Mechanical Properties and Equilibrium Swelling Characteristics of Some Polymer Composites Based on Ethylene Propylene Diene Terpolymer (EPDM) Reinforced with Hemp Fibers
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of EPDM/Hemp Fiber Composites
2.3. Measurements
3. Results and Discussion
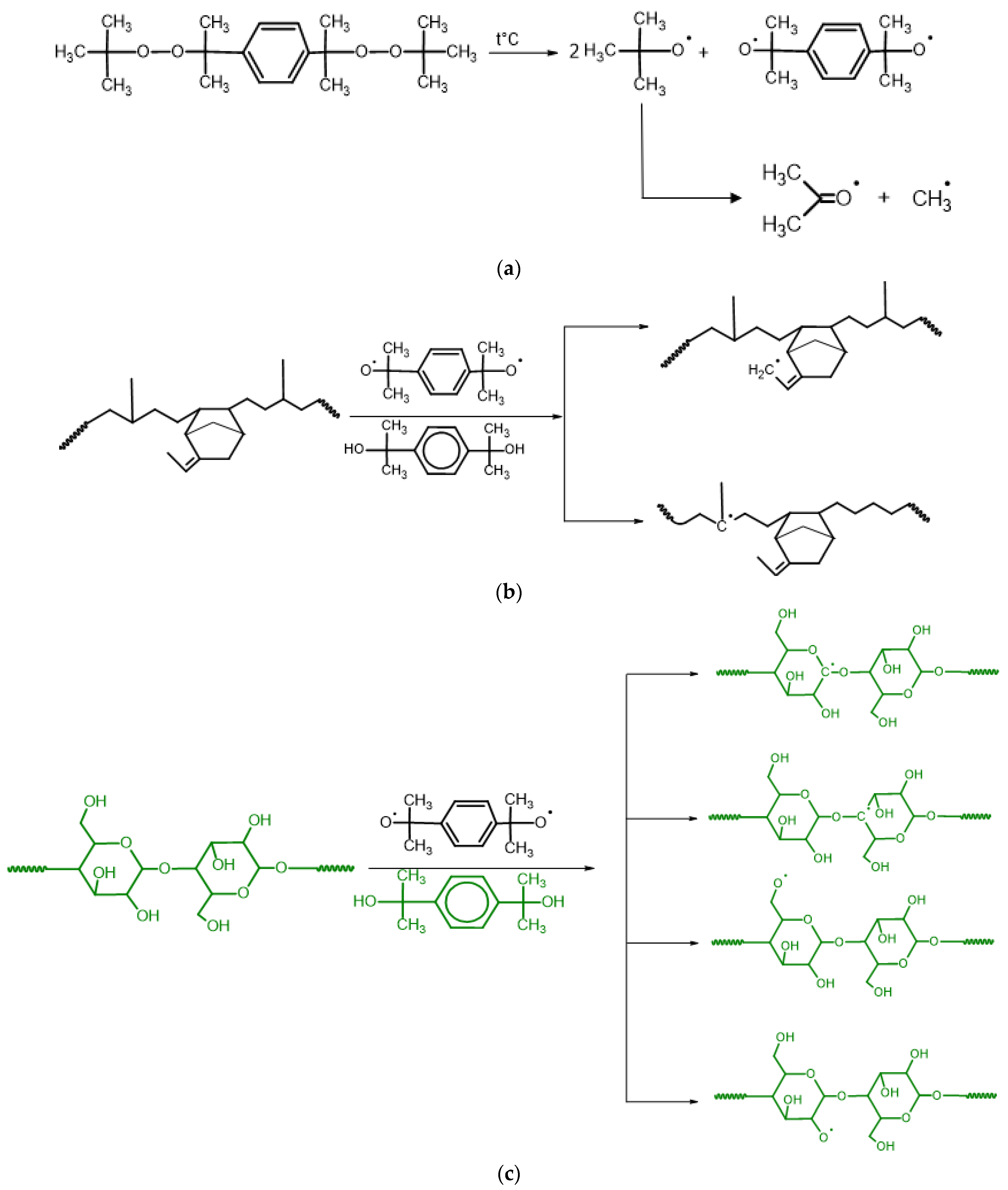
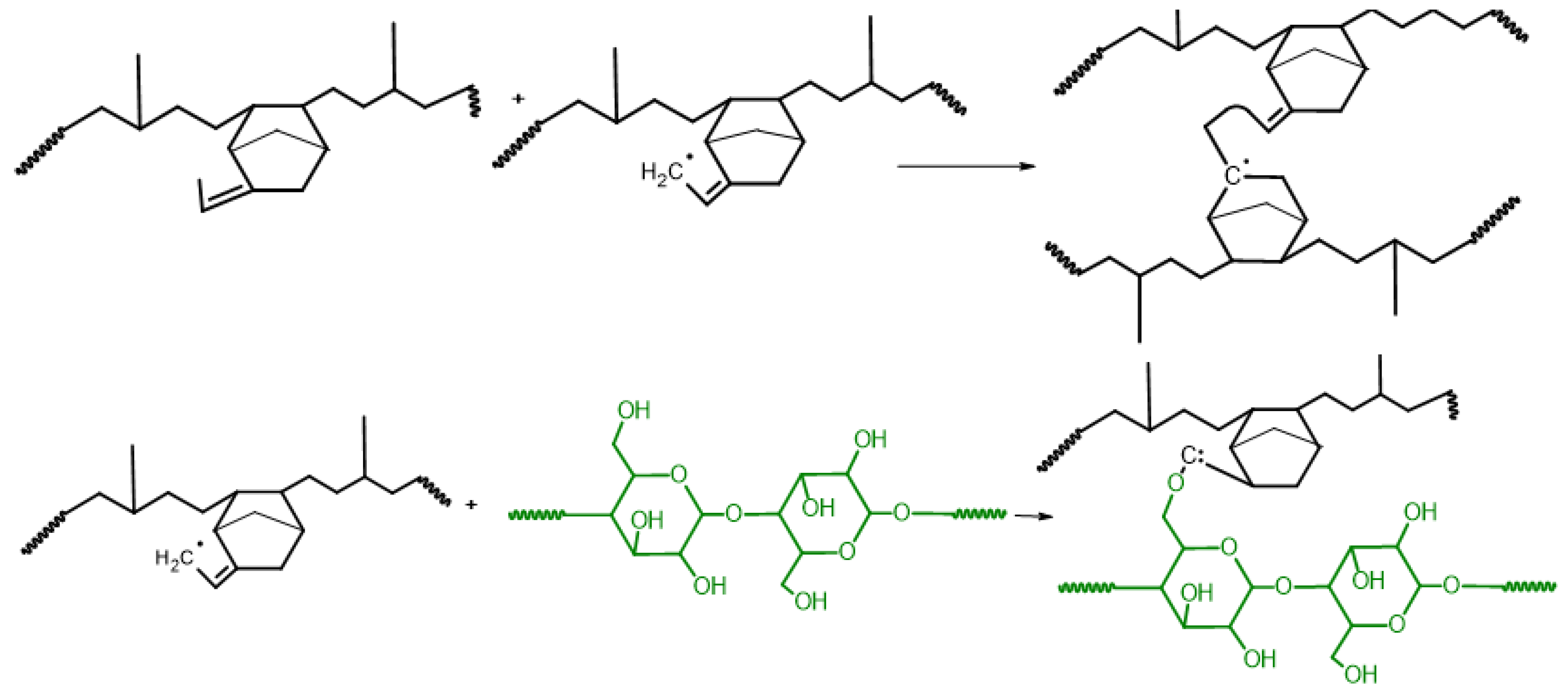
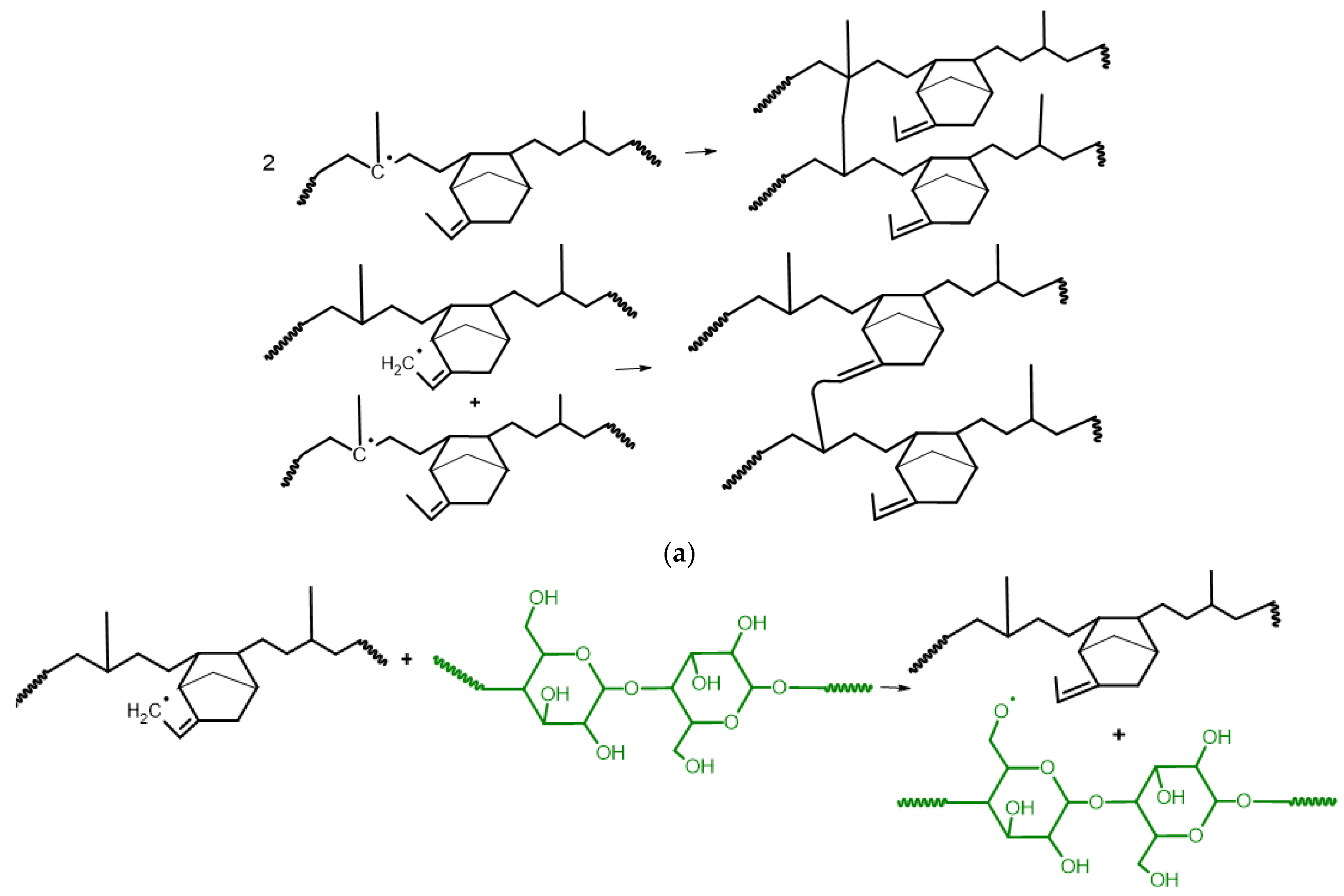
3.1. Reaction Mechanism
3.2. Mechanical Properties
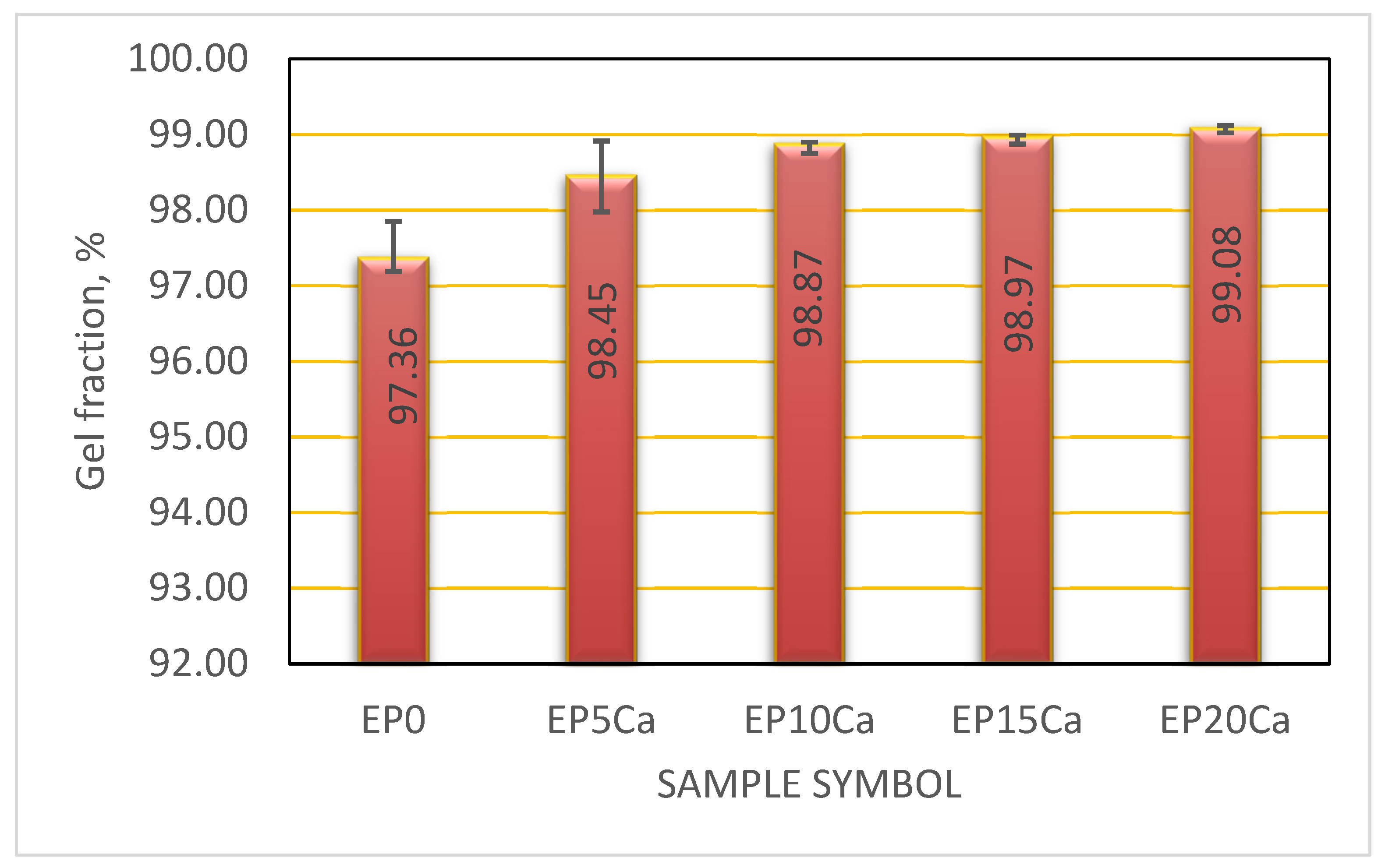
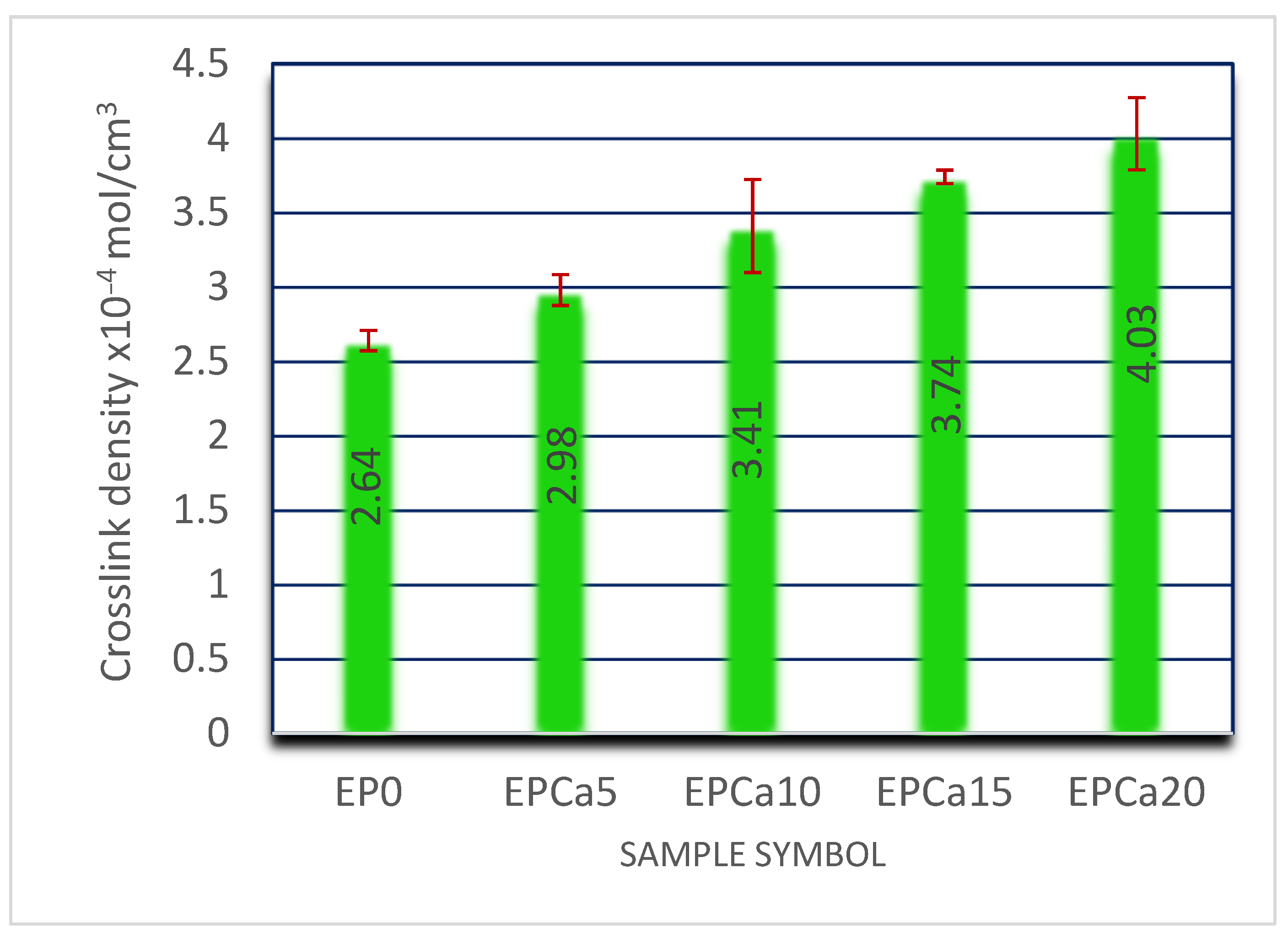
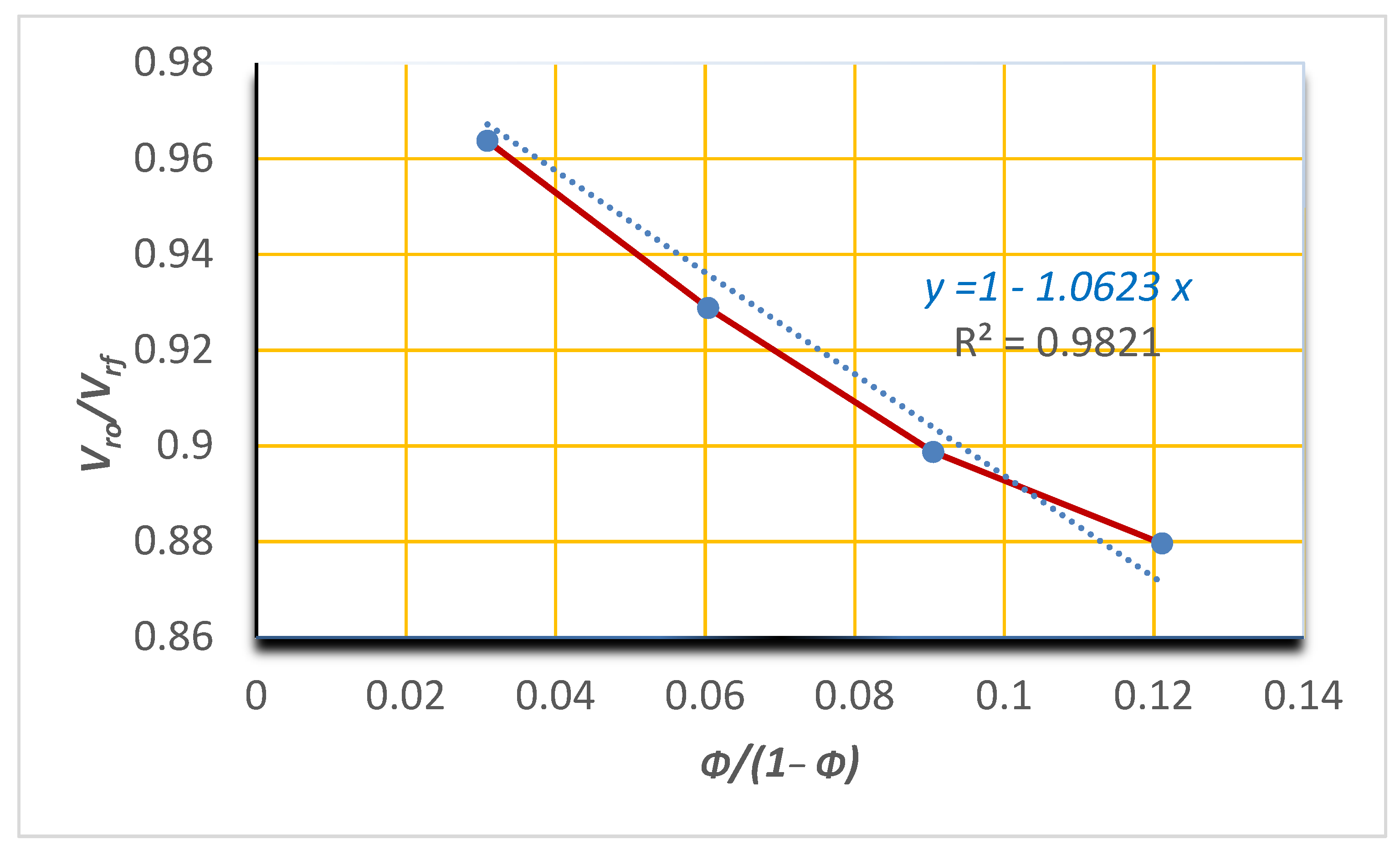
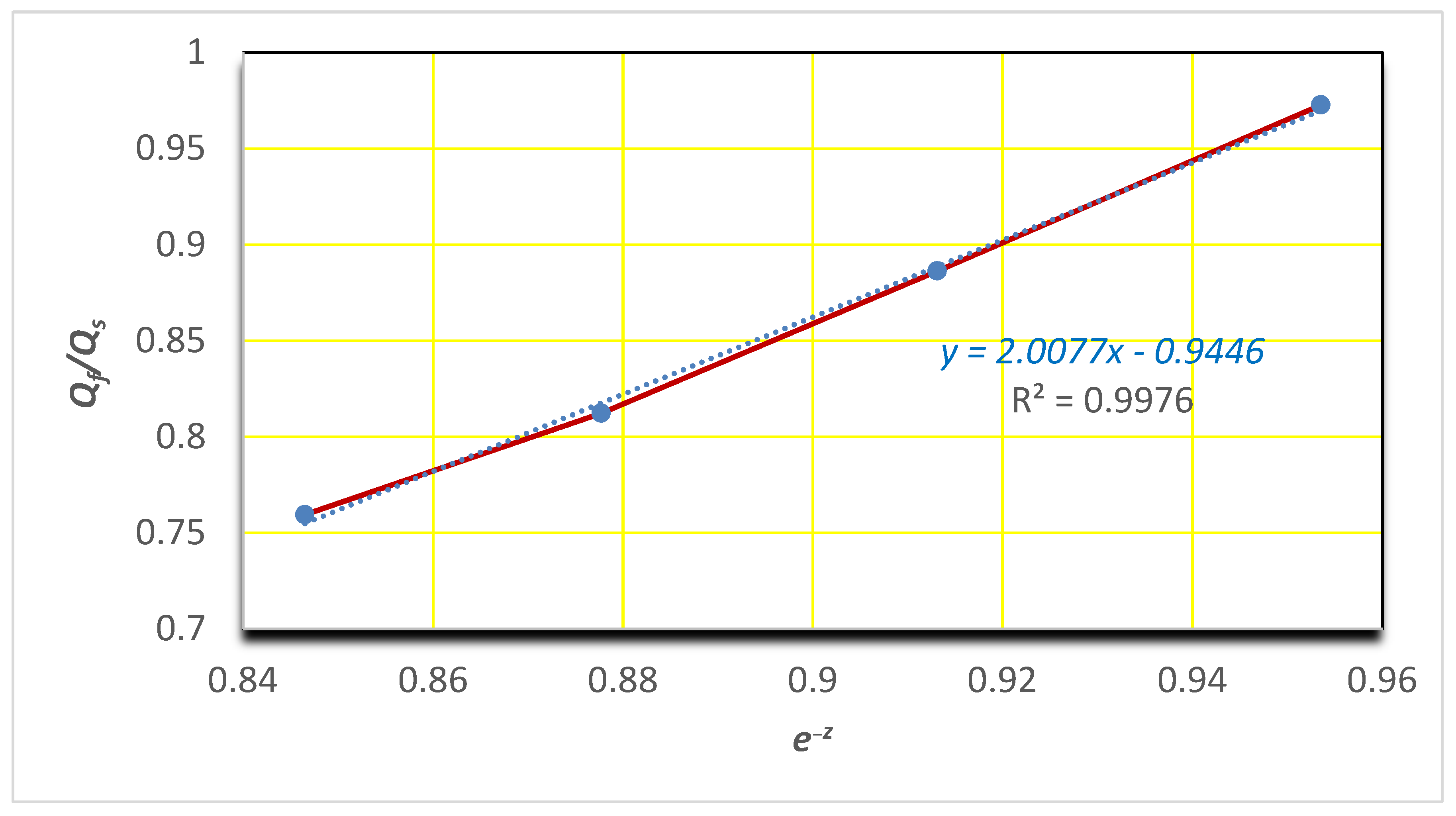
3.3. Equilibrium Swelling Studies
3.4. FTIR Spectra
3.5. SEM Analysis
3.6. Water Sorption
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, B.H.; Kim, H.J.; Yu, W.R. Fabrication of long and discontinuous natural fiber reinforced polypropylene biocomposites and their mechanical properties. Fibers Polym. 2009, 10, 83–90. [Google Scholar] [CrossRef]
- Mohammed, L.; Ansari, M.N.M.; Pua, G.; Jawaid, M.; Islam, M.S. A Review on natural fiber reinforced polymer composite and its applications. Int. J. Polym. Sci. 2015, 2015, 243947. [Google Scholar] [CrossRef]
- Faruk, O.; Bledzki, A.K.; Fink, H.P.; Sain, M. Progress report on natural fiber reinforced composites. Macromol. Mater. Eng. 2014, 299, 9–26. [Google Scholar] [CrossRef]
- Summerscales, J.; Dissanayake, N.P.J.; Virk, A.S.; Hall, W. A review of bast fibres and their composites, Part 1–Fibres as reinforcements. Composites A 2010, 41, 1329–1335. [Google Scholar] [CrossRef]
- Pickering, K.L.; Efendy, M.C.A.; Lee, T.M. A review of recent development in natural fibre composites. Composites A 2016, 83, 98–112. [Google Scholar] [CrossRef]
- Stelescu, M.D.; Manaila, E.; Georgescu, M.; Nituica, M. New materials based on ethylene propylene diene terpolymer and hemp fibres obtained by green reactive processing. Materials 2020, 13, 2067. [Google Scholar] [CrossRef]
- Mochane, M.J.; Mokhena, T.C.; Mokhothu, T.H.; Mtibe, A.; Sadiku, E.R.; Ray, S.S.; Ibrahim, I.D.; Daramola, O.O. Recent progress on natural fiber hybrid composites for advanced applications: A review. Express Polym. Lett. 2019, 13, 159–198. [Google Scholar] [CrossRef]
- Bledzki, A.; Gassan, J. Composites reinforced with cellulose based fibers. Prog. Polym. Sci. 1999, 24, 221–274. [Google Scholar] [CrossRef]
