Iodide Removal by Resorcinol-Formaldehyde Carbon Aerogels
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Synthesis
2.2. Characterization
2.3. Iodide Adsorption
2.3.1. Kinetics
2.3.2. Isotherms
3. Results and Discussion
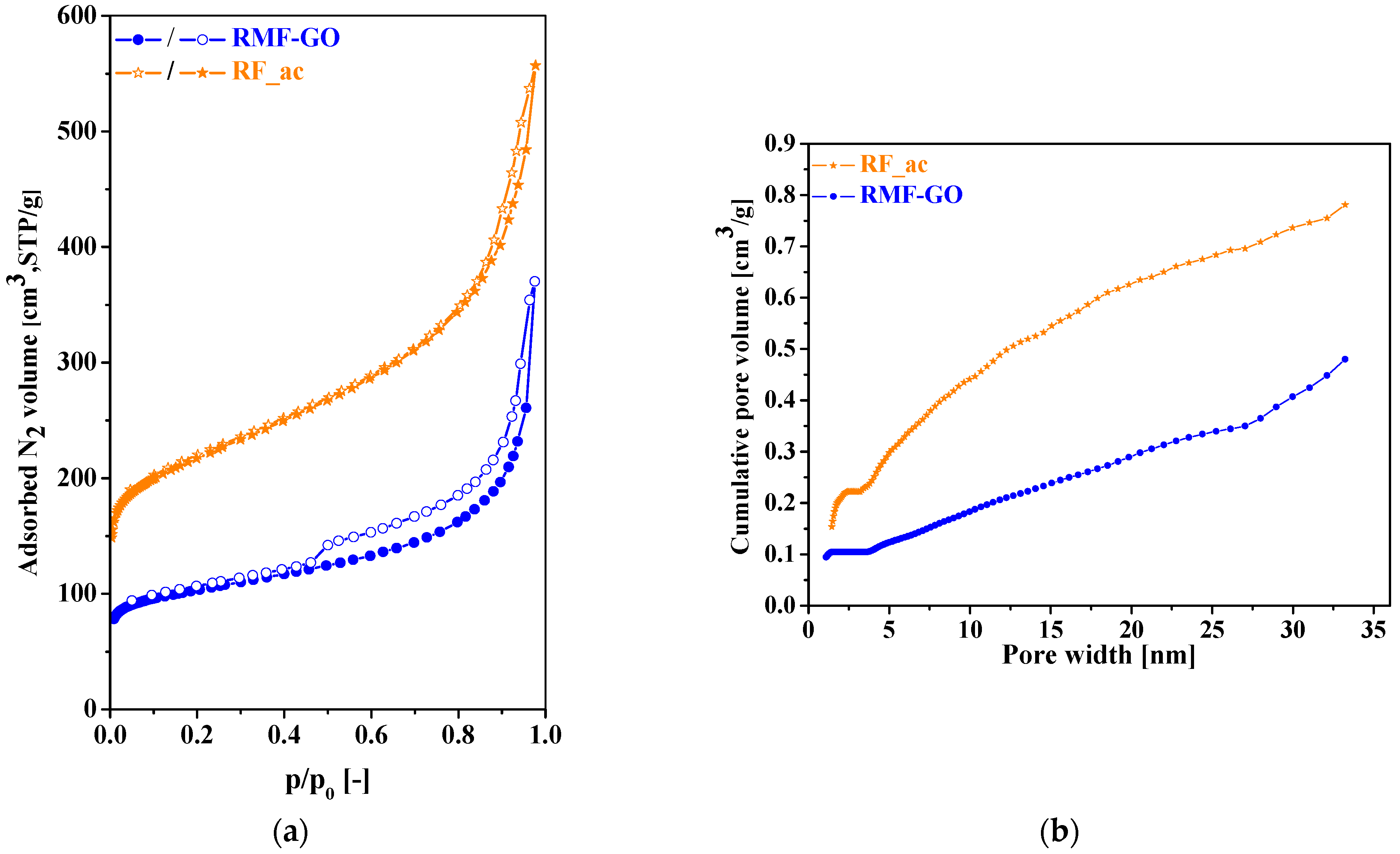
3.1. Characterization of the Carbon Aerogels
3.1.1. Morphology
3.1.2. Surface Chemistry
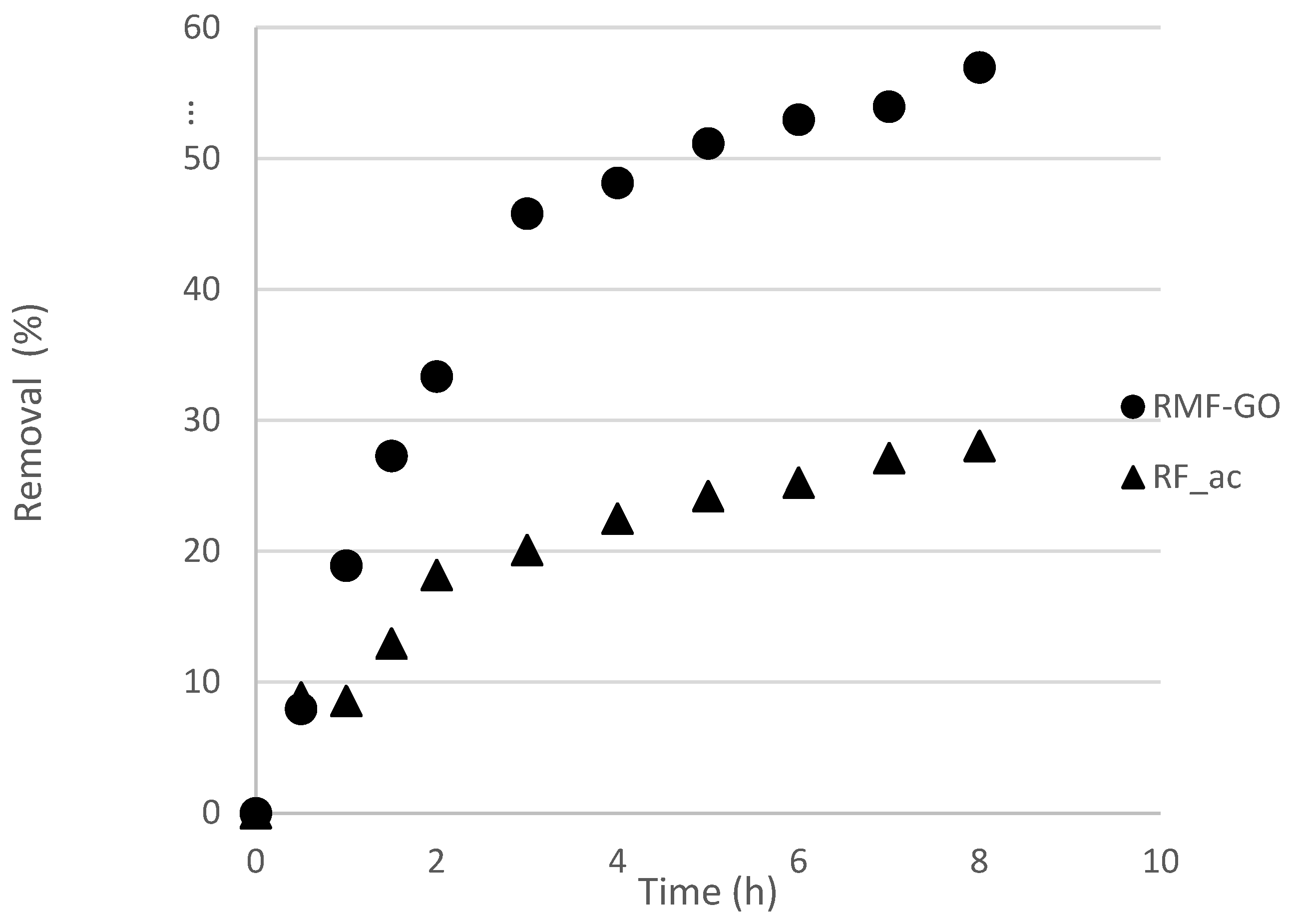
3.2. Iodide Adsorption
3.2.1. Experimental Results
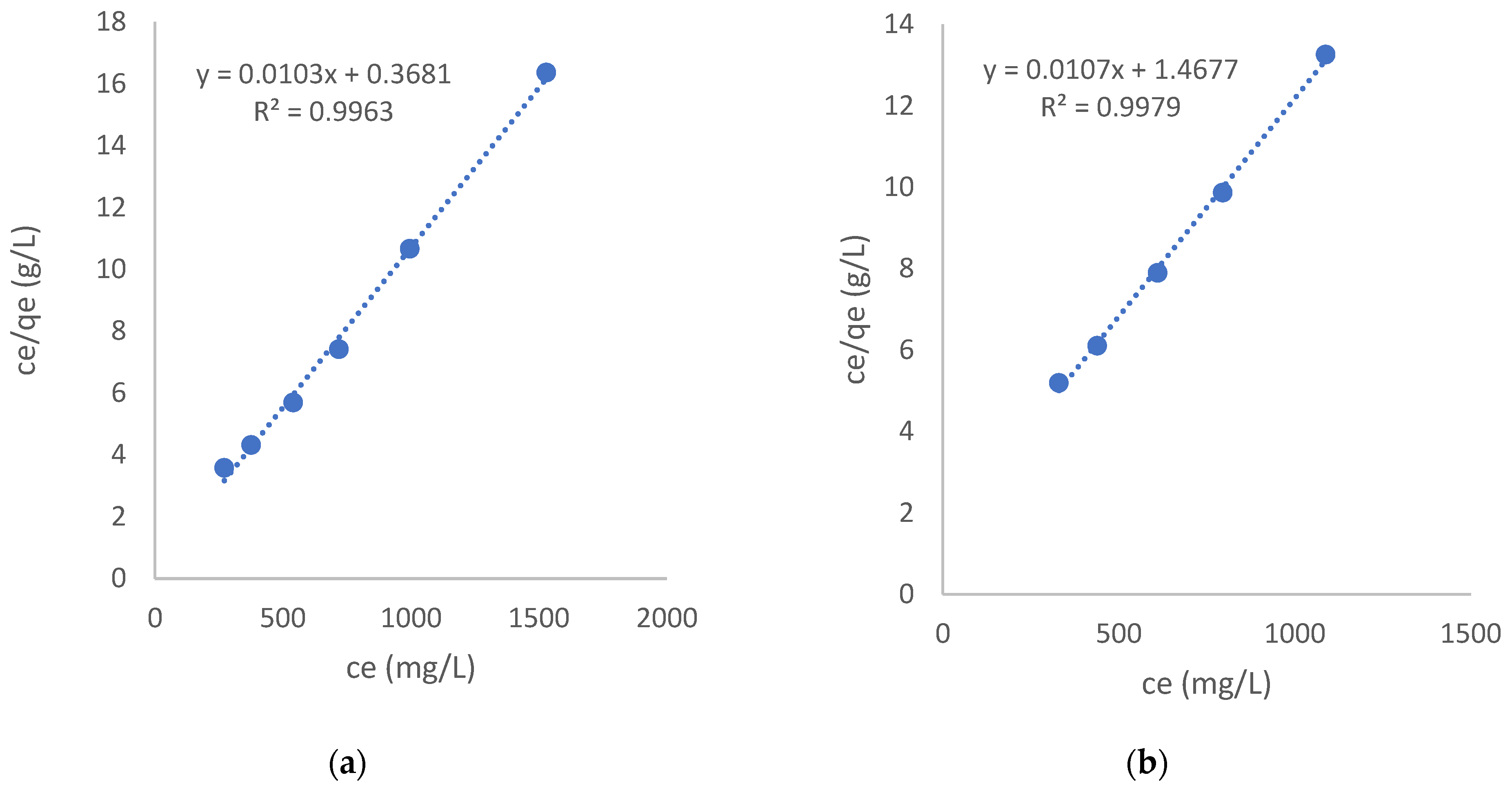
3.2.2. Isotherms Modeling
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shannon, M.A.; Bohn, P.W.; Elimelech, M.; Georgiadis, J.G.; Marĩas, B.J.; Mayes, A.M. Science and Technology for Water Purification in the Coming Decades. Nature 2008, 452, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Fuge, R.; Johnson, C.C. Iodine and Human Health, the Role of Environmental Geochemistry and Diet, a Review. Appl. Geochem. 2015, 63, 282–302. [Google Scholar] [CrossRef]
- MacKeown, H.; von Gunten, U.; Criquet, J. Iodide Sources in the Aquatic Environment and Its Fate during Oxidative Water Treatment—A Critical Review. Water Res. 2022, 217, 118417. [Google Scholar] [CrossRef]
- Zhang, X.; Gu, P.; Zhou, S.; Li, X.; Zhang, G.; Dong, L. Enhanced Removal of Iodide Ions by Nano Cu2O/Cu Modified Activated Carbon from Simulated Wastewater with Improved Countercurrent Two-Stage Adsorption. Sci. Total Environ. 2018, 626, 612–620. [Google Scholar] [CrossRef] [PubMed]
- Buesseler, K.O.; Jayne, S.R.; Fisher, N.S.; Rypina, I.I.; Baumann, H.; Baumann, Z.; Breier, C.F.; Douglass, E.M.; George, J.; Macdonald, A.M.; et al. Fukushima-Derived Radionuclides in the Ocean and Biota off Japan. Proc. Natl. Acad. Sci. USA 2012, 109, 5984–5988. [Google Scholar] [CrossRef]
- Woo, T.H. Atmospheric Modeling of Radioactive Material Dispersion and Health Risk in Fukushima Daiichi Nuclear Power Plants Accident. Ann. Nucl. Energy 2013, 53, 197–201. [Google Scholar] [CrossRef]
- González-García, C.M.; González, J.F.; Román, S. Removal Efficiency of Radioactive Methyl Iodide on TEDA-Impregnated Activated Carbons. Fuel Process. Technol. 2011, 92, 247–252. [Google Scholar] [CrossRef]
