Phosphogypsum-Based Ultra-Low Basicity Cementing Material
Abstract
:1. Introduction
2. Experimental Materials and Methods
2.1. Experimental Materials
2.2. Experimental Methods
3. Experimental Results and Discussion
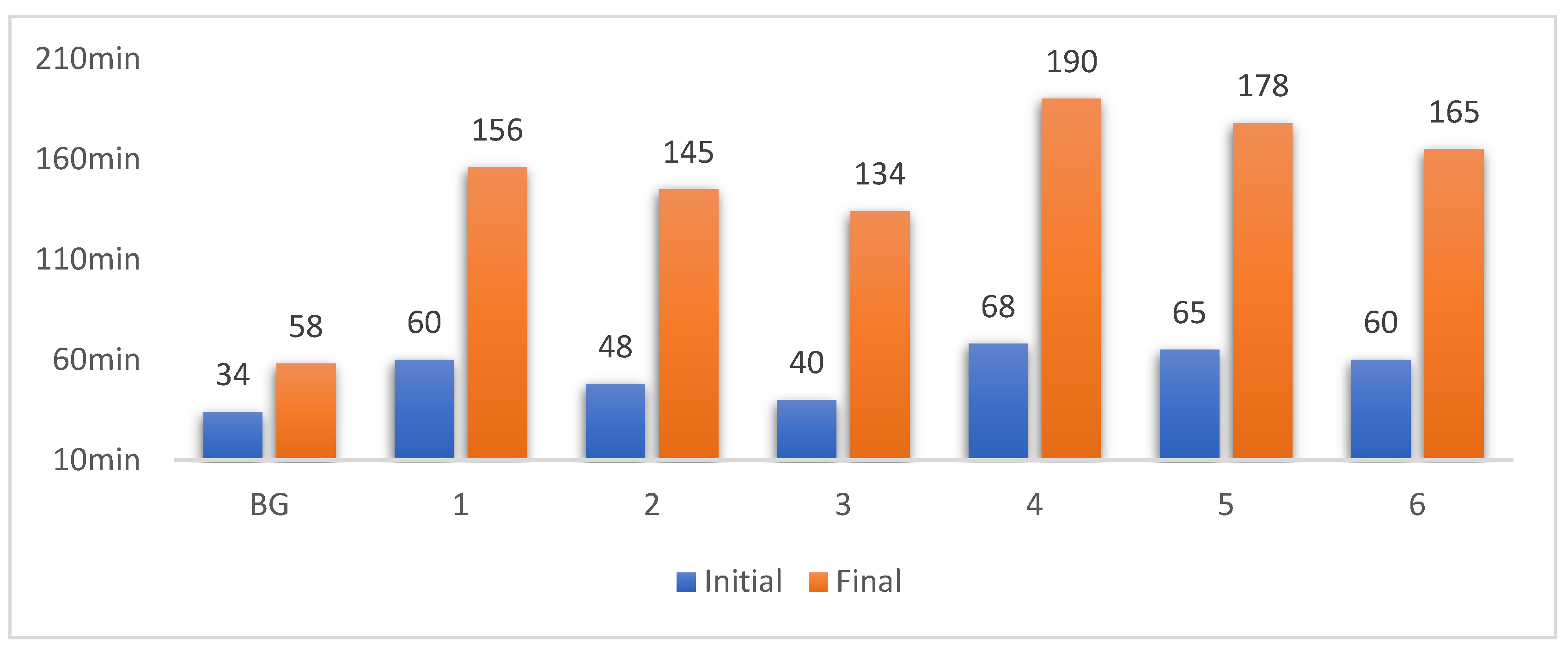
3.1. Setting Time of the Slurry