- Dhakal, H.N.; Hang, Z.Z. The use of hemp fibres as reinforcements in composites. In Biofiber Reinforcements in Composite Materials; Faruk, O., Sain, M., Eds.; Woodhead Publish.: Cambridge, UK, 2015; pp. 86–103. [Google Scholar]
- John, M.J.; Francis, B.; Varughese, K.T.; Thomas, S. Effect of chemical modification on properties of hybrid fiber biocomposites. Composites A 2008, 39, 352–363. [Google Scholar] [CrossRef]
- Nasir, M.; Gupta, A.; Beg, M.D.H.; Chua, G.K.; Kumar, A. Fabrication of medium density fibreboard from enzyme treated rubber wood (Hevea brasiliensis) fibre and modified organosolv lignin. Int. J. Adh. Adh. 2013, 44, 99–104. [Google Scholar] [CrossRef] [Green Version]
- George, M.; Mussone, P.G.; Bressler, D.C. Surface and thermal characterization of natural fibres treated with enzymes. Ind. Crops Prod. 2014, 53, 365–373. [Google Scholar] [CrossRef]
- Shalwan, A.; Yousif, B.F. In state of art: Mechanical and tribological behaviour of polymeric composites based on natural fibres. Mater. Des. 2013, 48, 14–24. [Google Scholar] [CrossRef]
- Xie, Y.; Hill, C.A.S.; Xiao, Z.; Militz, H.; Mai, C. Silane coupling agents used for natural fiber/polymer composites: A review. Composites A 2010, 41, 806–819. [Google Scholar] [CrossRef]
- Ray, S.S.; Bousmina, M. Biodegradable polymers and their layered silicate nanocomposites: In greening the 21st century materials world. Progr. Mater. Sci. 2005, 50, 962–1079. [Google Scholar]
- Koronis, G.; Silva, A.; Fontul, M. Green composites: A review of adequate materials for automotive applications. Composites B Eng. 2013, 44, 120–127. [Google Scholar] [CrossRef]
- Loureiro, N.C. Green composites on automotive interior parts: A solution using cellulosic fibers. In Green Composites for Automotive Applications; Koronis, G., Arlindo, S., Eds.; Woodhead Publishing: Cambridge, UK, 2019; pp. 81–97. [Google Scholar]
- Arockiam, N.J.; Jawaid, M.; Saba, N. Sustainable bio composites for aircraft components. In Sustainable Composites for Aerospace Applications; Jawaid, M., Thariq, M., Eds.; Woodhead Publishing: Cambridge, UK, 2018; pp. 109–123. [Google Scholar]
- Kopparthy, S.D.S.; Netravali, A.N. Review: Green composites for structural applications. Compos. Part C Open Access 2021, 6, 100169. [Google Scholar] [CrossRef]