- Awual, M.R.; Suzuki, S.; Taguchi, T.; Shiwaku, H.; Okamoto, Y.; Yaita, T. Radioactive Cesium Removal from Nuclear Wastewater by Novel Inorganic and Conjugate Adsorbents. Chem. Eng. J. 2014, 242, 127–135. [Google Scholar] [CrossRef]
- Fujiwara, H. Observation of Radioactive Iodine (131I,129I) in Cropland Soil after the Fukushima Nuclear Accident. Sci. Total Environ. 2016, 566–567, 1432–1439. [Google Scholar] [CrossRef] [PubMed]
- Kosaka, K.; Asami, M.; Kobashigawa, N.; Ohkubo, K.; Terada, H.; Kishida, N.; Akiba, M. Removal of Radioactive Iodine and Cesium in Water Purification Processes after an Explosion at a Nuclear Power Plant Due to the Great East Japan Earthquake. Water Res. 2012, 46, 4397–4404. [Google Scholar] [CrossRef]
- Kubota, T.; Fukutani, S.; Ohta, T.; Mahara, Y. Removal of Radioactive Cesium, Strontium, and Iodine from Natural Waters Using Bentonite, Zeolite, and Activated Carbon. J. Radioanal. Nucl. Chem. 2013, 296, 981–984. [Google Scholar] [CrossRef]
- Ohta, T.; Mahara, Y.; Kubota, T.; Fukutani, S.; Fujiwara, K.; Takamiya, K.; Yoshinaga, H.; Mizuochi, H.; Igarashi, T. Prediction of Groundwater Contamination with 137Cs and 131I from the Fukushima Nuclear Accident in the Kanto District. J. Environ. Radioact. 2012, 111, 38–41. [Google Scholar] [CrossRef]
- Dong, H.; Qiang, Z.; Richardson, S.D. Formation of Iodinated Disinfection Byproducts (I-DBPs) in Drinking Water: Emerging Concerns and Current Issues. Acc. Chem. Res. 2019, 52, 896–905. [Google Scholar] [CrossRef]
- Yu, F.; Chen, Y.; Wang, Y.; Liu, C.; Ma, W. Enhanced Removal of Iodide from Aqueous Solution by Ozonation and Subsequent Adsorption on Ag-Ag2O Modified on Carbon Spheres. Appl. Surf. Sci. 2018, 427, 753–762. [Google Scholar] [CrossRef]
- Singh, N.B.; Nagpal, G.; Agrawal, S. Rachna Water Purification by Using Adsorbents: A Review. Environ. Technol. Innov. 2018, 11, 187–240. [Google Scholar] [CrossRef]
- Tandekar, S.A.; Pande, M.A.; Shekhawat, A.; Fosso-Kankeu, E.; Pandey, S.; Jugade, R.M. Fe(III)–Chitosan Microbeads for Adsorptive Removal of Cr(VI) and Phosphate Ions. Minerals 2022, 12, 874. [Google Scholar] [CrossRef]
