Tuning the Porosity of Piezoelectric Zinc Oxide Thin Films Obtained from Molecular Layer-Deposited “Zincones”
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Molecular Layer-Deposited Zincone
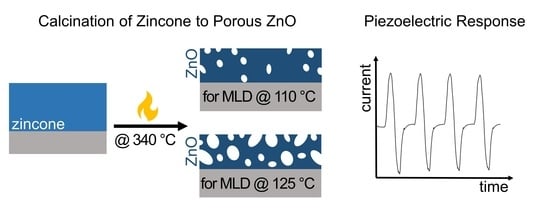
2.2. Synthesis of Porous ZnO from MLD Zincone
2.3. Optical Characterization
2.4. Chemical, Crystallographic, and Morphological Characterization
2.5. Piezoelectric Measurements of Porous ZnO
3. Results and Discussion
3.1. Growth of Zincone and Calcination to Porous ZnO
3.2. Morphological, Chemical, and Crystallographic Characterization of Zinc Oxide
3.3. Porosity Studies of Zinc Oxide
3.4. Piezoelectric Characterization of Porous Zinc Oxide
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ozgür, Ü.; Alivov, Y.I.; Liu, C.; Teke, A.; Reshchikov, M.A.; Doğan, S.; Avrutin, V.; Cho, S.-J.; Morkoç, H. A comprehensive review of ZnO materials and devices. J. Appl. Phys. 2005, 98, 041301. [Google Scholar] [CrossRef]
- Voiculescu, I.; Li, F.; Kowach, G.; Lee, K.-L.; Mistou, N.; Kastberg, R. Stretchable piezoelectric power generators based on ZnO thin films on elastic substrates. Micromachines 2019, 10, 661. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Li, T.; Li, Y.; Qiu, J.; Ma, X.; Chen, X.; Hu, X.; Zhang, W. A high power ZnO thin film piezoelectric generator. Appl. Surf. Sci. 2016, 364, 670–675. [Google Scholar] [CrossRef]
- Hirahara, N.; Onwona-Agyeman, B.; Nakao, M. Preparation of Al-doped ZnO thin films as transparent conductive substrate in dye-sensitized solar cell. Thin Solid Films 2012, 520, 2123–2127. [Google Scholar] [CrossRef]
- Hsu, C.-C.; Ting, W.-C.; Chen, Y.-T. Effects of substrate temperature on resistive switching behavior of planar ZnO resistive random access memories. J. Alloy. Compd. 2017, 691, 537–544. [Google Scholar] [CrossRef]
- Laurenti, M.; Cauda, V. ZnO Nanostructures for tissue engineering applications. Nanomaterials 2017, 7, 374. [Google Scholar] [CrossRef]
- Laurenti, M.; Cauda, V. Porous zinc oxide thin films: Synthesis approaches and applications. Coatings 2018, 8, 67. [Google Scholar] [CrossRef]
- Pauporté, T.; Rathouský, J. Electrodeposited mesoporous ZnO thin films as efficient photocatalysts for the degradation of dye pollutants. J. Phys. Chem. C 2007, 111, 7639–7644. [Google Scholar] [CrossRef]
- Choi, M.S.; Kim, M.Y.; Mirzaei, A.; Kim, H.-S.; Kim, S.-I.; Baek, S.-H.; Chun, D.W.; Jin, C.; Lee, K.H. Selective, sensitive, and stable NO2 gas sensor based on porous ZnO nanosheets. Appl. Surf. Sci. 2021, 568, 150910. [Google Scholar] [CrossRef]
- Zeng, Y.; Qiao, L.; Bing, Y.; Wen, M.; Zou, B.; Zheng, W.; Zhang, T.; Zou, G. Development of microstructure CO sensor based on hierarchically porous ZnO nanosheet thin films. Sens. Actuators B Chem. 2012, 173, 897–902. [Google Scholar] [CrossRef]
- Lee, P.-C.; Hsiao, Y.-L.; Dutta, J.; Wang, R.-C.; Tseng, S.-W.; Liu, C.-P. Development of porous ZnO thin films for enhancing piezoelectric nanogenerators and force sensors. Nano Energy 2021, 82, 105702. [Google Scholar] [CrossRef]
- Su, Y.-L.; Gupta, K.; Hsiao, Y.-L.; Wang, R.-C.; Liu, C.-P. Gigantic enhancement of electricity generation in piezoelectric semiconductors by creating pores as a universal approach. Energy Environ. Sci. 2019, 12, 410–417. [Google Scholar] [CrossRef]