- Nurazzi, N.M.; Asyraf, M.R.M.; Khalina, A.; Abdullah, N.; Aisyah, H.A.; Rafiqah, S.A.; Sabaruddin, F.A.; Kamarudin, S.H.; Norrrahim, M.N.F.; Ilyas, R.A.; et al. A review on natural fiber reinforced polymer composite for bullet proof and ballistic applications. Polymers 2021, 13, 646. [Google Scholar] [CrossRef]
- Davoodi, M.M.; Sapuan, S.M.; Ahmad, D.; Aidy, A.; Khalina, A.; Jonoobi, M. Concept selection of car bumper beam with developed hybrid bio-composite material. Mater. Des. 2011, 32, 4857–4865. [Google Scholar] [CrossRef]
- Sassoni, E.; Manzi, S.; Motori, A.; Montecchi, M.; Canti, M. Novel sustainable hemp-based composites for application in the building industry: Physical, thermal and mechanical characterization. Energy Build. 2014, 77, 219–226. [Google Scholar] [CrossRef]
- Tao, Y.; Ren, M.; Zhang, H.; Peijs, T. Recent progress in acoustic materials and noise control strategies—A review. Appl. Mater. Today 2021, 24, 101141. [Google Scholar] [CrossRef]
- Santoni, A.; Bonfiglio, P.; Fausti, P.; Marescotti, C.; Mazzanti, V.; Mollica, F.; Pompoli, F. Improving the sound absorption performance of sustainable thermal insulation materials: Natural hemp fibres. Appl. Acoust. 2019, 150, 279–289. [Google Scholar] [CrossRef]
- Zhu, W.; Chen, S.; Wang, Y.; Zhu, T.; Jiang, Y. Sound absorption behavior of polyurethane foam composites with different ethylene propylene diene monomer particles. Arch. Acoust. 2018, 43, 403–411. [Google Scholar]
- Stelescu, M.D.; Airinei, A.; Manaila, E.; Craciun, G.; Fifere, N.; Varganici, C.; Pamfil, D.; Doroftei, F. Effects of electron beam irradiation on the mechanical, thermal and surface properties of some EPDM/butyl rubber composites. Polymers 2018, 10, 1206. [Google Scholar] [CrossRef]
- Qu, P.; Gao, Y.; Wu, G.F.; Zhang, L.P. Nanocomposites of poly(lactic acid) reinforced with cellulose nanofibrils. BioResources 2010, 5, 1811–1823. [Google Scholar]
- Luo, S.; Cao, J.; Wang, X. Investigation of the interfacial compatibility of PEG and thermally modified wood flour/polypropylene composites using the stress relaxation approach. BioResources 2013, 8, 2064–2073. [Google Scholar] [CrossRef]
- Abdel-Hakim, A.; El-Gamal, A.A.; El-Zayat, M.M.; Sadek, A.M. Effect of novel sunrose based polyfunctional electrical properties of irradiated EPDM. Rad. Phys. Chem. 2021, 189, 109729. [Google Scholar] [CrossRef]
- Flory, P.J.; Rehner, J. Statistical mechanics of cross-linked polymer networks I. Rubberlike elasticity. J. Chem. Phys. 1943, 11, 512–521. [Google Scholar] [CrossRef]