- do Nascimento, F.H.; Masini, J.C. Vermiculite Modified with Fe3+ Polyhydroxy Cations Is a Low-Cost and Highly Available Adsorbent for the Removal of Phosphate Ions. Minerals 2022, 12, 1033. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Ahmad, H.; Parveen, T.; Ahmad, A.; Oves, M.; Ismail, I.M.I.; Qari, H.A.; Umar, K.; Mohamad Ibrahim, M.N. Recent Advances in Metal Decorated Nanomaterials and Their Various Biological Applications: A Review. Front. Chem. 2020, 8, 341. [Google Scholar] [CrossRef] [PubMed]
- Watson, K.; Farré, M.J.; Knight, N. Strategies for the Removal of Halides from Drinking Water Sources, and Their Applicability in Disinfection by-Product Minimisation: A Critical Review. J. Environ. Manag. 2012, 110, 276–298. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Dong, Q.; Yu, W.; Qin, Z.; Nie, X.; Wan, Q.; Chen, X. Preparation of Halloysite/Ag2O Nanomaterials and Their Performance for Iodide Adsorption. Minerals 2022, 12, 304. [Google Scholar] [CrossRef]
- Tauanov, Z.; Inglezakis, V.J. Removal of Iodide from Water Using Silver Nanoparticles-Impregnated Synthetic Zeolites. Sci. Total Environ. 2019, 682, 259–270. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Parveen, T.; Umar, K.; Ibrahim, M.N.M. Role of Nanomaterials in the Treatment of Wastewater: A Review. Water 2020, 12, 495. [Google Scholar] [CrossRef]
- Sánchez-Polo, M.; Rivera-Utrilla, J.; Salhi, E.; von Gunten, U. Removal of Bromide and Iodide Anions from Drinking Water by Silver-Activated Carbon Aerogels. J. Colloid Interface Sci. 2006, 300, 437–441. [Google Scholar] [CrossRef]
- Sánchez-Polo, M.; Rivera-Utrilla, J.; Salhi, E.; von Gunten, U. Ag-Doped Carbon Aerogels for Removing Halide Ions in Water Treatment. Water Res. 2007, 41, 1031–1037. [Google Scholar] [CrossRef]
- Sánchez-Polo, M.; Rivera-Utrilla, J.; Méndez-Díaz, J.; López-Peñalver, J. Metal-Doped Carbon Aerogels New Materials for Water Treatments. Ind. Eng. Chem. Res. 2008, 47, 6001–6005. [Google Scholar] [CrossRef]
- Zhang, H.; Hu, Y.; Ye, X.; Liu, H.; Li, Q.; Guo, M.; Wu, Z. Iodide Adsorption from Aqueous Solutions by Bis(Trimethoxysilylpropyl)Amine Polycondensate/Silver Chloride Composites. Desalination Water Treat. 2013, 51, 3930–3937. [Google Scholar] [CrossRef]