- Yagüe, J.L.; Coclite, A.M.; Petruczok, C.; Gleason, K.K. Chemical Vapor deposition for solvent-free polymerization at surfaces. Macromol. Chem. Phys. 2013, 214, 302–312. [Google Scholar] [CrossRef]
- Sundberg, P.; Karppinen, M. Organic and inorganic–organic thin film structures by molecular layer deposition: A review. Beilstein J. Nanotechnol. 2014, 5, 1104–1136. [Google Scholar] [CrossRef] [PubMed]
- Leskela, M.; Ritala, M. Atomic layer deposition (ALD): From precursors to thin film structures. Thin Solid Films 2002, 409, 138–146. [Google Scholar] [CrossRef]
- Sundberg, P.; Karppinen, M. Organic–inorganic thin films from TiCl4 and 4-aminophenol precursors: A Model case of ALD/MLD hybrid-material growth? Eur. J. Inorg. Chem. 2014, 2014, 968–974. [Google Scholar] [CrossRef]
- Lee, B.H.; Anderson, V.R.; George, S.M. Molecular layer deposition of zircone and ZrO2/Zircone alloy films: Growth and properties. Chem. Vap. Depos. 2013, 19, 204–212. [Google Scholar] [CrossRef]
- Dameron, A.A.; Seghete, D.; Burton, B.B.; Davidson, S.D.; Cavanagh, A.S.; Bertrand, J.A.; George, S.M. Molecular layer deposition of alucone polymer films using trimethylaluminum and ethylene glycol. Chem. Mater. 2008, 20, 3315–3326. [Google Scholar] [CrossRef]
- Tanskanen, A.; Karppinen, M. Iron-based inorganic–organic hybrid and superlattice thin films by ALD/MLD. Dalton Trans. 2015, 44, 19194–19199. [Google Scholar] [CrossRef]
- Lee, B.H.; Anderson, V.R.; George, S.M. Growth and properties of hafnicone and HfO2/Hafnicone nanolaminate and alloy films using molecular layer deposition techniques. ACS Appl. Mater. Interfaces 2014, 6, 16880–16887. [Google Scholar] [CrossRef]
- Meng, X.; Lau, K.C.; Zhou, H.; Ghosh, S.K.; Benamara, M.; Zou, M. Molecular layer deposition of crosslinked polymeric lithicone for superior lithium metal anodes. Energy Mater. Adv. 2021, 2021, 9786201. [Google Scholar] [CrossRef]
- Zhu, H.; Shiraz, M.H.A.; Yao, L.; Adair, K.; Wang, Z.; Tong, H.; Song, X.; Sham, T.-K.; Arjmand, M.; Song, X.; et al. Molecular-layer-deposited tincone: A new hybrid organic–inorganic anode material for three-dimensional microbatteries. Chem. Commun. 2020, 56, 13221–13224. [Google Scholar] [CrossRef] [PubMed]
- Van de Kerckhove, K.; Mattelaer, F.; Deduytsche, D.; Vereecken, P.M.; Dendooven, J.; Detavernier, C. Molecular layer deposition of “titanicone”, a titanium-based hybrid material, as an electrode for lithium-ion batteries. Dalton Trans. 2016, 45, 1176–1184. [Google Scholar] [CrossRef] [PubMed]
- Abdulagatov, A.I.; Hall, R.A.; Sutherland, J.L.; Lee, B.H.; Cavanagh, A.S.; George, S.M. Molecular Layer Deposition of Titanicone Films using TiCl4 and Ethylene Glycol or Glycerol: Growth and Properties. Chem. Mater. 2012, 24, 2854–2863. [Google Scholar] [CrossRef]
- Van de Kerckhove, K.; Mattelaer, F.; Dendooven, J.; Detavernier, C. Molecular layer deposition of “vanadicone”, a vanadium-based hybrid material, as an electrode for lithium-ion batteries. Dalton Trans. 2017, 46, 4542–4553. [Google Scholar] [CrossRef]