- Bala, P.; Samantaray, B.K.; Srivastava, S.K.; Nando, G.B. Organomodified montmorillonite as filler in natural and synthetic rubber. J. Appl. Polym. Sci. 2004, 92, 3583–3592. [Google Scholar] [CrossRef]
- Hait, S.; De, D.; Ghosh, P.; Chanda, J.; Mukhopadhyay, R.; Dasgupta, S.; Sallat, A.; Al Aiti, M.; Stöckelhuber, K.W.; Wießner, S.; et al. Understanding the coupling effect between lignin and polybutadiene elastomer. J. Compos. Sci. 2021, 5, 154. [Google Scholar] [CrossRef]
- Ellis, B.; Welding, G.N. Estimation, from swelling, of the structural contribution of chemical reactions to the vulcanization of natural rubber. Part I. General method. Rubber Chem. Technol. 1964, 37, 563–570. [Google Scholar] [CrossRef]
- Ellis, B.; Welding, G.N. Estimation, from swelling, of the structural contribution of chemical reactions to the vulcanization of natural rubber. Part II. Estimation of equilibrium degree of swelling. Rubber Chem. Technol. 1964, 37, 571–575. [Google Scholar] [CrossRef]
- Valentın, J.L.; Carretero-Gonzalez, J.; Mora-Barrantes, I.; Chasse, W.; Saalwachter, K. Uncertainties in the determination of cross-link density by equilibrium swelling experiments in natural rubber. Macromolecules 2008, 41, 4717–4729. [Google Scholar] [CrossRef]
- Blanks, R.F.; Prausnitz, J.M. Thermodynamics of polymer solubility in polar and nonpolar systems. Ind. Eng. Chem. Fundam. 1964, 3, 1–8. [Google Scholar] [CrossRef]
- Marzocca, A.J. Evaluation of the polymer–solvent interaction parameter for the system cured styrene butadiene rubber and toluene. Eur. Polym. J. 2007, 43, 2682–2689. [Google Scholar] [CrossRef]
- Marzocca, A.J.; Garraza, A.R.; Mansilla, M. Evaluation of the polymer–solvent interaction parameter for the system cured polybutadiene rubber and toluene. Polym. Test. 2010, 29, 119–126. [Google Scholar] [CrossRef]
- Zielińska, M.; Seyger, R.; Dierkes, W.K.; Bielinski, D.; Noordermeer, J.W.M. Swelling of EPDM rubbers for oil-well applications as influenced by medium composition and temperature Part I. Literature and theoretical background. Elastomery 2016, 20, 6–12. [Google Scholar]
- Simon, D.A.; Barany, T. Effective thermomechanical devulcanization of ground tire rubber with a co-rotating twin-screw extruder. Polym. Degrad. Stab. 2021, 190, 109626. [Google Scholar] [CrossRef]
- van Duin, M. Chemistry of EPDM cross-linking. Kautsch. Gummi Kunstst. 2002, 55, 150–156. [Google Scholar]