- Ikari, M.; Matsui, Y.; Suzuki, Y.; Matsushita, T.; Shirasaki, N. Removal of Iodide from Water by Chlorination and Subsequent Adsorption on Powdered Activated Carbon. Water Res. 2015, 68, 227–237. [Google Scholar] [CrossRef]
- Hoskins, J.S.; Karanfil, T.; Serkiz, S.M. Removal and Sequestration of Iodide Using Silver-Impregnated Activated Carbon. Environ. Sci. Technol. 2002, 36, 784–789. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Kaplan, D.I.; Knox, A.S.; Crapse, K.P.; Diprete, D.P. Aqueous 99Tc, 129I and 137Cs Removal from Contaminated Groundwater and Sediments Using Highly Effective Low-Cost Sorbents. J. Environ. Radioact. 2014, 136, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Zhang, Y.; Ye, X.; Liu, H.; Zhang, H.; Wu, A.; Wu, Z. Removal of I- from Aqueous Solutions Using a Biomass Carbonaceous Aerogel Modified with KH-560. ACS Sustain. Chem. Eng. 2017, 5, 7700–7708. [Google Scholar] [CrossRef]
- Balsley, S.D.; Brady, P.V.; Krumhansl, J.L.; Anderson, H.L. Anion Scavengers for Low-Level Radioactive Waste Repository Backfills. Soil Sediment Contam. 1998, 7, 125–141. [Google Scholar] [CrossRef]
- Chen, B.; Ma, Q.; Tan, C.; Lim, T.T.; Huang, L.; Zhang, H. Carbon-Based Sorbents with Three-Dimensional Architectures for Water Remediation. Small 2015, 11, 3319–3336. [Google Scholar] [CrossRef]
- Ma, Q.; Yu, Y.; Sindoro, M.; Fane, A.G.; Wang, R.; Zhang, H. Carbon-Based Functional Materials Derived from Waste for Water Remediation and Energy Storage. Adv. Mater. 2017, 29, 1605361. [Google Scholar] [CrossRef]
- Dos Santos, M.C.; Maynart, M.C.; Aveiro, L.R.; da Paz, E.C.; dos Santos Pinheiro, V. Carbon-Based Materials: Recent Advances, Challenges, and Perspectives. In Reference Module in Materials Science and Materials Engineering; Elsevier Ltd.: Amsterdam, The Netherlands, 2017; pp. 1–12. ISBN 9780128035818. [Google Scholar]