- Yoon, B.; Lee, B.H.; George, S.M. Highly Conductive and Transparent Hybrid Organic–Inorganic Zincone Thin Films Using Atomic and Molecular Layer Deposition. J. Phys. Chem. C 2012, 116, 24784–24791. [Google Scholar] [CrossRef]
- Choudhury, D.; Rajaraman, G.; Sarkar, S.K. Stability of molecular layer deposited zincone films: Experimental and theoretical exploration. RSC Adv. 2015, 5, 29947–29952. [Google Scholar] [CrossRef]
- Yoon, B.; O’Patchen, J.L.; Seghete, D.; Cavanagh, A.S.; George, S.M. Molecular Layer Deposition of Hybrid Organic-Inorganic Polymer Films using Diethylzinc and Ethylene Glycol. Chem. Vap. Depos. 2009, 15, 112–121. [Google Scholar] [CrossRef]
- Peng, Q.; Gong, B.; VanGundy, R.M.; Parsons, G.N. “Zincone” Zinc Oxide−Organic Hybrid Polymer Thin Films Formed by Molecular Layer Deposition. Chem. Mater. 2009, 21, 820–830. [Google Scholar] [CrossRef]
- Liang, X.; Weimer, A.W. An overview of highly porous oxide films with tunable thickness prepared by molecular layer deposition. Curr. Opin. Solid State Mater. Sci. 2015, 19, 115–125. [Google Scholar] [CrossRef]
- Liang, X.; Yu, M.; Li, J.; Jiang, Y.-B.; Weimer, A.W. Ultra-thin microporous–mesoporous metal oxide films prepared by molecular layer deposition (MLD). Chem. Commun. 2009, 46, 7140–7142. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Li, P.; Wang, G.; Yu, Y.; Duan, F.; Chen, C.; Song, W.; Qin, Y.; Knez, M. Nanoporous Nitrogen-Doped Titanium Dioxide with Excellent Photocatalytic Activity under Visible Light Irradiation Produced by Molecular Layer Deposition. Angew. Chem. 2013, 125, 9366–9370. [Google Scholar] [CrossRef]
- Kint, J.; Mattelaer, F.; Vandenbroucke, S.S.T.; Muriqi, A.; Minjauw, M.M.; Nisula, M.; Vereecken, P.; Nolan, M.; Dendooven, J.; Detavernier, C. Molecular Layer Deposition of “Magnesicone”, a Magnesium-based Hybrid Material. Chem. Mater. 2020, 32, 4451–4466. [Google Scholar] [CrossRef]
- Liang, X.; Jiang, Y.-B.; Weimer, A.W. Nanocoating zinc alkoxide (zincone) hybrid polymer films on particles using a fluidized bed reactor. J. Vac. Sci. Technol. A Vacuum Surfaces Films 2012, 30, 01A108. [Google Scholar] [CrossRef]
- Perrotta, A.; Pilz, J.; Pachmajer, S.; Milella, A.; Coclite, A.M. On the transformation of “zincone”-like into porous ZnO thin films from sub-saturated plasma enhanced atomic layer deposition. Beilstein J. Nanotechnol. 2019, 10, 746–759. [Google Scholar] [CrossRef]
- Perrotta, A.; Berger, R.; Muralter, F.; Coclite, A.M. Mesoporous ZnO thin films obtained from molecular layer deposited “zincones”. Dalton Trans. 2019, 48, 14178–14188. [Google Scholar] [CrossRef]
- Berger, R.; Seiler, M.; Perrotta, A.; Coclite, A. Study on Porosity in Zinc Oxide Ultrathin Films from Three-Step MLD Zn-Hybrid Polymers. Materials 2021, 14, 1418. [Google Scholar] [CrossRef]
- Bui, Q.C.; Salem, B.; Roussel, H.; Mescot, X.; Guerfi, Y.; Jiménez, C.; Consonni, V.; Ardila, G. Effects of thermal annealing on the structural and electrical properties of ZnO thin films for boosting their piezoelectric response. J. Alloys Compd. 2021, 870, 159512. [Google Scholar] [CrossRef]
- Abu Ali, T.; Pilz, J.; Schäffner, P.; Kratzer, M.; Teichert, C.; Stadlober, B.; Coclite, A.M. Piezoelectric Properties of Zinc Oxide Thin Films Grown by Plasma-Enhanced Atomic Layer Deposition. Phys. Status Solidi Appl. Mater. Sci. 2020, 217, 2000319. [Google Scholar] [CrossRef]