- Stelescu, M.D. High-Performance Thermoplastic Elastomers Based on Ethylene-Propylene Terpolymer Rubber (EPDM) Which Can Be Used in the Footwear Industry; Performantica Publishing House: Iasi, Romania, 2011. [Google Scholar]
- Orza, R.A.; Magusin, P.C.M.M.; Litvinov, V.M.; van Duin, M.; Michels, M.A.J. Mechanism for peroxide cross-linking of EPDM rubber from MAS 13C NMR spectroscopy. Macromolecules 2009, 42, 8914–8924. [Google Scholar] [CrossRef]
- Granström, M. Cellulose Derivatives: Synthesis, Properties and Applications. Ph.D. Thesis, University of Helsinki, Helsinki, Finland, 2009. [Google Scholar]
- Chavan, R.B.; Rathi, S.; Sainaga Jyothi, V.G.S.; Shastri, N.R. Cellulose based polymers in development of amorphous solid dispersions. Asian J. Pharm. Sci. 2018, 14, 248–264. [Google Scholar] [CrossRef]
- Hofmann, W. Rubber Technology Handbook; Hanser Publishers: Munnich, Germany, 1989; pp. 93–100. [Google Scholar]
- Lee, S.H.; Park, S.Y.; Chung, K.H.; Jang, K.S. Phlogopite-reinforced natural rubber (NR)/ethylene-propylene diene monomer rubber (EPDM) composites with aminosilane compatibilizer. Polymers 2021, 13, 2318. [Google Scholar] [CrossRef] [PubMed]
- Conant, F.S. Physical testing of vulcanizates, In Rubber Technology, 3rd ed.; Morton, M., Ed.; Springer Science: Dordrecht, The Netherlands, 1999; pp. 134–178. [Google Scholar]
- Nor, N.A.M.; Othman, N. Effect of filler loading on curing characteristic and tensile properties of palygorskite natural rubber nanocomposites. Procedia Chem. 2016, 19, 351–358. [Google Scholar] [CrossRef]
- Wang, J.; Wu, W.; Wang, W.; Zhang, J. Efect of a coupling agent on the properties of hemp-hurd-powder-filled styrene-butadiene rubber. J. Appl. Polym. Sci. 2011, 121, 681–689. [Google Scholar] [CrossRef]
- Stelescu, M.D.; Airinei, A.; Manaila, E.; Craciun, G.; Fifere, N.; Varganici, C. Property correlations for composites based on ethylene propylene diene rubber reinforced with flax fibers. Polym. Test. 2017, 59, 75–83. [Google Scholar] [CrossRef]
- Azahari, N.A.; Othman, N.; Ismail, H. Effect of attapulgite clay on biodegradability and tensile properties of polyvinyl alcohol/corn starch blend film. Int. J. Polym. Mater. 2012, 61, 1065–1078. [Google Scholar] [CrossRef]
- Cristea, M.; Airinei, A.; Ionita, D.; Stelescu, M.D. Relaxation behavior of flax reinforced ethylene-propylene ediene rubber. High. Perform. Polym. 2015, 27, 676–682. [Google Scholar] [CrossRef]
