Mechanical Properties, Crystallization Behaviors and Phase Morphologies of PLA/GTR Blends by Reactive Compatibilization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Samples Preparation
2.3. Characterizations and Evaluations
3. Results
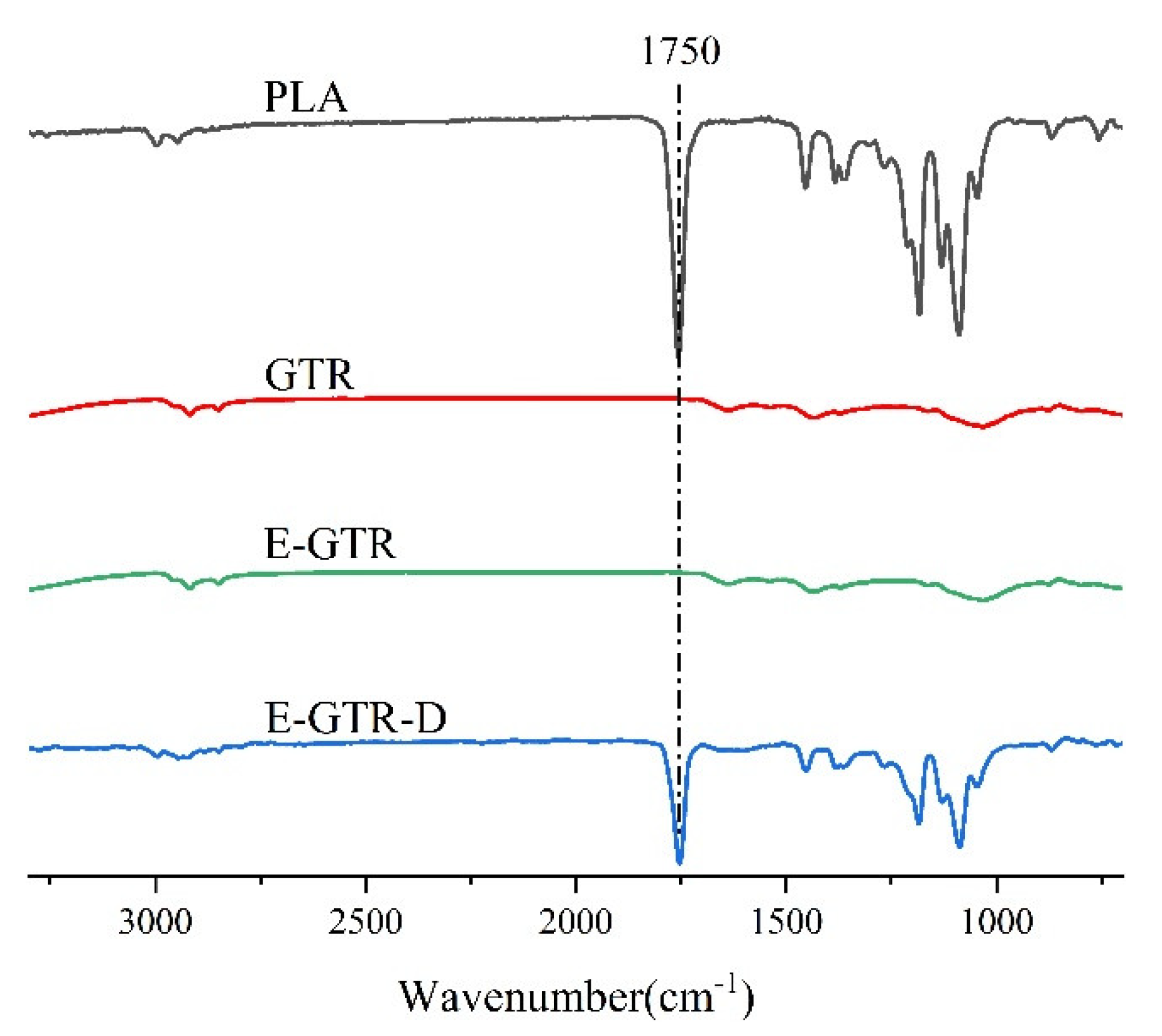
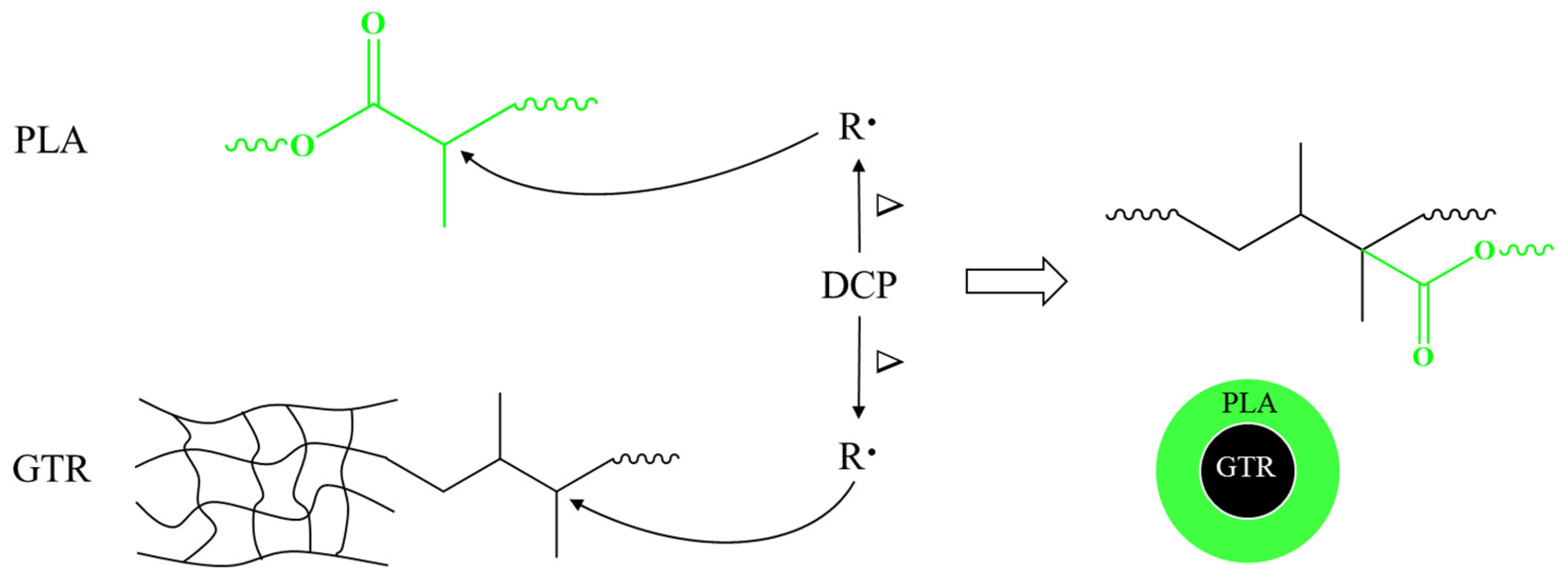
3.1. Interaction between PLA and GTR
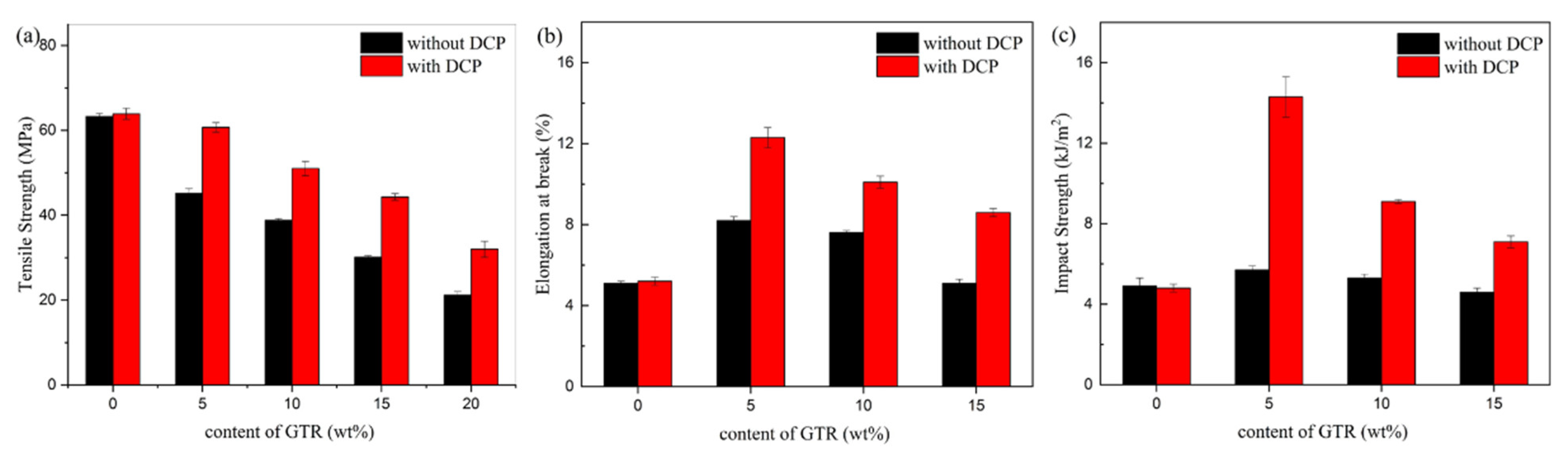
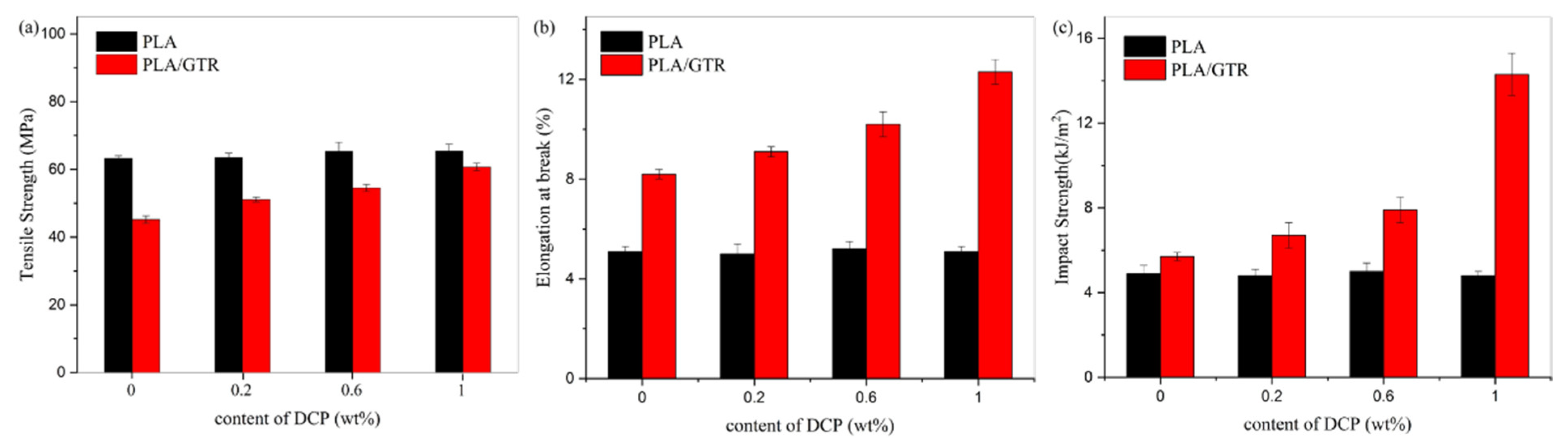
3.2. Mechanical Properties
3.3. Crystallization Behavior
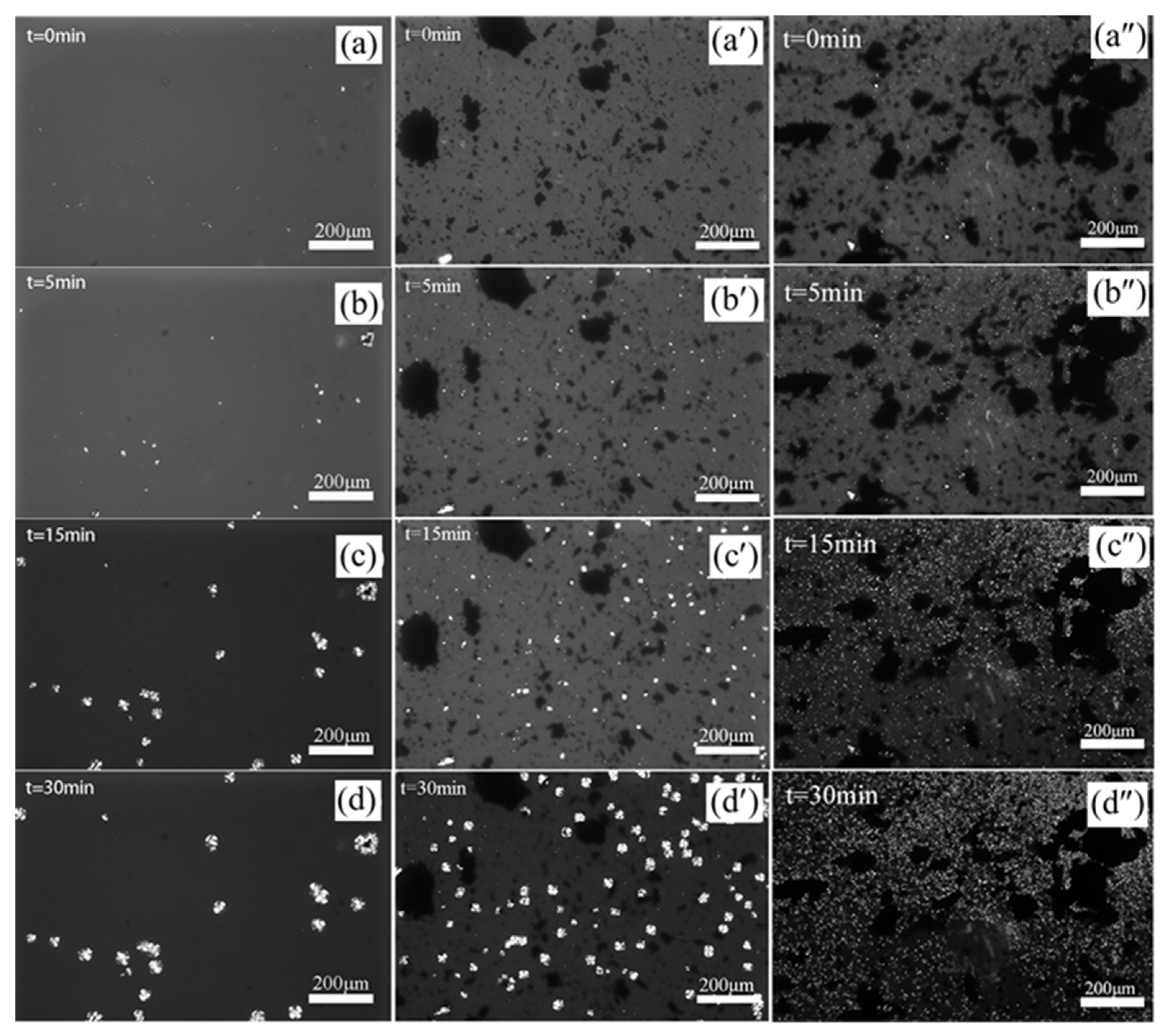
3.4. POM Observation
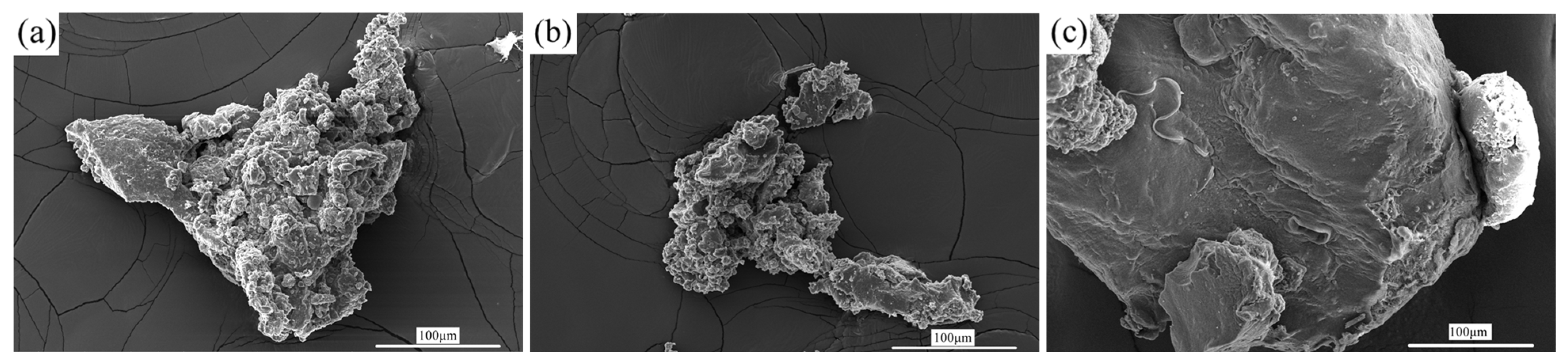
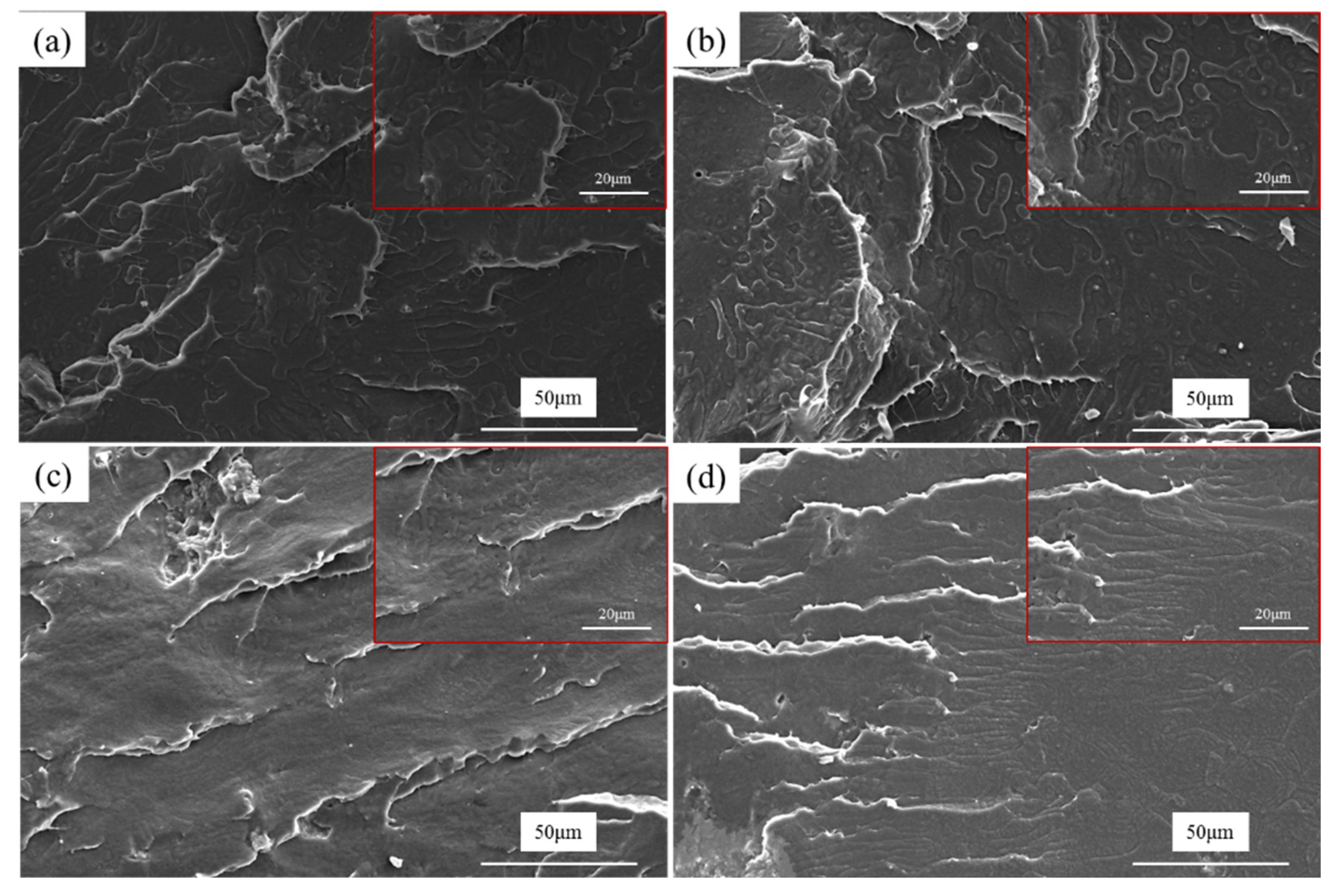
3.5. Morphology Observation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, 1700782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Auta, H.S.; Emenike, C.U.; Fauziah, S.H. Distribution and importance of microplastics in the marine environment: A review of the sources, fate, effects, and potential solutions. Environ. Int. 2017, 102, 165–176. [Google Scholar] [CrossRef] [PubMed]
- De-la-Torre, G.E. Microplastics: An emerging threat to food security and human health. J. Food. Sci. Technol. 2020, 57, 1601–16088. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wang, Q.; Zhang, C.; Zhang, J.; Dong, Z.; Xu, Q. Aging simulation of thin-film plastics in different environments to examine the formation of microplastic. Water Res. 2021, 202, 117462. [Google Scholar] [CrossRef]