3.2. Mortar Strength Test
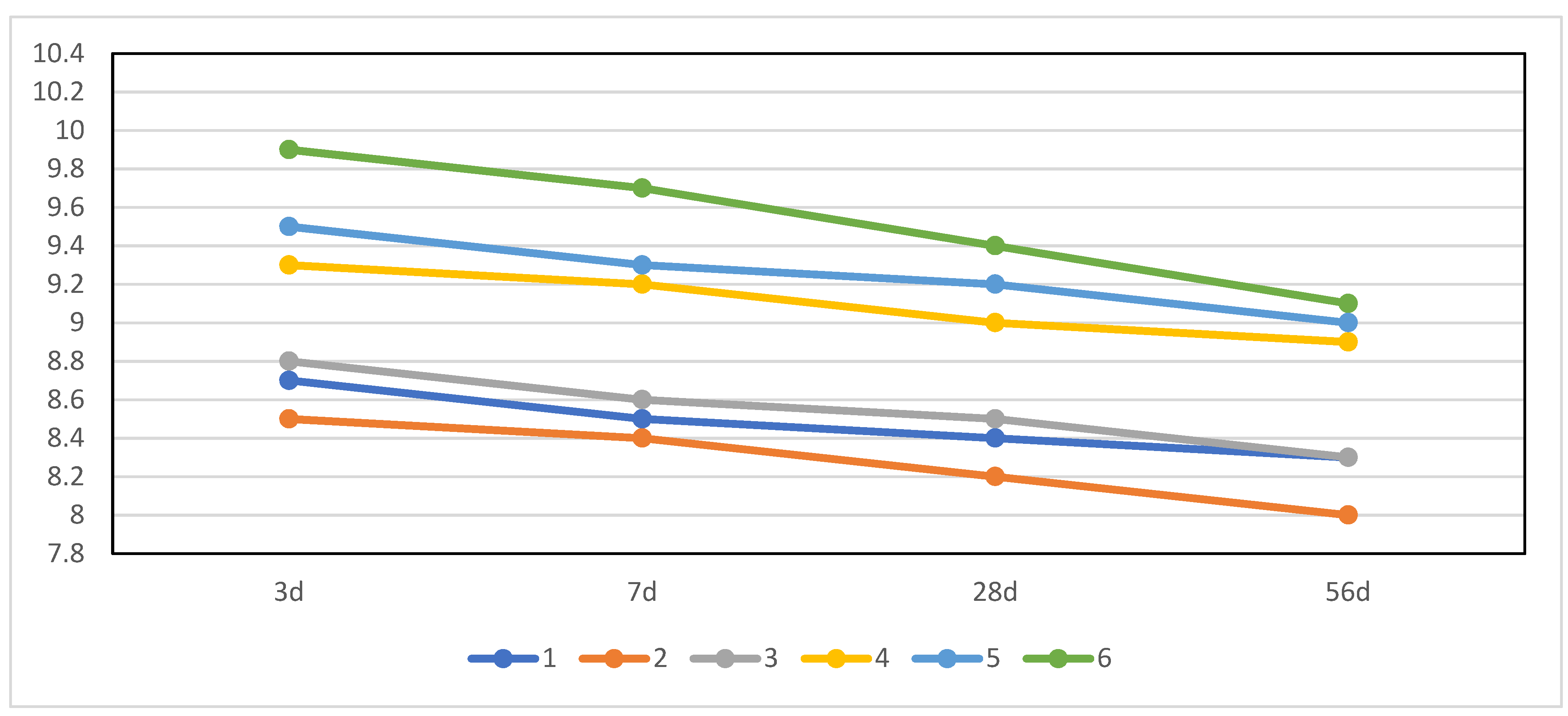
3.3. Pore Solution pH Value
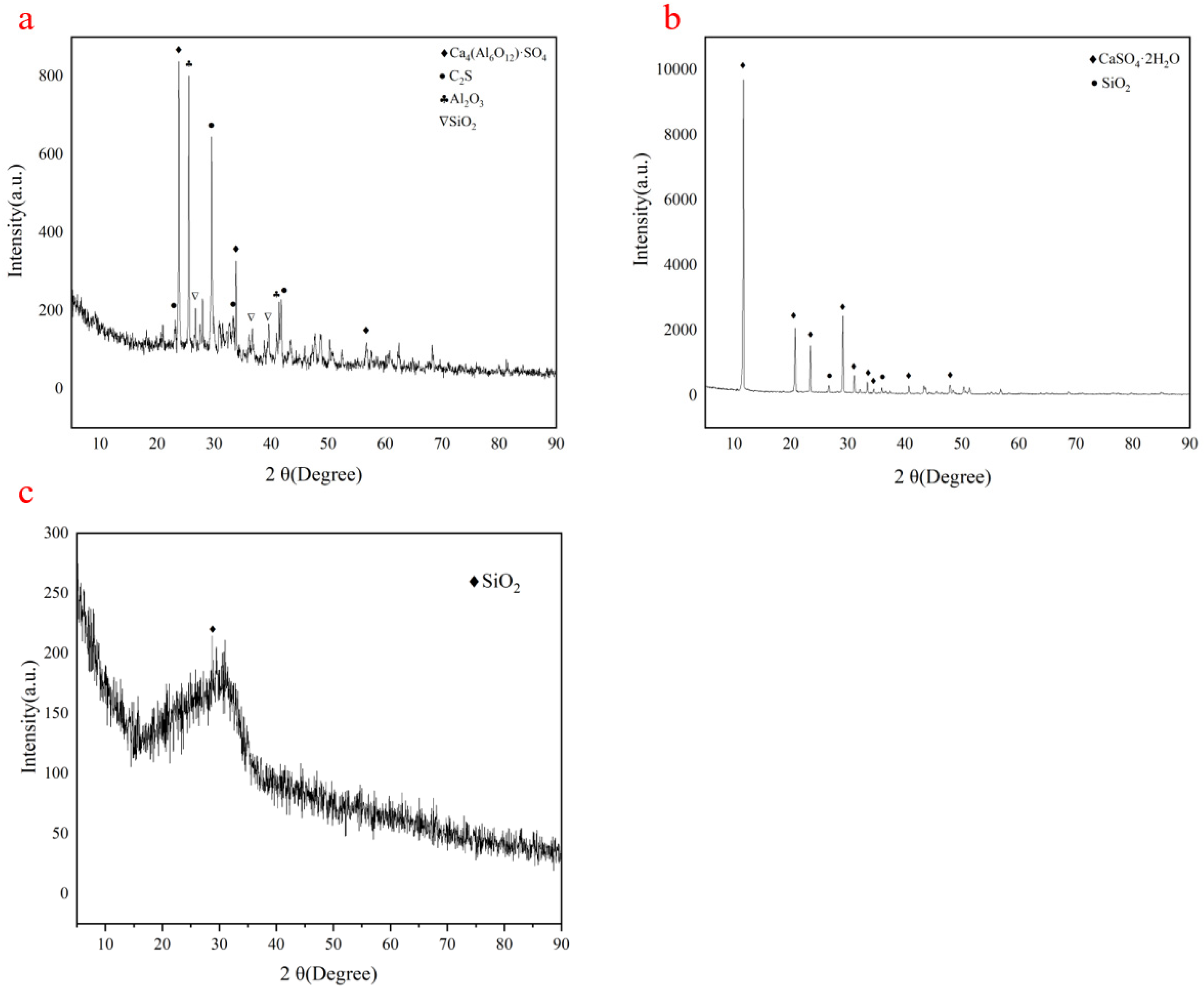
3.4. XRD Analysis of Hydration Products
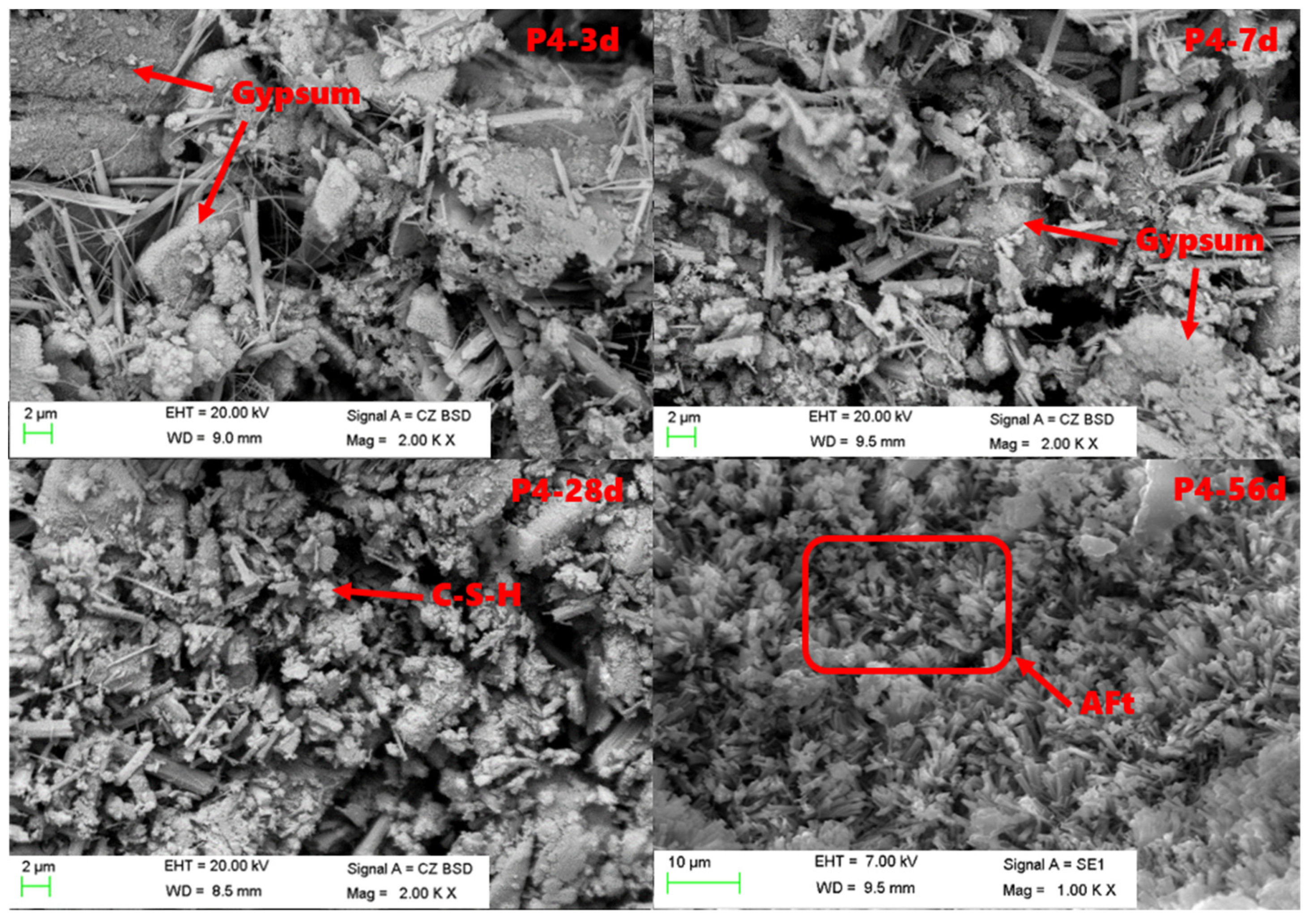
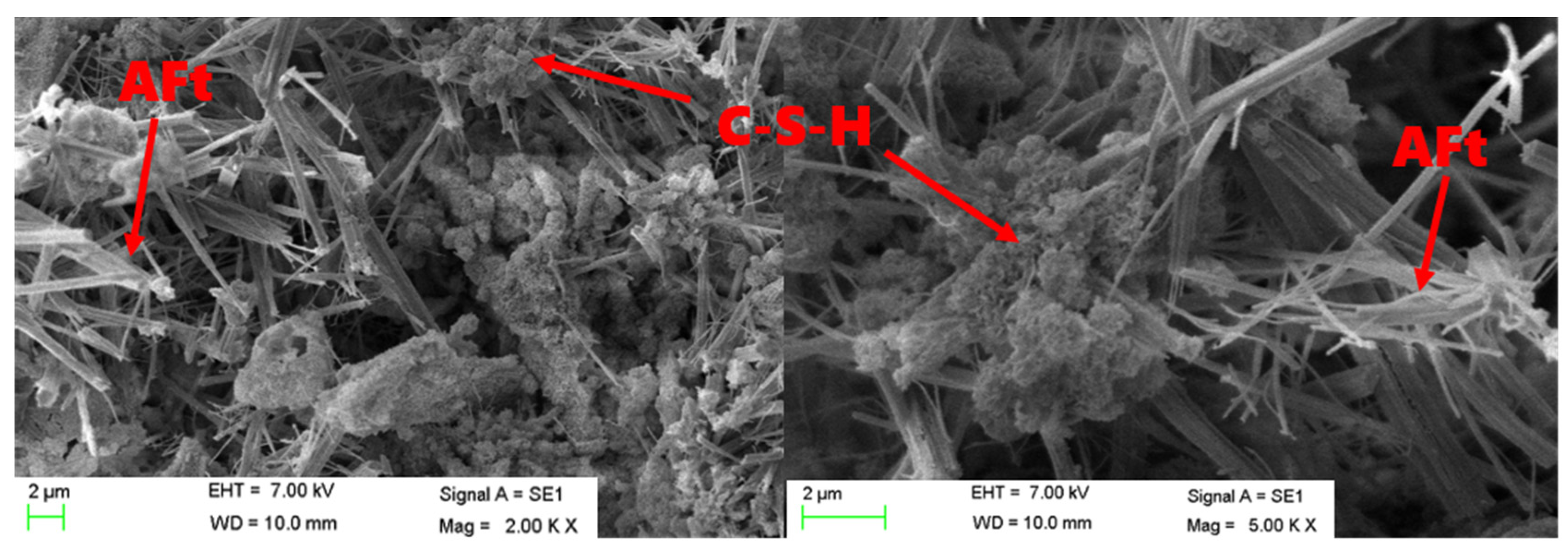