- Jawaid, M.; Ahmad, A.; Ismail, N.; Rafatullah, M. Green Energy and Technology Environmental Remediation Through Carbon Based Nano Composites; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Zuo, L.; Zhang, Y.; Zhang, L.; Miao, Y.E.; Fan, W.; Liu, T. Polymer/Carbon-Based Hybrid Aerogels: Preparation, Properties and Applications. Materials 2015, 8, 6806–6848. [Google Scholar] [CrossRef]
- Hu, L.; He, R.; Lei, H.; Fang, D. Carbon Aerogel for Insulation Applications: A Review. Int. J. Thermophys. 2019, 40, 1–25. [Google Scholar] [CrossRef]
- Nagy, B.; Ábrahám, D.; Dobos, G.; Madarász, J.; Onyestyák, G.; Sáfrán, G.; Geissler, E.; László, K. Molybdenum Doped Carbon Aerogels with Catalytic Potential. Carbon 2014, 66, 210–218. [Google Scholar] [CrossRef]
- Seredych, M.; László, K.; Rodríguez-Castellón, E.; Bandosz, T.J. S-Doped Carbon Aerogels/GO Composites as Oxygen Reduction Catalysts. J. Energy Chem. 2016, 25, 236–245. [Google Scholar] [CrossRef]
- Biener, J.; Stadermann, M.; Suss, M.; Worsley, M.A.; Biener, M.M.; Rose, K.A.; Baumann, T.F. Advanced Carbon Aerogels for Energy Applications. Energy Environ. Sci. 2011, 4, 656–667. [Google Scholar] [CrossRef]
- Maleki, H. Recent Advances in Aerogels for Environmental Remediation Applications: A Review. Chem. Eng. J. 2016, 300, 98–118. [Google Scholar] [CrossRef]
- Smirnova, I.; Gurikov, P. Aerogel Production: Current Status, Research Directions, and Future Opportunities. J. Supercrit. Fluids 2018, 134, 228–233. [Google Scholar] [CrossRef]
- Pekala, R.W. Organic Aerogels from the Polycondensation of Resorcinol with Formaldehyde. J. Mater. Sci. 1989, 24, 3221–3227. [Google Scholar] [CrossRef]
- Fathy, N.A.; Rizk, M.S.; Awad, R.M.S. Pore Structure and Adsorption Properties of Carbon Xerogels Derived from Carbonization of Tannic Acid-Resorcinol-Formaldehyde Resin. J. Anal. Appl. Pyrolysis 2016, 119, 60–68. [Google Scholar] [CrossRef]
- Li, F.; Xie, L.; Sun, G.; Kong, Q.; Su, F.; Cao, Y.; Wei, J.; Ahmad, A.; Guo, X.; Chen, C.M. Resorcinol-Formaldehyde Based Carbon Aerogel: Preparation, Structure and Applications in Energy Storage Devices. Microporous Mesoporous Mater. 2019, 279, 293–315. [Google Scholar] [CrossRef]
- Elkhatat, A.M.; Al-Muhtaseb, S.A. Advances in Tailoring Resorcinol-Formaldehyde Organic and Carbon Gels. Adv. Mater. 2011, 23, 2887–2903. [Google Scholar] [CrossRef]