- Rasal, R.M.; Janorkar, A.V.; Hirt, D.E. Poly(lactic acid) modifications. Prog. Polym. Sci. 2010, 35, 338–356. [Google Scholar] [CrossRef]
- Gao, T.; Zhang, Z.M.; Li, L.; Bao, R.Y.; Liu, Z.Y.; Xie, B.H. Tailoring Crystalline Morphology by High-Efficiency Nucleating Fiber: Toward High-Performance Poly(l-lactide) Biocomposites. ACS Appl. Mater. Interfaces 2018, 10, 20044–20054. [Google Scholar] [CrossRef]
- Naser, A.Z.; Deiab, I.; Darras, B.M. Poly(lactic acid) (PLA) and polyhydroxyalkanoates (PHAs), green alternatives to petroleum-based plastics: A review. RSC Adv. 2021, 11, 17151–17196. [Google Scholar] [CrossRef]
- Cvek, M.; Paul, U.C.; Zia, J.; Mancini, G.; Sedlarik, V.; Athanassiou, A. Biodegradable Films of PLA/PPC and Curcumin as Packaging Materials and Smart Indicators of Food Spoilage. ACS Appl. Mater. Interfaces 2022, 14, 14654–14667. [Google Scholar] [CrossRef]
- Wang, W.; Huang, J.; Gong, Z.; Fan, J.; Cao, L.; Chen, Y. Biobased PLA/NR-PMMA TPV with balanced stiffness-toughness: In-situ interfacial compatibilization, performance and toughening model. Polym. Test. 2020, 81, 106268. [Google Scholar] [CrossRef]
- McCutcheon, C.J.; Zhao, B.; Ellison, C.J.; Bates, F.S. Crazing and Toughness in Diblock Copolymer-Modified Semicrystalline Poly(l-lactide). Macromolecules 2021, 54, 11154–11169. [Google Scholar] [CrossRef]