3.5. SEM Analysis of Hydration Products
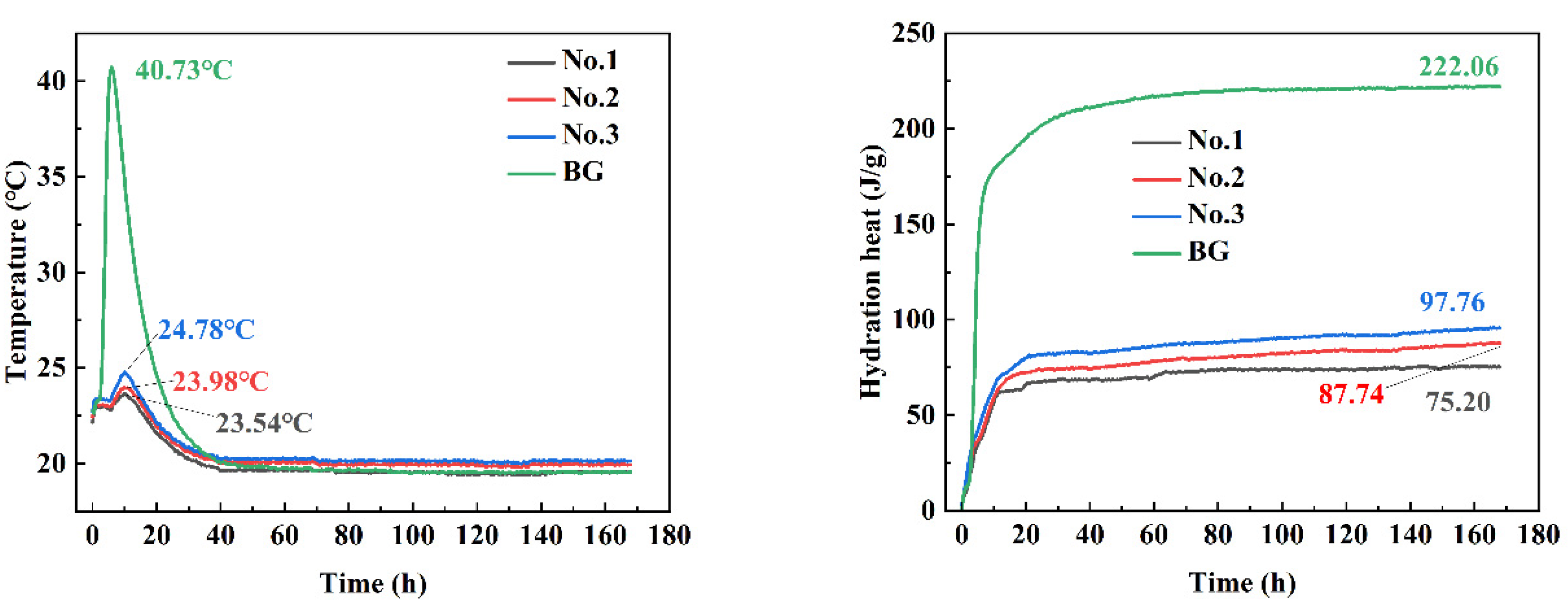
3.6. Heat of Hydration Analysis
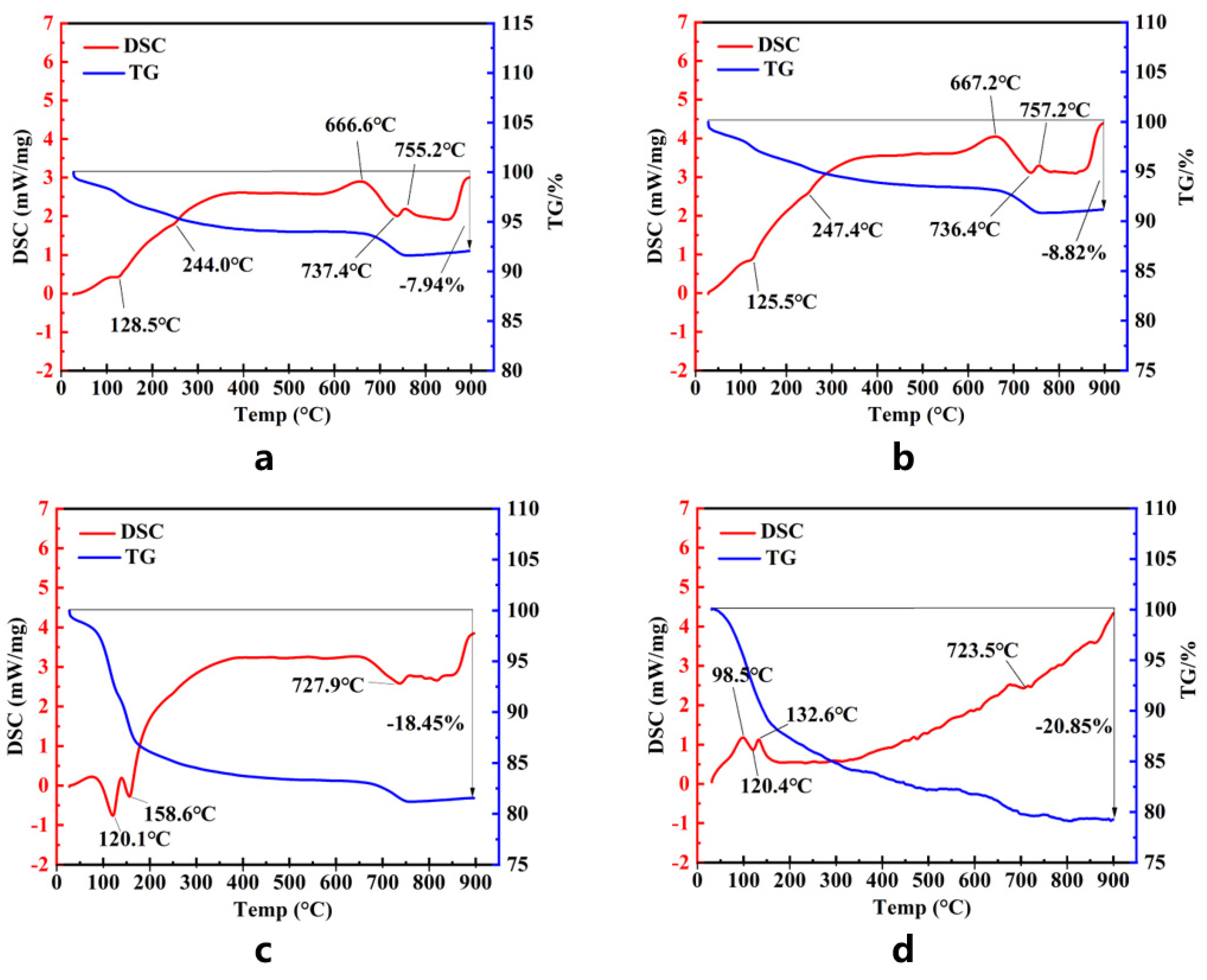
3.7. TG-DSC Analysis
3.8. Toxicity Leaching Test Analysis
4. Reaction Mechanism Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, R.; Wang, S.; Shu, G.; Wei, J.; Yu, Q. Study on the modification method of alkali environment in the pore of vegetation-type ecological concrete. New Build. Mater. 2014, 41, 11–14. [Google Scholar]
- Liang, K.; Lin, Q.; Sun, G. Research Status of Low Alkalinity Cement-Based Gel Materials Science and Technology Innovation and Application. 2014. Available online: https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&dbname=CJFD2014&filename=CXYY201426240&uniplatform=NZKPT&v=2hofb7lVC2eZU-uMcZfG4xjZSr0uEGP6De37F4Kmx_ZQvy-4tcVVp4DHuMIZ6XCV (accessed on 18 September 2014).