- Puch, F.; Hopmann, C. Experimental investigation of the influence of the compounding process and the composite composition on the mechanical properties of a short-flax fiber reinforced polypropylene composite. Polym. Compos. 2015, 36, 2282–2290. [Google Scholar] [CrossRef]
- Hoy, K.L. Solubility parameter as a design parameter for water borne polymers and coatings. J. Paint Technol. 1970, 42, 115–118. [Google Scholar] [CrossRef]
- Bakhtiarian, E.; Foot, P.J.S.; Miller Tate, P.C. Conductive poly(epichlorohydrin)-polyaniline dodecylbenzenessulfonate in solution. Progr. Rubber Plast Recycl. Technol. 2016, 32, 183–200. [Google Scholar] [CrossRef]
- Fried, J.R. Polymer Science and Technology, 3rd ed.; Prentice Hall: New York, NY, USA, 2014; pp. 101–152. [Google Scholar]
- Kraus, G. Degree of cure in filler-reinforced vulcanizates by the swelling method. Rubber Chem. Technol. 1957, 30, 928–951. [Google Scholar] [CrossRef]
- Kraus, G. Swelling of filler-reinforced vulcanizates. J. Appl. Polym. Sci. 1963, 7, 861–871. [Google Scholar] [CrossRef]
- Mathew, L.; Joseph, K.U.; Joseph, R. Swelling behaviour of isora/natural rubber composites in oils used in automobiles. Bull. Mater. Sci. 2006, 29, 91–99. [Google Scholar] [CrossRef]
- Parks, C.; Lorenz, O. Crosslinking efficiency in the reaction of dicumyl peroxide with dimethyloctadiene. J. Polym. Sci. 1961, 50, 287–298. [Google Scholar] [CrossRef]
- Jacob, M.; Thomas, S.; Varughese, K.T. Mechanical properties of sisal/oil palm hybrid fiber reinforced natural rubber composites. Comp. Sci. Technol. 2004, 64, 955–965. [Google Scholar] [CrossRef]
- Das, A.; Stockel Huber, K.W.; Wang, D.Y.; Galiatstos, V.; Heinrich, G. Understanding the reinforcing behavior of expanded clay partides in natural rubber compounds. Soft Matter 2013, 9, 3798–3808. [Google Scholar]
- Pejića, B.M.; Kramara, A.D.; Obradovićb, B.M.; Kuraicab, M.M.; Žekićb, A.A.; Kostića, M.M. Effect of plasma treatment on chemical composition, structure and sorption properties of lignocellulosic hemp fibers (Cannabis sativa L.). Carbohydr. Polym. 2020, 236, 116000. [Google Scholar] [CrossRef]
- Haghighatnia, T.; Abbasian, A.; Morshedian, J. Hemp fiber reinforced thermoplastic polyurethane composite: An investigation in mechanical properties. Ind. Crops Prod. 2017, 108, 853–863. [Google Scholar] [CrossRef]