- Chen, J.; Rong, C.; Lin, T.; Chen, Y.; Wu, J.; You, J. Stable Co-Continuous PLA/PBAT Blends Compatibilized by Interfacial Stereocomplex Crystallites: Toward Full Biodegradable Polymer Blends with Simultaneously Enhanced Mechanical Properties and Crystallization Rates. Macromolecules 2021, 54, 2852–2861. [Google Scholar] [CrossRef]
- Dominguez-Candela, I.; Gomez-Caturla, J.; Cardona, S.C.; Lora-García, J.; Fombuena, V. Novel compatibilizers and plasticizers developed from epoxidized and maleinized chia oil in composites based on PLA and chia seed flour. Eur. Polym. J. 2022, 173, 111289. [Google Scholar] [CrossRef]
- Garcia-Garcia, D.; Carbonell-Verdu, A.; Arrieta, M.P.; López-Martínez, J.; Samper, M.D. Improvement of PLA film ductility by plasticization with epoxidized karanja oil. Polym. Degrad. Stab. 2020, 179, 109259. [Google Scholar] [CrossRef]
- Zheng, M.; Zhang, S.; Chen, Y.; Wu, Q.; Li, Q.; Wang, S. Structure evolution of bio-based PLA/ENR thermoplastic vulcanizates during dynamic vulcanization processing. Polym. Test. 2020, 82, 106324. [Google Scholar] [CrossRef]
- Zafar, R.; Lee, W.; Kwak, S.Y. A facile strategy for enhancing tensile toughness of poly(lactic acid) (PLA) by blending of a cellulose bio-toughener bearing a highly branched polycaprolactone. Eur. Polym. J. 2022, 175, 111376. [Google Scholar] [CrossRef]