- Czakkel, O.; Marthi, K.; Geissler, E.; László, K. Influence of Drying on the Morphology of Resorcinol-Formaldehyde-Based Carbon Gels. Microporous Mesoporous Mater. 2005, 86, 124–133. [Google Scholar] [CrossRef]
- Nagy, B.; Domán, A.; Menyhárd, A.; László, K. Influence of Graphene Oxide Incorporation on Resorcinol-Formaldehyde Polymer and Carbon Aerogels. Period. Polytech. Chem. Eng. 2018, 62, 441–449. [Google Scholar] [CrossRef]
- White, R.J.; Yoshizawa, N.; Titirici, M. Sustainable Synthesis of Nitrogen-Doped Carbon Aerogels. Green Chem. 2011, 13, 2428–2434. [Google Scholar] [CrossRef]
- Wang, C.C.; Lu, S.Y. Carbon Black-Derived Graphene Quantum Dots Composited with Carbon Aerogel as a Highly Efficient and Stable Reduction Catalyst for the Iodide/Tri-Iodide Couple. Nanoscale 2015, 7, 1209–1215. [Google Scholar] [CrossRef]
- Ying, T.Y.; Yang, K.L.; Yiacoumi, S.; Tsouris, C. Electrosorption of Ions from Aqueous Solutions by Nanostructured Carbon Aerogel. J. Colloid Interface Sci. 2002, 250, 18–27. [Google Scholar] [CrossRef]
- Xu, P.; Drewes, J.E.; Heil, D.; Wang, G. Treatment of Brackish Produced Water Using Carbon Aerogel-Based Capacitive Deionization Technology. Water Res. 2008, 42, 2605–2617. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Polo, M.; Rivera-Utrilla, J.; von Gunten, U. Bromide and Iodide Removal from Waters under Dynamic Conditions by Ag-Doped Aerogels. J. Colloid Interface Sci. 2007, 306, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Ritter, J.A. Effect of Synthesis PH on the Structure of Carbon Xerogels. Carbon 1997, 35, 1271–1278. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved Synthesis of Graphene Oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef] [PubMed]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Dubinin, M.M.; Radushkevich, L.V. The Equation of the Characteristic Curve of Activated Charcoal. Proc. Acad. Sci. USSR Phys. Chem. Sect. 1947, 55, 327–329. [Google Scholar]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- László, K.; Tombácz, E.; Josepovits, K. Effect of Activation on the Surface Chemistry of Carbons from Polymer Precursors. Carbon 2001, 39, 1217–1228. [Google Scholar] [CrossRef]