- Liu, Z. Research on Low Basicity Cementing Material of Eco-Concrete; Nanjing Institute of Hydraulic Research: Nanjing, China, 2004. [Google Scholar]
- Yu, X.; Liu, X.; Guo, J. Saline-alkali land improvement and landscaping construction technology. J. Eng. Technol. Res. 2020, 5, 58–59. [Google Scholar]
- Du, D. Saline Conditions. Study of Effects of Plant Rhizosphere Bacteria Grows. Master’s Thesis, Shandong Agricultural University, Tai’an, China, 2020. [Google Scholar]
- Xu, W.; Liu, H.; Xu, D.; Lian, X.; Liang, Y.; Hong, J. Research on suitable basicity of eco-fish reef concrete. Concr. J. World 2020, 71–73. [Google Scholar]
- Gao, T. Research on Alkali Reduction Technology of Ecological Porous Concrete; Central South University of Forestry and Technology: Changsha, China, 2017. [Google Scholar]
- Gao, T.; Yin, J.; Sang, Z.; Hu, X.; Li, S. Research progress of eco-concrete. J. Shang Qiu Norm. Univ. 2017, 33, 46–50. [Google Scholar]
- Zhang, L.; Guo, L.; Li, W. Effect of mineral admixtures on basicity and strength of cement pore solution and its mechanism. China Min. 2020, 29, 141–146. [Google Scholar]
- Mousavi, M.A.; Sadeghi-Nik, A.; Bahari, A.; Ashour, A.; Khayat, K.H. Cement Paste Modified by Nano-Montmorillonite and Carbon Nanotubes. ACI Mater. J. 2022, 119, 173–185. [Google Scholar]
- Onyelowe, K.C.; Kontoni, D.N.; Ebid, A.M.; Dabbaghi, F.; Soleymani, A.; Jahangir, H.; Nehdi, M.L. Multi-Objective Optimization of Sustainable Concrete Containing Fly Ash Based on Environmental and Mechanical Considerations. Buildings 2022, 12, 948. [Google Scholar] [CrossRef]
- Mousavi, M.A.; Sadeghi-Nik, A.; Bahari, A.; Jin, C.; Ahmed, R.; Ozbakkaloglu, T.; de Brito, J. Strength optimization of cementitious composites reinforced by carbon nanotubes and Titania nanoparticles. Constr. Build. Mater. 2021, 303, 124510. [Google Scholar] [CrossRef]
- Bahari, A.; Sadeghi-Nik, A.; Cerro-Prada, E.; Sadeghi-Nik, A.; Roodbari, M.; Zhuge, Y. One-step random-walk process of nanoparticles in cement-based materials. J. Cent. South Univ. 2021, 28, 1679–1691. [Google Scholar] [CrossRef]
- Malhotra, V.M.; Mehta, P.K. High Performance, High Volume Fly Ash Concrete: Materials, Mixture Proportioning, Properties, Construction Practice, and Case Histories; Marquardt Printing: Ottawa, ON, Canada, 2002. [Google Scholar]
- Wang, J.; Xiao, J.; Chen, L.; Zhao, J. Effect of fly ash on cement hydration and strength. Compr. Use Fly Ash 2009, 5, 34–36. [Google Scholar]
- Li, W.; Su, S.; Yue, S. Study on the influence of fly ash content on the strength, alkalinity, and carbonization resistance of high-performance concrete. Coal Eng. 2005, 37, 53–55. [Google Scholar]
- Calvo, J.L.G.; Hidalgo, A. Development of low-pH cementitious materials for HL RW repositories: Resistance against ground waters aggression. Cem. Concr. Res. 2010, 40, 1290–1297. [Google Scholar] [CrossRef]
- Kim, H.; Son, H.M.; Lee, H.K. Review on recent advances in securing the long-term durability of calcium aluminate cement (CAC)-based systems. Funct. Compos. Struct. 2021, 3, 035002. [Google Scholar] [CrossRef]
- Chang, Y. Study on Water Resistance and Steel Corrosion Resistance of Magnesium Phosphate Cement. Master’s Thesis, Hunan University, Changsha, China, 2014. [Google Scholar]
- Xie, R.; Wu, Y.; Wang, X.; Yang, K.; Wang, T. Phosphogypsum leach liquor on the acute toxicity of zebrafish and oxidative stress damage. J. Environ. Sci. 2021, 9, 1101–1110. [Google Scholar]
- Guerrero, J.L.; Gutiérrez-Álvarez, I.; Hierro, A.; Pérez-Moreno, S.M.; Olías, M.; Bolívar, J.P. Seasonal evolution of natural radionuclides in two rivers affected by acid mine drainage and phosphogypsum pollution. Catena 2021, 197, 104978. [Google Scholar] [CrossRef]
- Chen, A.N.; He, K. Overall promotion of phosphogypsum pollution prevention and comprehensive utilization. China Environment News, 15 September 2021; p.3. [Google Scholar]
- Zhang, L.-Z.; Zhang, Y.-X.; Zhang, X.-F.; Tan, X.-M.; Lu, Z.-H. Advances in the comprehensive utilization of phosphogypsum resources in China. Prot. Util. Miner. Resour. 2019, 39, 14–18+92. [Google Scholar]