- Li, X.; Han, L.; Liu, X.; Chu, C.; Ju, J.; Bai, J. A study on the impact behaviors of Mg wires/PLA composite for orthopedic implants. J. Mater. Sci. 2019, 54, 14545–14553. [Google Scholar] [CrossRef]
- Xu, P.; Tan, S.; Niu, D.; Yang, W.; Ma, P. Highly Toughened Sustainable Green Polyglycolic Acid/Polycaprolactone Blends with Balanced Strength: Morphology Evolution, Interfacial Compatibilization, and Mechanism. ACS Appl. Mater. Interfaces 2022, 4, 5772–5780. [Google Scholar] [CrossRef]
- Mulchandani, N.; Masutani, K.; Kumar, S.; Yamane, H.; Sakurai, S.; Kimura, Y. Toughened PLA-b-PCL-b-PLA triblock copolymer based biomaterials: Effect of self-assembled nanostructure and stereocomplexation on the mechanical properties. Polym. Chem. 2021, 12, 3806–3824. [Google Scholar] [CrossRef]
- Ecker, J.V.; Burzic, I.; Haider, A.; Hild, S.; Rennhofer, H. Improving the impact strength of PLA and its blends with PHA in fused layer modelling. Polym. Test. 2019, 78, 105929. [Google Scholar] [CrossRef]
- Briassoulis, D.; Athanasoulia, I.G.; Tserotas, P. PHB/PLA plasticized by olive oil and carvacrol solvent-cast films with optimised ductility and physical ageing stability. Polym. Degrad. Stab. 2022, 200, 109958. [Google Scholar] [CrossRef]