- Célino, A.; Gonçalves, O.; Jacquemin, F.; Fréour, S. Qualitative and quantitative assessment of water sorption in natural fibres using ATR-FTIR spectroscopy. Carbohydr. Polym. 2014, 101, 163–170. [Google Scholar] [CrossRef]
- Lazorenko, G.; Kasprzhitskii, A.; Yavna, V.; Mischinenko, V.; Kukharskii, A.; Kruglikov, A.; Kolodina, A.; Yalovega, G. Effect of pre-treatment of flax tows on mechanical properties and microstructure of natural fiber reinforced geopolymer composites. Env. Technol. Innov. 2020, 20, 101105. [Google Scholar] [CrossRef]
- Abidi, N.; Cabrales, L.; Haigler, C.H. Changes in the cell wall and cellulose content of developing cotton fibers investigated by FTIR spectroscopy. Carbohydr. Polym. 2014, 100, 9–16. [Google Scholar] [CrossRef]
- Gunasekaram, S.; Natarajan, R.K.; Kala, A. FTIR spectra and mechanical strength analysis of some selected rubber derivatives. Spectrochim. Acta Part A 2007, 68, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Litvinov, V.M.; De, P.P. Spectroscopy of Rubber and Rubbery Materials; RAPRA Technol. Ltd.: Shawbury, UK, 2002. [Google Scholar]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems—With special reference to the determination of surface area and porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Dhakal, H.N.; Zhang, Z.Y.; Richardson, M.O.W. Effect of water absorption on the mechanical properties of hemp fibre reinforced un saturated polyester composites. Compos. Sci. Technol. 2007, 67, 1674–1683. [Google Scholar] [CrossRef]
- Brunauer, S.; Deming, L.S.; Deming, W.E.; Teller, E. On a theory of the van der Waals adsorption of gases. J. Am. Chem. Soc. 1940, 62, 1723–1732. [Google Scholar] [CrossRef]
- Murray, K.L.; Seaton, N.A.; Day, M.A. An adsorption-based method for the characterization of pore networks containing both mesopores and macropores. Langmuir 1999, 15, 6728–6737. [Google Scholar] [CrossRef]
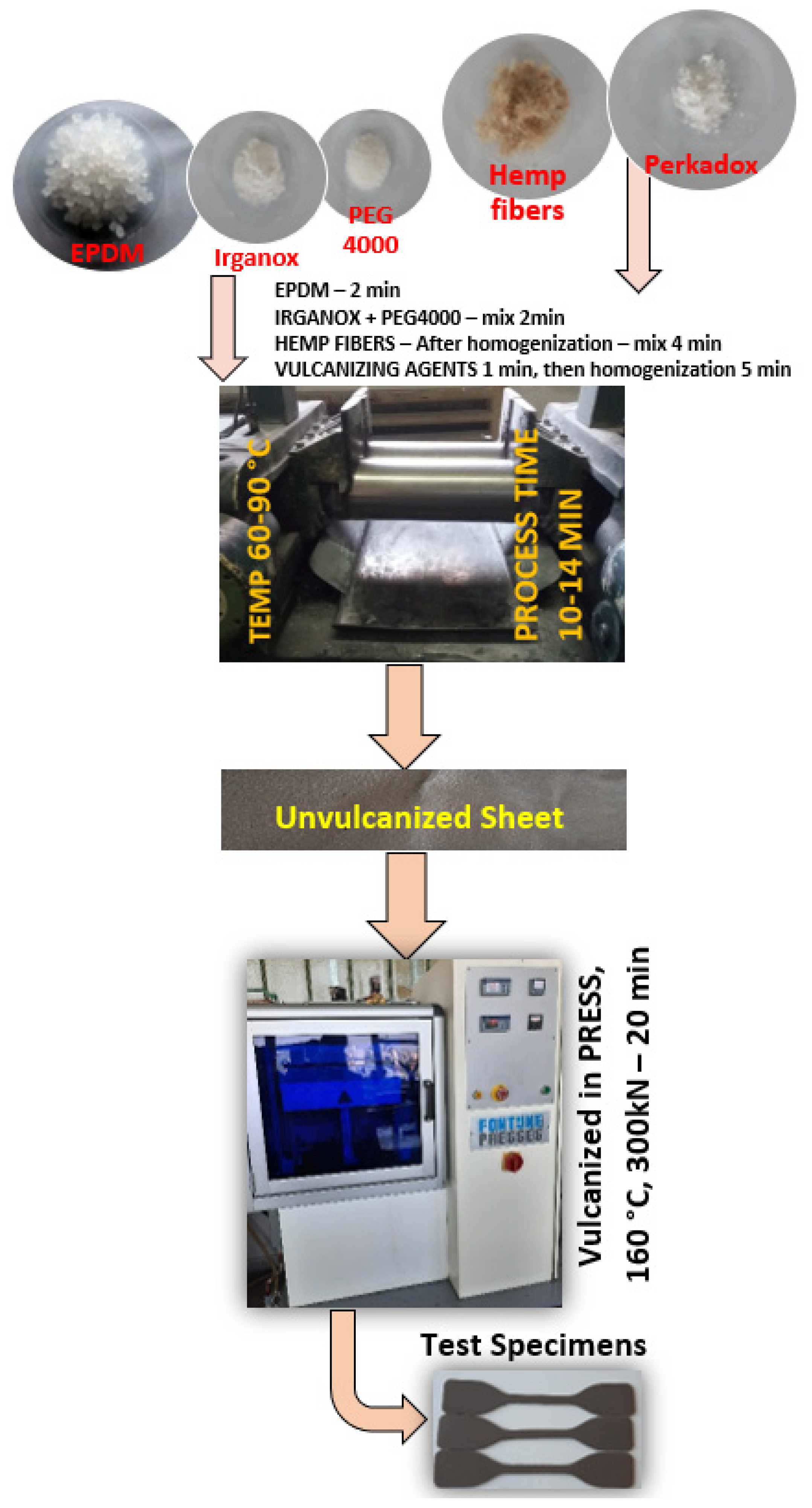




| Ingredients | Sample | ||||
|---|---|---|---|---|---|
| EP0 | EP5Ca | EP10Ca | EP15Ca | EP20Ca | |
| Polymer matrix, EPDM | 100 | 100 | 100 | 100 | 100 |
| reinforcing agent, hemp | 0 | 5 | 10 | 15 | 20 |
| Lubricating agent, PEG 4000 | 3 | 3 | 3 | 3 | 3 |
| Antioxidant, Irganox 1010 | 1 | 1 | 1 | 1 | 1 |
| Vulcanizing agent, Perkadox 14-40B | 8 | 8 | 8 | 8 | 8 |
| Property | Sample | ||||
|---|---|---|---|---|---|
| EP0 | EP5Ca | EP10Ca | EP15Ca | EP20Ca | |