- Perrotta, A.; Poodt, P.; Bruele, F.J.F.V.D.; Kessels, W.M.M.E.; Creatore, M. Characterization of nano-porosity in molecular layer deposited films. Dalton Trans. 2018, 47, 7649–7655. [Google Scholar] [CrossRef]
- Boscher, N.D.; Wang, M.; Perrotta, A.; Heinze, K.; Creatore, M.; Gleason, K.K. Metal-Organic Covalent Network Chemical Vapor Deposition for Gas Separation. Adv. Mater. 2016, 28, 7479–7485. [Google Scholar] [CrossRef] [PubMed]
- Aghaee, M.; Niemelä, J.-P.; Kessels, W.M.M.; Creatore, M. On the role of micro-porosity in affecting the environmental stability of atomic/molecular layer deposited (ZnO)a(Zn–O–C6H4–O)b films. Dalton Trans. 2019, 48, 3496–3505. [Google Scholar] [CrossRef] [PubMed]
- Pilz, J.; Tazreiter, M.; Coclite, A.M. Universal software for the real-time control of sequential processing techniques. J. Vac. Sci. Technol. A. 2019, 37, 063201. [Google Scholar] [CrossRef]
- Baklanov, M.R.; Mogilnikov, K.P.; Polovinkin, V.; Dultsev, F.N. Determination of pore size distribution in thin films by ellipsometric porosimetry. J. Vac. Sci. Technol. B Microelectron. Nanometer Struct. 2000, 18, 1385–1391. [Google Scholar] [CrossRef]
- Scherrer, P. Bestimmung der inneren Struktur und der Größe von Kolloidteilchen mittels Röntgenstrahlen. Göttingen Nachr. Math. Phys. 1918, 2, 98–100. [Google Scholar] [CrossRef]
- Neuschitzer, M.; Moser, A.; Neuhold, A.; Kraxner, J.; Stadlober, B.; Oehzelt, M.; Salzmann, I.; Resel, R.; Novák, J. Grazing-incidence in-plane X-ray diffraction on ultra-thin organic films using standard laboratory equipment. J. Appl. Crystallogr. 2012, 45, 367–370. [Google Scholar] [CrossRef]
- Christian, P.; Ehmann, H.M.; Coclite, A.M.; Werzer, O. Polymer encapsulation of an amorphous pharmaceutical by initiated chemical vapor deposition for enhanced stability. ACS Appl. Mater. Interfaces 2016, 8, 21177–21184. [Google Scholar] [CrossRef]
- Saha, S.; Mehan, N.; Sreenivas, K.; Gupta, V. Temperature dependent optical properties of (002) oriented ZnO thin film using surface plasmon resonance. Appl. Phys. Lett. 2009, 95, 071106. [Google Scholar] [CrossRef]
- Liang, X.; Evanko, B.W.; Izar, A.; King, D.M.; Jiang, Y.-B.; Weimer, A.W. Ultrathin highly porous alumina films prepared by alucone ABC molecular layer deposition (MLD). Microporous Mesoporous Mater. 2012, 168, 178–182. [Google Scholar] [CrossRef]
- Kim, M.T. Deposition behavior of hexamethydisiloxane films based on the FTIR analysis of Si–O–Si and Si–CH3 bonds. Thin Solid Films 1997, 311, 157–163. [Google Scholar] [CrossRef]
- Mawhinney, D.B.; Glass, J.J.A.; Yates, J.J.T. FTIR Study of the oxidation of porous silicon. J. Phys. Chem. B 1997, 101, 1202–1206. [Google Scholar] [CrossRef]
- Abrahams, S.C.; Bernstein, J.L. Remeasurement of the structure of hexagonal ZnO. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1969, 25, 1233–1236. [Google Scholar] [CrossRef]
- Guziewicz, E.; Godlewski, M.; Wachnicki, L.; Krajewski, T.A.; Luka, G.; Gieraltowska, S.; Jakiela, R.; Stonert, A.; Lisowski, W.; Krawczyk, M.; et al. ALD grown zinc oxide with controllable electrical properties. Semicond. Sci. Technol. 2012, 27, 074011. [Google Scholar] [CrossRef]