- Yang, H.R.; Jia, G.; Wu, H.; Ye, C.; Yuan, K.; Liu, S. Design of Fully Biodegradable Super-toughened PLA/PBAT Blends with Asymmetric Composition via Reactive Compatibilization and Controlling Morphology. Mater. Lett. 2022, 133067, in press. [Google Scholar] [CrossRef]
- Song, X.; Zhang, Y.; Zou, T.; Li, Y.; Wang, P.; Hu, D. The reinforcement and toughening of jute/PLA (lactic acid) composite material with modified-rubber/SiO2 core-shell particles. Mater. Today Sustain. 2022, 19, 100198. [Google Scholar] [CrossRef]
- Maroufkhani, M.; Katbab, A.; Bizhani, H.; Zhang, J. Toward morphology development and impact strength of Co-continuous supertough dynamically vulcanized rubber toughened PLA blends: Effect of sulfur content. Polymer 2021, 217, 123439. [Google Scholar] [CrossRef]
- Wu, N.; Zhang, H.; Fu, G. Super-tough Poly(lactide) Thermoplastic Vulcanizates Based on Modified Natural Rubber. ACS Sustain. Chem. Eng. 2017, 5, 78–84. [Google Scholar] [CrossRef]
- Wang, Q.; Huang, Z.; Liu, Z. Overview of High-Value Reuse and Grinding at Sub-Zero Temperature of Scrap Rubber Tires. IOP Conf. Ser. Mater. Sci. Eng. 2019, 472, 012071. [Google Scholar] [CrossRef]
- Fazli, A.; Rodrigue, D. Waste Rubber Recycling: A Review on the Evolution and Properties of Thermoplastic Elastomers. Materials 2020, 13, 782. [Google Scholar] [CrossRef] [Green Version]
