4133. [Google Scholar] [CrossRef]
- Neville, A. Properties of Concrete, 5th ed.; Pearson Education Limited: Harlow, UK, 2011. [Google Scholar]
- Hu, B.; Zhou, Y.; Xing, F.; Sui, L.; Luo, M. Experimental and theoretical investigation on the hybrid CFRP-ECC flexural strengthening of RC beams with corroded longitudinal reinforcement. Eng. Struct. 2019, 200, 109717. [Google Scholar] [CrossRef]
- Zhou, Y.; Zheng, Y.; Sui, L.; Hu, B.; Huang, X. Study on the Flexural Performance of Hybrid-Reinforced Concrete Beams with a New Cathodic Protection System Subjected to Corrosion. Materials 2020, 13, 234. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Chen, X.; Wang, X.; Sui, L.; Huang, X.; Guo, M.; Hu, B. Seismic performance of large rupture strain FRP retrofitted RC columns with corroded steel reinforcement. Eng. Struct. 2020, 216, 110744. [Google Scholar] [CrossRef]
- Hong, K.; Hooton, R. Effects of cyclic chloride exposure on penetration of concrete cover. Cement Concrete Res. 1999, 29, 1379–1386. [Google Scholar] [CrossRef]
- Chang, H.; Mu, S.; Xie, D.; Wang, P. Influence of pore structure and moisture distribution on chloride “maximum phenomenon” in surface layer of specimens exposed to cyclic drying-wetting condition. Constr. Build. Mater. 2017, 131, 16–30. [Google Scholar] [CrossRef]
- Sun, C.; Yuan, L.; Zhai, X.; Qu, F.; Li, Y.; Hou, B. Numerical and experimental study of moisture and chloride transport in unsaturated concrete. Constr. Build. Mater. 2018, 189, 1067–1075. [Google Scholar] [CrossRef]
- Guo, M.; Zhong, Q.; Zhou, Y.; Hu, B.; Huang, Z.; Yue, Y. Influence of flexural loading and chloride exposure on the fatigue behavior of high-performance lightweight engineered cementitious composites. Constr. Build. Mater. 2020, 249, 118512. [Google Scholar] [CrossRef]
- Saetta, A.; Scotta, R.; Vitaliani, R. Analysis of Chloride Diffusion into Partially Saturated Concrete. ACI Mater. J. 1993, 90, 441–451. [Google Scholar] [CrossRef]
- Martın-Pérez, B. Service Life Modelling of RC Highway Structures Exposed to Chlorides. Ph.D. Thesis, University of Toronto, Toronto, ON, Canada, 1999. [Google Scholar]
- Meijers, S.J.H.; Bijen, J.M.J.M.; de Borst, R.; Fraaij, A.L.A. Computational results of a model for chloride ingress in concrete including convection, drying-wetting cycles and carbonation. Mater. Struct. 2005, 38, 145–154. [Google Scholar] [CrossRef]
- Bastidas-Arteaga, E.; Chateauneuf, A.; Sánchez-Silva, M.; Bressolette, P.; Schoefs, F. A comprehensive probabilistic model of chloride ingress in unsaturated concrete. Eng. Struct. 2011, 33, 720–730. [Google Scholar] [CrossRef]
- Sleiman, H.; Amiri, O.; Ait-Mokhtar, A.; Loche, J.-M. Chloride transport through unsaturated concrete: Chloride profile simulations and experimental validation. Mag. Concr. Res. 2012, 64, 351–359. [Google Scholar] [CrossRef]
- Ranaivomanana, H.; Verdier, J.; Sellier, A.; Bourbon, X. Toward a better comprehension and modeling of hysteresis cycles in the water sorption–desorption process for cement-based materials. Cement Concrete Res. 2011, 41, 817–827. [Google Scholar] [CrossRef]
- Huang, Q.; Jiang, Z.; Gu, X.; Zhang, W.; Guo, B. Numerical simulation of moisture transport in concrete based on a pore size distribution model. Cement Concrete Res. 2015, 67, 31–43. [Google Scholar] [CrossRef]
- Chaube, R.; Kishi, T.; Maekawa, K. Modelling of Concrete Performance: Hydration, Microstructure and Mass Transport; CRC Press: New York, NY, USA, 2005. [Google Scholar] [CrossRef]
- Iqbal, P.O.; Ishida, T. Modeling of chloride transport coupled with enhanced moisture conductivity in concrete exposed to marine environment. Cement Concrete Res. 2009, 39, 329–339. [Google Scholar] [CrossRef]
- Zhang, Y.; Jin, W. Distribution of Chloride Accumulation in Marine Tidal Zone along Altitude. ACI Mater. J. 2011, 108, 1–9. [Google Scholar] [CrossRef]
- Promentilla, M.A.B.; Sugiyama, T.; Hitomi, T.; Takeda, N. Quantification of tortuosity in hardened cement pastes using synchrotron-based X-ray computed microtomography. Cement Concrete Res. 2009, 39, 548–557. [Google Scholar] [CrossRef]