- Jung, T.-H.; Park, J.-S.; Kim, D.-H.; Jeong, Y.; Park, S.-G.; Kwon, J.-D. Effect of in situ hydrogen plasma treatment on zinc oxide grown using low temperature atomic layer deposition. J. Vac. Sci. Technol. A Vac. Surf. Films 2013, 31, 01A124. [Google Scholar] [CrossRef]
- Pilz, J.; Perrotta, A.; Christian, P.; Tazreiter, M.; Resel, R.; Leising, G.; Griesser, T.; Coclite, A.M. Tuning of material properties of ZnO thin films grown by plasma-enhanced atomic layer deposition at room temperature. J. Vac. Sci. Technol. A Vac. Surf. Films 2018, 36, 01A109. [Google Scholar] [CrossRef]
- Fan, J.C.C.; Goodenough, J.B. X-ray photoemission spectroscopy studies of Sn-doped indium-oxide films. J. Appl. Phys. 1977, 48, 3524–3531. [Google Scholar] [CrossRef]
- Sotomayor, F.J.; Cychosz, K.A.; Thommes, M. Characterization of micro/mesoporous materials by physisorption: Concepts and case studies. Acc. Mater. Surf. Res. 2018, 3, 34–50. [Google Scholar]
- Schneider, P. Adsorption isotherms of microporous-mesoporous solids revisited. Appl. Catal. A Gen. 1995, 129, 157–165. [Google Scholar] [CrossRef]
- Lu, Y.; Emanetoglu, N.W.; Chen, Y. ZnO piezoelectric devices. In Processing, Properties and Applications; Elsevier: Amsterdam, The Netherlands, 2006; pp. 443–489. [Google Scholar] [CrossRef]
- D’Agostino, D.; Di Giorgio, C.; Di Trolio, A.; Guarino, A.; Cucolo, A.M.; Vecchione, A.; Bobba, F. Piezoelectricity and charge trapping in ZnO and Co-doped ZnO thin films. AIP Adv. 2017, 7, 055010. [Google Scholar] [CrossRef] [Green Version]










| Tcalc. (°C) | Sample Condition | Oxygen (at%) (±1%) | Zinc (at%) (±1%) | Carbon (at%) (±1%) | Zn/O Ratio (±0.1%) | Corrected Zn/O Ratio |
|---|---|---|---|---|---|---|
| 340 °C | pristine | 34 | 18 | 48 | 0.55 | 1.0 ± 0.1 |
| sputtered | 43 | 56 | 1 | 1.31 | ||
| 400 °C | pristine | 37 | 27 | 36 | 0.73 | 1.1 ± 0.1 |
| sputtered | 42 | 57 | 1 | 1.35 | ||
| 500 °C | pristine | 39 | 37 | 24 | 0.97 | 0.9 ± 0.1 |
| sputtered | 45 | 54 | 1 | 1.21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kräuter, M.; Abu Ali, T.; Stadlober, B.; Resel, R.; Unger, K.; Coclite, A.M. Tuning the Porosity of Piezoelectric Zinc Oxide Thin Films Obtained from Molecular Layer-Deposited “Zincones”. Materials 2022, 15, 6786. https://doi.org/10.3390/ma15196786
Kräuter M, Abu Ali T, Stadlober B, Resel R, Unger K, Coclite AM. Tuning the Porosity of Piezoelectric Zinc Oxide Thin Films Obtained from Molecular Layer-Deposited “Zincones”. Materials. 2022; 15(19):6786. https://doi.org/10.3390/ma15196786
Chicago/Turabian StyleKräuter, Marianne, Taher Abu Ali, Barbara Stadlober, Roland Resel, Katrin Unger, and Anna Maria Coclite. 2022. "Tuning the Porosity of Piezoelectric Zinc Oxide Thin Films Obtained from Molecular Layer-Deposited “Zincones”" Materials 15, no. 19: 6786. https://doi.org/10.3390/ma15196786
APA StyleKräuter, M., Abu Ali, T., Stadlober, B., Resel, R., Unger, K., & Coclite, A. M. (2022). Tuning the Porosity of Piezoelectric Zinc Oxide Thin Films Obtained from Molecular Layer-Deposited “Zincones”. Materials, 15(19), 6786. https://doi.org/10.3390/ma15196786