- Hejna, A.; Korol, J.; Przybysz-Romatowska, M.; Zedler, Ł.; Chmielnicki, B.; Formela, K. Waste tire rubber as low-cost and environmentally-friendly modifier in thermoset polymers—A review. Waste Manag. 2020, 108, 106–118. [Google Scholar] [CrossRef]
- Sofi, A. Effect of waste tire rubber on mechanical and durability properties of concrete–A review. Ain Shams Eng. J. 2018, 9, 2691–2700. [Google Scholar] [CrossRef]
- Zedler, Ł.; Colom, X.; Saeb, M.R.; Formela, K. Preparation and characterization of natural rubber composites highly filled with brewers’ spent grain/ground tire rubber hybrid reinforcement. Compos. Part B Eng. 2018, 145, 182–188. [Google Scholar] [CrossRef]
- Coiai, S.; Passaglia, E.; Ciardelli, F.; Tirelli, D.; Peruzzotti, F.; Resmini, E. Modification of Cross-Linked Rubber Particles by Free Radical Polymerization. Macromol. Symp. 2006, 234, 193–202. [Google Scholar] [CrossRef]
- Phiri, M.M.; Phiri, M.J.; Formela, K.; Hlangothi, S.P. Chemical surface etching methods for ground tire rubber as sustainable approach for environmentally-friendly composites development—A review. Compos. Part B Eng. 2021, 204, 108429. [Google Scholar] [CrossRef]
- Phiri, M.M.; Phiri, M.J.; Formela, K.; Hlangothi, S.P. Grafting and reactive extrusion technologies for compatibilization of ground tyre rubber composites: Compounding, properties, and applications. J. Clean. Prod. 2022, 369, 133084. [Google Scholar] [CrossRef]