- Nielsen, E.P.; Geiker, M.R. Chloride diffusion in partially saturated cementitious material. Cement Concrete Res. 2003, 33, 133–138. [Google Scholar] [CrossRef]
- Climent, M.; de Vera, G.; López, J.F.; Viqueira, E.; Andrade, C. A test method for measuring chloride diffusion coefficients through nonsaturated concrete: Part I. The instantaneous plane source diffusion case. Cement Concrete Res. 2002, 32, 1113–1123. [Google Scholar] [CrossRef]
- de Vera, G.; Climent, M.A.; Viqueira, E.; Antón, C.; Andrade, C. A test method for measuring chloride diffusion coefficients through partially saturated concrete. Part II: The instantaneous plane source diffusion case with chloride binding consideration. Cement Concrete Res. 2007, 37, 714–724. [Google Scholar] [CrossRef]
- Olsson, N.; Baroghel-Bouny, V.; Nilsson, L.-O.; Thiery, M. Non-saturated ion diffusion in concrete—A new approach to evaluate conductivity measurements. Cement Concrete Compos. 2013, 40, 40–47. [Google Scholar] [CrossRef]
- Mindess, S.; Young, F.; Darwin, D. Concrete, 2nd ed.; Pearson Education, Inc.: Upper Saddle River, NJ, USA, 2003. [Google Scholar]
- Park, K.-B.; Noguchi, T.; Plawsky, J. Modeling of hydration reactions using neural networks to predict the average properties of cement paste. Cement Concrete Res. 2005, 35, 1676–1684. [Google Scholar] [CrossRef]
- Maruyama, I.; Matsushita, T.; Noguchi, T. Numerical modeling of Portland cement hydration. Proc. Int. RILEM Symp. CONMOD 2008, 8, 155–163. [Google Scholar]
- Fan, W.-J.; Wang, X.-Y.; Park, K.-B. Evaluation of the Chemical and Mechanical Properties of Hardening High-Calcium Fly Ash Blended Concrete. Materials 2015, 8, 5933–5952. [Google Scholar] [CrossRef] [PubMed]
- Hansen, T.C. Physical structure of hardened cement paste. A classical approach. Mater. Struct. 1986, 19, 423–436. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Negishi, K.; Hoshino, S.; Tanaka, T. Modeling of diffusive mass transport in micropores in cement-based materials. Cement Concrete Res. 2009, 39, 1149–1155. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Lee, H.-S. A model for predicting the carbonation depth of concrete containing low-calcium fly ash. Constr. Build. Mater. 2009, 23, 725–733. [Google Scholar] [CrossRef]
- Garboczi, E.J.; Bentz, D.P. Computer simulation of the diffusivity of cement-based materials. J. Mater. Sci. 1992, 27, 2083–2092. [Google Scholar] [CrossRef]
- El-Dieb, A.; Hooton, R.D. Evaluation of the Katz-Thompson model for estimating the water permeability of cement-based materials from mercury intrusion porosimetry data. Cement Concrete Res. 1994, 24, 443–455. [Google Scholar] [CrossRef]
- Quenard, D.; Sallee, H. Water vapour adsorption and transfer in cement-based materials: A network simulation. Mater. Struct. 1992, 25, 515–522. [Google Scholar] [CrossRef]
- Song, H.W.; Lee, C.H.; Jung, M.S.; Ann, K.Y. Development of chloride binding capacity in cement pastes and influence of the pH of hydration products. Can. J. Civ. Eng. 2008, 35, 1427–1434. [Google Scholar] [CrossRef]
- Bejaoui, S.; Bary, B. Modeling of the link between microstructure and effective diffusivity of cement pastes using a simplified composite model. Cement Concrete Res. 2007, 37, 469–480. [Google Scholar] [CrossRef]
- Akita, H.; Fujiwara, T.; Ozaka, Y. A practical procedure for the analysis of moisture transfer within concrete due to drying. Mag. Concr. Res. 1997, 49, 129–137. [Google Scholar] [CrossRef]
- Ishida, T.; Iqbal, P.O.; Anh, H.T.L. Modeling of chloride diffusivity coupled with non-linear binding capacity in sound and cracked concrete. Cement Concrete Res. 2009, 39, 913–923. [Google Scholar] [CrossRef]
- Aldred, J.; Rangan, B.; Buenfeld, N. Effect of initial moisture content on wick action through concrete. Cement Concrete Res. 2004, 34, 907–912. [Google Scholar] [CrossRef]
- Puyate, Y.; Lawrence, C. Effect of solute parameters on wick action in concrete. Chem. Eng. Sci. 1999, 54, 4257–4265. [Google Scholar] [CrossRef]
- Li, K.; Li, C.; Chen, Z. Influential depth of moisture transport in concrete subject to drying–wetting cycles. Cement Concrete Compos. 2009, 31, 693–698. [Google Scholar] [CrossRef]
- BS8110; Structural Use of Concrete. British Standards Institution: London, UK, 1986.