- Ye, X. Utilization status and situation analysis of phosphogypsum in China in 2019. Phosphate Fertil. Compd. Fertil. 2020, 35, 1–3. [Google Scholar]
- Du, B.X.; Huang, K. The Research on Comprehensive Utilization of Phosphogypsum. Adv. Mater. Res. 2014, 850, 1368–1371. [Google Scholar] [CrossRef]
- Ma, G.; Yang, P. Analysis of comprehensive treatment of phosphogypsum. Chem. Fertil. Des. 2018, 56, 42–45. [Google Scholar]
- Guo, Y.; Zhao, Z.; Zhang, Z.; Yan, Y.; Luo, H. Discussion on comprehensive utilization of phosphogypsum. China Non-Met. Miner. Ind. Guide 2021, 4–7. [Google Scholar]
- Li, M. Study on Influencing Factors of Phosphogypsum Quality and Resource Utilization of Building Materials. Ph.D. Thesis, Chongqing University, Chongqing, China, 2012. [Google Scholar]
- Hu, W. Modified Phosphorus Gypsum as Cement Retarder Research. Master’s Thesis, Xi’an Building University of Science and Technology, Xi’an, China, 2019. [Google Scholar]
- Tang, Y.H. Application of industrial by-product gypsum (phosphogypsum) as a retarder in cement. Jiangsu Build. Mater. 2021, 37–39. [Google Scholar]
- Shen, W.; Gan, G.; Dong, R.; Chen, H.; Tan, Y.; Zhou, M. Utilization of solidified phosphogypsum as Portland cement retarder. J. Mater. Cycles Waste Manag. 2012, 14, 228–233. [Google Scholar] [CrossRef]
- Calderón-Morales, B.R.; García-Martínez, A.; Pineda, P.; García-Tenório, R. Valorization of phosphogypsum in cement-based materials: Limits and potential in eco-efficient construction. J. Build. Eng. 2021, 44, 102506. [Google Scholar] [CrossRef]
- Ji, L.; Zhao, H. Utilization of industrial by-product gypsum from the perspective of circular economy. Sulfuric Acid Ind. 2021, 1–8. [Google Scholar]
- Wu, Z.; Zhang, Y.; Zhang, L.; Zhang, Z. Phosphogypsum step of beta-type building gypsum powder prepared by the experimental study. J. Chem. Miner. Processing 2021, 50, 36–40. [Google Scholar]
- Han, S.; Zhao, Z.M.; Cheng, Y.H.; Liu, Y.C.; Quan, S.C. On Pretreatment Experimental Study of Yunnan Phosphorus Building Gypsum. Adv. Mater. Res. 2014, 1025, 837–841. [Google Scholar] [CrossRef]
- Liu, S.; Wang, L.; Yu, B. Review on hydration mechanism and engineering application of supersulfate cement. Concr. World 2018, 46–51. [Google Scholar]
- Wu, Q.; Xue, Q.; Yu, Z. The Research status of super sulfate cement. J. Clean. Prod. 2021, 294, 126228. [Google Scholar] [CrossRef]
- Chen, Y.; Ji, J.-R.; Zhou, Z.; Wu, S.-L.; Chen, H.-X. Influence factors of early strength of supersulfate cement and ways to improve it. Bull. Chin. Ceram. Soc. 2021, 40, 1413–1419. [Google Scholar]
- Yousry, M.Z.M.; Mahmoud, Y.Z.M.; Mahmoud, Y.Z.M. Developments in polyester composite materials—An in-depth review on natural fibres and nano fillers. Compos. Struct. 2021, 278, 114698. [Google Scholar]
- Mohammed, F.; Yousry, Z.M.M. Investigation of Electrophoretic Deposition of PANI Nano fibers as a Manufacturing Technology for corrosion protection. Prog. Org. Coat. 2022, 171, 107015. [Google Scholar]
- Mohammed, F.; Yousry, Z.M.M. Statistical and qualitative analyses of the kinetic models using electrophoretic deposition of polyaniline. J. Ind. Eng. Chem. 2022, 113, 475–487. [Google Scholar]
- Li, Y.; Yang, Z. Development trend of phosphogypsum comprehensive Utilization Technology. Phosphate Fertil. Compd. Fertil. 2018, 33, 1–6. [Google Scholar]
- Ou Yang, J.; Liu, S. Modification mechanism of phosphogypsum and its application in supersulfate cement. Concr. World 2019, 60–66. [Google Scholar]
- Gao, Y.; Yu, B.; Wang, J. Hydration products and pore structure characteristics of supersulfate cement. Civ. Archit. Environ. Eng. 2014, 36, 118–122. [Google Scholar]
- Jiang, C.; Mei, J.; Liu, Z. Research Progress of ecological concrete artificial reef. Concr. Cem. Prod. 2021, 7, 43–46. [Google Scholar]
- Kafi, M.A.; Sadeghi-Nik, A.; Bahari, A.; Sadeghi-Nik, A.; Mirshafiei, E. Microstructural Characterization and Mechanical Properties of Cementitious Mortar Containing Montmorillonite Nanoparticles. J. Mater. Civ. Eng. 2016, 28, 04016155. [Google Scholar] [CrossRef]