- Nagy, B.; Villar-Rodil, S.; Tascón, J.M.D.; Bakos, I.; László, K. Nitrogen Doped Mesoporous Carbon Aerogels and Implications for Electrocatalytic Oxygen Reduction Reactions. Microporous Mesoporous Mater. 2016, 230, 135–144. [Google Scholar] [CrossRef]
- Radovic, L.R. Chemistry of Activated Carbon Materials. In Surfaces of Nanoparticles and Porous Materials; Scharz, J.A., Contescu, C.I., Eds.; Markel Dekker: New York, NY, USA, 1999; pp. 529–564. [Google Scholar]
- Everett, D.H. Reporting Data on Adsorption from Solution at the Solid/Solution Interface (Recommendations 1986). Pure Appl. Chem. 1986, 58, 967–984. [Google Scholar] [CrossRef]
- Nightingale, E.R. Phenomenological Theory of Ion Solvation. Effective Radii of Hydrated Ions. J. Phys. Chem. 1959, 63, 1381–1387. [Google Scholar] [CrossRef]




| Carbon Type | Iodide Concentration (mmol/L) | Dosage (g/L) | Maximum Loading (mmol/g) | Reference |
|---|---|---|---|---|
| Ag-doped carbon aerogels | 0.15 (pH = 7) | - | 5.8 × 10−3 | [23] |
| Resorcinol–formaldehyde carbon aerogel impregnated with silver nanoparticles | 0.1–10 × 10−3 (pH = 6.5–7) | 1 | 2 × 10−3 | [24] |
| Resorcinol–formaldehyde carbon aerogel impregnated with silver nanoparticles | 0–10 × 10−3 (pH = 6.5–7) | 1 | 2 × 10−3 | [25] |
| TSPA * carbon aerogel precursor impregnated with silver chloride nanoparticles | 2–14 (pH = 1–9) | 1 | 5 | [26] |
| Powdered activated carbon | 10 × 10−3 (pH = 7) | 0.1–0.6 | 0.23 | [27] |
| Granular activated carbon and 1.05 wt% silver-impregnated granular activated carbon | 8–1576 × 10−3 (pH = 5) | 1 | 0.9–1 | [28] |
| Activated carbon and bone char | 18.5 Bq/mL 129I (pH = 7.4–9.6) | 10 | - | [29] |
| Silver- and silver-oxide-modified carbon spheres | - (pH = 1.5–2) | 0.6 | 1.97 | [14] |
| Biomass carbonaceous aerogel modified with KH-560 ** | 1–20 (pH = 1–5.4) | - | 2.4 | [30] |
| Sub-bituminous coal | 0.01 (pH = 6.2) | 1–20 | - | [31] |
| Lignite | 0.01 (pH = 3.9) | 1–20 | - | [31] |
| Sample | SBET [m2/g] | Vtot [cm3/g] | Vmicro [cm3/g] | Vmeso [cm3/g] | Vmicro/Vtot | davg [nm] |