- da Costa, A.; Fenaux, M.; Fernández, J.; Sánchez, E.; Moragues, A. Modelling of chloride penetration into non-saturated concrete: Case study application for real marine offshore structures. Constr. Build. Mater. 2013, 43, 217–224. [Google Scholar] [CrossRef]
- Pack, S.-W.; Jung, M.S.; Song, H.-W.; Kim, S.-H.; Ann, K.Y. Prediction of time dependent chloride transport in concrete structures exposed to a marine environment. Cement Concrete Res. 2010, 40, 302–312. [Google Scholar] [CrossRef]
- Thomas, M.D.A.; Bamforth, P.B. Modelling chloride diffusion in concrete: Effect of fly ash and slag. Cement Concrete Res. 1999, 29, 487–495. [Google Scholar] [CrossRef]
- Moradllo, M.K.; Shekarchi, M.; Hoseini, M. Time-dependent performance of concrete surface coatings in tidal zone of marine environment. Constr. Build. Mater. 2012, 30, 198–205. [Google Scholar] [CrossRef]
- Glass, G.K.; Buenfeld, N.R. The influence of chloride binding on the chloride induced corrosion risk in reinforced concrete. Corros. Sci. 2000, 42, 329–344. [Google Scholar] [CrossRef]
- Ben Fraj, A.; Bonnet, S.; Khelidj, A. New approach for coupled chloride/moisture transport in non-saturated concrete with and without slag. Constr. Build. Mater. 2012, 35, 761–771. [Google Scholar] [CrossRef]
- Song, H.W.; Lee, C.H.; Ann, K.Y. Factors influencing chloride transport in concrete structures exposed to marine environments. Cement Concrete Compos. 2008, 30, 113–121. [Google Scholar] [CrossRef]
- Ehlen, M.; Thomas, M.; Bentz, E. Life-365 service life prediction modelTM version 2.0. Concr. Int. 2009, 31, 41–46. [Google Scholar]
- Zhang, Y.; Zhang, M. Transport properties in unsaturated cement-based materials—A review. Constr. Build. Mater. 2014, 72, 367–379. [Google Scholar] [CrossRef]

| Property | Notation | Gel Pores | Capillary Pores | |
|---|---|---|---|---|
| i = 1 | i = 2 (Small) | i = 3 (Large) | ||
| Pore distribution | 0.03 | 0.05 | 0.12 | |
| (nm) | 0.75 | 3.99 | 50.47 | |
| Tortuosity | - | 0.26 | ||
| 8.94 | ||||
| Effective pores | 0.47 | |||
| (m2/s) | 2.09 × 10−12 | |||
| Parameter | Relative Humidity at External Condition (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 60 | 65 | 70 | 75 | 80 | 85 | 90 | 95 | 100 | |
| MSE (%2) | 0.0759 | 0.0274 | 0.0180 | 0.0143 | 0.0123 | 0.0110 | 0.0101 | 0.0092 | 0.0083 |
| m | 0.4780 | 0.2032 | 0.1209 | 0.0840 | 0.0646 | 0.0538 | 0.0479 | 0.0442 | 0.0427 |
| k (%/ln(yr)) | 0.0055 | 0.0883 | 0.1239 | 0.1407 | 0.1483 | 0.1508 | 0.1493 | 0.1445 | 0.1331 |
| Temperature (°C) | Humidity (%) | Tide Level (m) | |
|---|---|---|---|
| Minimum | 5.5 | 24 | 3.5 |
| Maximum | 28.9 | 97 | 12.0 |
| Input | 20.0 | 70 | 7.7 |
| Property | Notation | Gel Pores | Capillary Pores | |
|---|---|---|---|---|
| i = 1 | i = 2 (Small) | i = 3 (Large) | ||
| Pore structure | 0.09 | 0.01 | 0.09 | |
| (nm) | 0.75 | 3.91 | 51.23 | |
| - | 0.37 | |||
| 6.22 | ||||
| (m2/s) | 3.28 × 10−12 | |||
| Chloride binding | α | 1.15 | ||
| β | 0.28 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.-M.; Hong, S.I.; Yang, H.J.; Jung, D.-H. Numerical Modeling of Chloride Transport in Concrete under Cyclic Exposure to Chloride. Materials 2022, 15, 5966. https://doi.org/10.3390/ma15175966
Lee J-M, Hong SI, Yang HJ, Jung D-H. Numerical Modeling of Chloride Transport in Concrete under Cyclic Exposure to Chloride. Materials. 2022; 15(17):5966. https://doi.org/10.3390/ma15175966
Chicago/Turabian StyleLee, Jae-Min, Sung In Hong, Hee Jun Yang, and Dong-Hyuk Jung. 2022. "Numerical Modeling of Chloride Transport in Concrete under Cyclic Exposure to Chloride" Materials 15, no. 17: 5966. https://doi.org/10.3390/ma15175966