- Sonnier, R.; Leroy, E.; Clerc, L.; Bergeret, A.; Lopez-Cuesta, J.M.; Bretelle, A.S. Compatibilizing thermoplastic/ground tyre rubber powder blends: Efficiency and limits. Polym. Test. 2008, 27, 901–907. [Google Scholar] [CrossRef]
- Zedler, Ł.; Przybysz, M.; Klein, M.; Saeb, M.R.; Formela, K. Processing, physico-mechanical and thermal properties of reclaimed GTR and NBR/reclaimed GTR blends as function of various additives. Polym. Degrad. Stab. 2017, 143, 186–195. [Google Scholar] [CrossRef]
- Lorenzo, A.T.; Arnal, M.L.; Albuerne, J.; Müller, A.J. DSC isothermal polymer crystallization kinetics measurements and the use of the Avrami equation to fit the data: Guidelines to avoid common problems. Polym. Test. 2007, 26, 222–231. [Google Scholar] [CrossRef]
- Li, H.; Huneault, M.A. Effect of nucleation and plasticization on the crystallization of poly(lactic acid). Polymer 2007, 48, 6855–6866. [Google Scholar] [CrossRef]
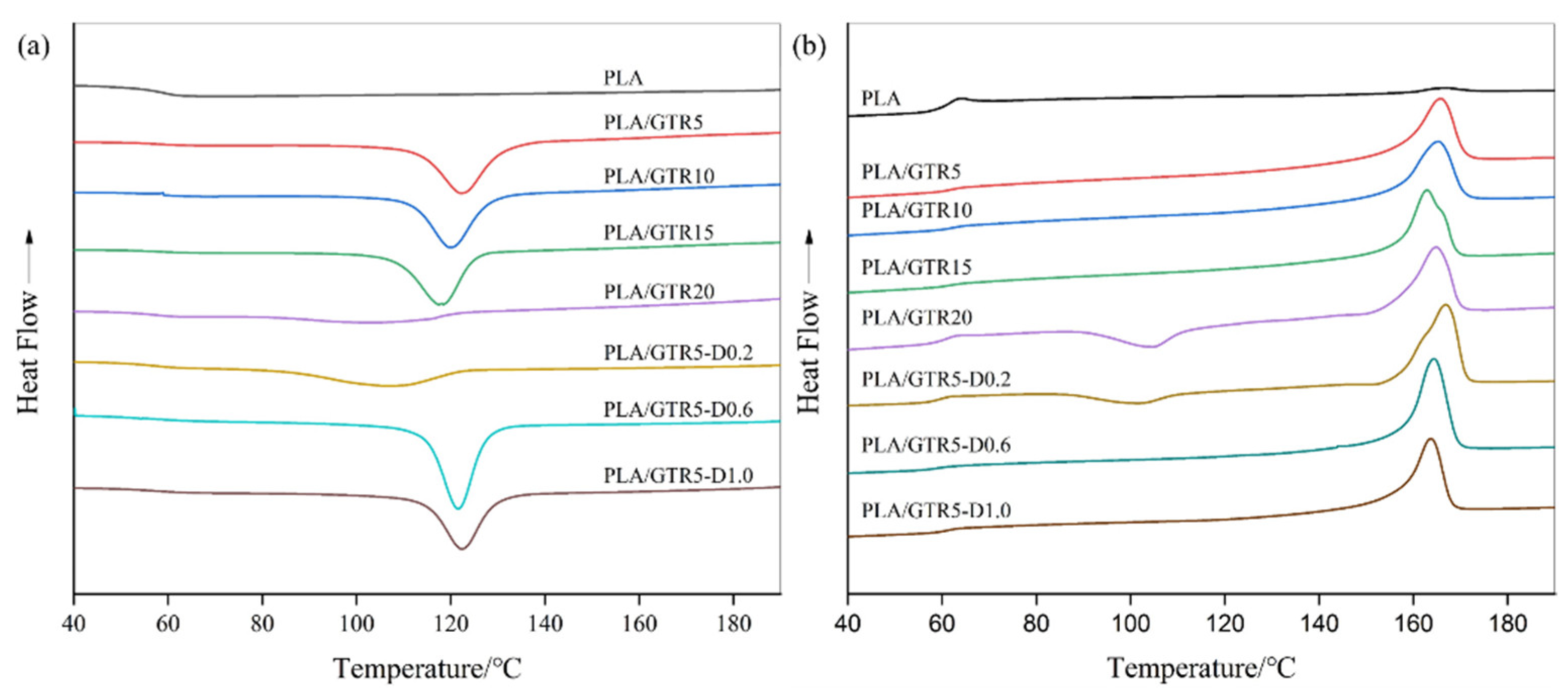

| Samples | Tg (°C) | Tcc (°C) | Tc (°C) | Tonset (°C) | Tonset -Tc (°C) | Tm (°C) | ∆Hcc (J/g) | ∆Hc (J/g) | ∆Hm (J/g) | Xc (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| PLA | 61.8 | - | - | - | - | 166.4 | - | - | 1.6 | 1.7 |
| PLA/GTR5 | 61.2 | - | 122.4 | 131.2 | 8.8 | 165.8 | - | 33.4 | 32.2 | 36.4 |