- Ximena, G.; Victoria, B.M.; Jordi, P.; María, M.J.; Iván, T.J. Mineralogical evolution of cement pastes at early ages based on thermogravimetric analysis (TG). J. Therm. Anal. Calorim. 2018, 132, 39–46. [Google Scholar]
- Wu, M.; Sui, S.; Zhang, Y.; Jia, Y.; She, W.; Liu, Z.; Yang, Y. Analyzing the filler and activity effect of fly ash and slag on the early hydration of blended cement based on calorimetric test. Constr. Build. Mater. 2021, 276, 122201. [Google Scholar] [CrossRef]
- Guo, C.Z. Study on the Influence Mechanism of Fluorine and Phosphorus on Mineral Hydration Process of Portland Cement Clinker. Ph.D. Thesis, Wuhan University of Technology, Wuhan, China, 2012. [Google Scholar]
- Dong, C.; Sun, Z. Characteristics of phosphogypsum and its application status in new building Materials. Compr. Util. Fly Ash 2014, 4, 51–56. [Google Scholar]

| CaO | SO3 | SiO2 | Al2O3 | Fe2O3 | MgO | P2O5 | K2O | F | |
|---|---|---|---|---|---|---|---|---|---|
| phosphogypsum | 36.05 | 52.35 | 8.83 | 0.17 | 1.56 | - | 1.18 | 0.27 | 0.71 |
| S95 GGBS | 40.56 | - | 33.89 | 14.47 | 0.36 | 7.43 | - | - | - |
| Sulphoaluminate cement | 44.63 | 8.64 | 23.68 | 31.28 | 1.65 | 1.36 | - | 1.6 | - |
| Specific Surface Area (m2/kg) | Setting Time/ min | Flexural Strength/MPa | Compressive Strength/MPa | pH | |||
|---|---|---|---|---|---|---|---|
| Initial | Final | 3 d | 28 d | 3 d | 28 d | ||
| 380 | 34 | 58 | 4.6 | 7.3 | 41.0 | 53.1 | 11.20 |
| Density/(g/cm3) | Specific Surface Area/(m2/kg) | The Activation Index/% | Fluidity/% | |
|---|---|---|---|---|
| 7 d | 28 d | |||
| ≥2.8 | 429 | 90 | 102 | ≥95 |
| Formaldehyde Content/% | Net Slurry Fluidity/mm | Sodium Sulfate Content/% | Chloride Ion Content/% | Alkali Content/% |
|---|---|---|---|---|
| 0.003 | 280 | 1.0 | 0.01 | 1.1 |
| NO. | Sulphoaluminate Cement/wt% | S95 GGBS/wt% | Phosphogypsum/wt% | Water–Binder Ratio | Water Reducing Agent/wt% |
|---|---|---|---|---|---|
| 1 | 15.0 | 35.0 | 50.0 | 0.5 | 0.5 |
| 2 | 20.0 | 30.0 | 50.0 | 0.5 | 0.5 |
| 3 | 25.0 | 25.0 | 50.0 | 0.5 | 0.5 |
| 4 | 15.0 | 35.0 | 50.0 | 0.6 | 0.5 |
| 5 | 20.0 | 30.0 | 50.0 | 0.6 | 0.5 |
| 6 | 25.0 | 25.0 | 50.0 | 0.6 | 0.5 |
| BG | 100.0 | - | - | 0.5 | 0.5 |
| No. | Strength of 3 d (MPa) | Strength of 7 d (MPa) | Strength of 28 d (MPa) | Strength of 56 d (MPa) | ||||
|---|---|---|---|---|---|---|---|---|
| Flexural | Compressive | Flexural | Compressive | Flexural | Compressive | Flexural | Compressive | |
| 1 | 2.6 | 17.8 | 3.7 | 21.4 | 4.0 | 22.3 | 4.2 | 23.5 |
| 2 | 2.9 | 18.6 | 4.1 | 25.1 | 4.2 | 27.6 | 4.6 | 29.5 |
| 3 | 2.8 | 18.2 | 4.0 | 24.7 | 4.3 | 26.9 | 4.5 | 27.6 |
| 4 | 1.4 | 8.9 | 1.6 | 11.3 | 2.2 | 13.4 | 2.3 | 14.2 |
| 5 | 1.5 | 9.7 | 2.2 | 11.9 | 2.6 | 14.7 | 2.7 | 16.4 |
| 6 | 1.7 | 11.3 | 2.3 | 13.3 | 2.7 | 16.8 | 2.9 | 18.5 |
| BG | 4.6 | 41.0 | 5.6 | 47.6 | 7.3 | 53.1 | 7.9 | 56.4 |
| Type of Toxic Ion | Control Group/(mg·kg−1) | Concentration of Toxic Ions of 3 d/(mg·kg−1) | Concentration of Toxic Ions of 56 d/(mg·kg−1) | Reference Standard of Mass Concentration Detection Limit/(mg·kg−1) |
|---|---|---|---|---|
| GB 5085.3-2007 | ||||
| Total Ba | 0.711 | 0.067 | 0.053 | 0.4≤ |
| Total Cr | 0.752 | 0.191 | 0.125 | 0.4≤ |
| Inorganic fluoride | 87.925 | 9.021 | 8.125 | 100≤ |
| Total As | 9.704 | 7.924 | 6.851 | 15≤ |
| Inorganic phosphate | 1.728 | 0.155 | 0.107 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, P.; Zhang, X.; Zhong, M.; Fan, Z.; Xiong, J.; Zhang, Z. Phosphogypsum-Based Ultra-Low Basicity Cementing Material. Materials 2022, 15, 6601. https://doi.org/10.3390/ma15196601
Li P, Zhang X, Zhong M, Fan Z, Xiong J, Zhang Z. Phosphogypsum-Based Ultra-Low Basicity Cementing Material. Materials. 2022; 15(19):6601. https://doi.org/10.3390/ma15196601
Chicago/Turabian StyleLi, Pengping, Xinxing Zhang, Mingfeng Zhong, Zhihong Fan, Jianbo Xiong, and Zhijie Zhang. 2022. "Phosphogypsum-Based Ultra-Low Basicity Cementing Material" Materials 15, no. 19: 6601. https://doi.org/10.3390/ma15196601
APA StyleLi, P., Zhang, X., Zhong, M., Fan, Z., Xiong, J., & Zhang, Z. (2022). Phosphogypsum-Based Ultra-Low Basicity Cementing Material. Materials, 15(19), 6601. https://doi.org/10.3390/ma15196601