| Hardness, °ShA | 62 ± 0.58 | 67 ± 1.00 | 74 ± 1.00 | 76 ± 0.58 | 78 ± 0.58 |
| Elasticity, % | 64 ± 0.57 | 56 ± 0.57 | 50 ± 0.57 | 46 ± 0.57 | 46 ± 0.57 |
| 100% modulus, N/mm2 | 1.1 ± 0.03 | 1.4 ± 0.07 | 1.4 ± 0.01 | 1.4 ± 0.01 | 1.3 ± 0.03 |
| Tensile strength at break, N/mm2 | 1.9 ± 0.12 | 1.8 ± 0.06 | 1.8 ± 0.03 | 1.8 ± 0.03 | 1.7 ± 0.03 |
| Elongation at break, % | 287 ± 6.67 | 200 ± 5.77 | 193 ± 6.67 | 220 ± 1.67 | 200 ± 2.89 |
| Group | E (MPa1/2 cm3mol−1) |
|---|---|
| −CH3 | 303 |
| −CH2− | 269 |
| >CH− | 176 |
| >CH=CH− | 422 |
| Sample | W (%) | (nm) | BET Data * | |
|---|---|---|---|---|
| Area (m2/g) | Monolayer (g/g) | |||
| EP0 | 0.7573 | 2.23 | 6.784 | 0.0019 |
| EPCa5 | 1.0091 | 2.67 | 7.561 | 0.0022 |
| EPCa10 | 1.4261 | 1.62 | 17.639 | 0.0050 |
| EPCa15 | 1.7594 | 2.93 | 12.009 | 0.0034 |
| EPCa20 | 2.0199 | 2.09 | 19.329 | 0.0055 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stelescu, M.D.; Airinei, A.; Bargan, A.; Fifere, N.; Georgescu, M.; Sonmez, M.; Nituica, M.; Alexandrescu, L.; Stefan, A. Mechanical Properties and Equilibrium Swelling Characteristics of Some Polymer Composites Based on Ethylene Propylene Diene Terpolymer (EPDM) Reinforced with Hemp Fibers. Materials 2022, 15, 6838. https://doi.org/10.3390/ma15196838
Stelescu MD, Airinei A, Bargan A, Fifere N, Georgescu M, Sonmez M, Nituica M, Alexandrescu L, Stefan A. Mechanical Properties and Equilibrium Swelling Characteristics of Some Polymer Composites Based on Ethylene Propylene Diene Terpolymer (EPDM) Reinforced with Hemp Fibers. Materials. 2022; 15(19):6838. https://doi.org/10.3390/ma15196838
Chicago/Turabian StyleStelescu, Maria Daniela, Anton Airinei, Alexandra Bargan, Nicusor Fifere, Mihai Georgescu, Maria Sonmez, Mihaela Nituica, Laurentia Alexandrescu, and Adriana Stefan. 2022. "Mechanical Properties and Equilibrium Swelling Characteristics of Some Polymer Composites Based on Ethylene Propylene Diene Terpolymer (EPDM) Reinforced with Hemp Fibers" Materials 15, no. 19: 6838. https://doi.org/10.3390/ma15196838
APA StyleStelescu, M. D., Airinei, A., Bargan, A., Fifere, N., Georgescu, M., Sonmez, M., Nituica, M., Alexandrescu, L., & Stefan, A. (2022). Mechanical Properties and Equilibrium Swelling Characteristics of Some Polymer Composites Based on Ethylene Propylene Diene Terpolymer (EPDM) Reinforced with Hemp Fibers. Materials, 15(19), 6838. https://doi.org/10.3390/ma15196838