|---|---|---|---|---|---|---|
| RF_ac | 790 | 0.86 | 0.31 | 0.55 | 0.36 | 4.4 |
| RMF-GO | 375 | 0.57 | 0.15 | 0.42 | 0.26 | 6.1 |
| Sample | C | O | N | O/C | N/C | (O + N)/C |
|---|---|---|---|---|---|---|
| Atomic% | ||||||
| RF_ac | 88.3 | 11.3 | - * | 0.128 | 0.128 | |
| RMF-GO | 94.7 | 3.4 | 1.3 | 0.036 | 0.014 | 0.149 |
| Peak Position, eV | 285.2 | 286.5 | 287.3 | 288.1 | 289.5 | 291.8 |
|---|---|---|---|---|---|---|
| RF_ac | 65.3 | 18.2 | 2.0 | 5.3 | 4.5 | 2.9 |
| RMF-GO | 70.4 | 12.1 | 3.5 | 5.0 | 3.8 | 3.0 |
| Peak Position, eV | O1s | N1s | |||
|---|---|---|---|---|---|
| 530.9–531.2 | 532.8–533.0 | 534.2–534.5 | 399.2 | 401.7 | |
| RF_ac | 5.8 | 60.0 | 34.2 | - | - |
| RMF-GO | 13.3 | 46.3 | 40.4 | 28.5 | 71.5 |
| Sample | k, 1/h | t1/2 *, h | R2 |
|---|---|---|---|
| RF_ac | 0.357 | 1.94 | 0.97302 |
| RMF-GO | 0.408 | 1.70 | 0.99357 |
| Sample | qm | KL, L/mg | Surface Coverage, I− ion/m2 | Area Available, nm2/I− ion | |
|---|---|---|---|---|---|
| mg/g | μmol/g | ||||
| RF_ac | 97.1 | 765 | 0.028 | 5.83 × 1017 | 17 |
| RMF-GO | 93.5 | 736 | 0.073 | 1.18 × 1018 | 8.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domán, A.; Battalgazy, B.; Dobos, G.; Kiss, G.; Tauanov, Z.; László, K.; Zorpas, A.A.; Inglezakis, V.J. Iodide Removal by Resorcinol-Formaldehyde Carbon Aerogels. Materials 2022, 15, 6885. https://doi.org/10.3390/ma15196885
Domán A, Battalgazy B, Dobos G, Kiss G, Tauanov Z, László K, Zorpas AA, Inglezakis VJ. Iodide Removal by Resorcinol-Formaldehyde Carbon Aerogels. Materials. 2022; 15(19):6885. https://doi.org/10.3390/ma15196885
Chicago/Turabian StyleDomán, Andrea, Bekassyl Battalgazy, Gábor Dobos, Gábor Kiss, Zhandos Tauanov, Krisztina László, Antonis A. Zorpas, and Vassilis J. Inglezakis. 2022. "Iodide Removal by Resorcinol-Formaldehyde Carbon Aerogels" Materials 15, no. 19: 6885. https://doi.org/10.3390/ma15196885
APA StyleDomán, A., Battalgazy, B., Dobos, G., Kiss, G., Tauanov, Z., László, K., Zorpas, A. A., & Inglezakis, V. J. (2022). Iodide Removal by Resorcinol-Formaldehyde Carbon Aerogels. Materials, 15(19), 6885. https://doi.org/10.3390/ma15196885