| PLA/GTR10 | 61.5 | - | 120.1 | 128.5 | 7.4 | 165.2 | - | 31.9 | 30.8 | 36.8 |
| PLA/GTR15 | 61.5 | - | 118.4 | 125.2 | 6.8 | 163.1 | - | 31.6 | 30.6 | 38.7 |
| PLA/GTR20 | 61.2 | 104.4 | 105.4 | 121.4 | 16.0 | 164.8 | 12.8 | 11.4 | 28.0 | 20.5 |
| PLA/GTR5-D0.2 | 59.3 | 101.6 | 106.8 | 123.4 | 16.6 | 166.8 | 8.6 | 19.4 | 33.5 | 28.2 |
| PLA/GTR5-D0.6 | 60.3 | - | 121.5 | 127.7 | 6.2 | 164.5 | - | 40.1 | 35.6 | 40.5 |
| PLA/GTR5-D1.0 | 61.6 | - | 122.5 | 129.6 | 7.1 | 163.8 | - | 31.4 | 31.3 | 35.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, H.; Hu, Y.; Lin, Z.; Meng, F.; Ju, G. Mechanical Properties, Crystallization Behaviors and Phase Morphologies of PLA/GTR Blends by Reactive Compatibilization. Materials 2022, 15, 7095. https://doi.org/10.3390/ma15207095
Shen H, Hu Y, Lin Z, Meng F, Ju G. Mechanical Properties, Crystallization Behaviors and Phase Morphologies of PLA/GTR Blends by Reactive Compatibilization. Materials. 2022; 15(20):7095. https://doi.org/10.3390/ma15207095
Chicago/Turabian StyleShen, Hongwang, Yongxiang Hu, Zhitao Lin, Fantao Meng, and Guannan Ju. 2022. "Mechanical Properties, Crystallization Behaviors and Phase Morphologies of PLA/GTR Blends by Reactive Compatibilization" Materials 15, no. 20: 7095. https://doi.org/10.3390/ma15207095
APA StyleShen, H., Hu, Y., Lin, Z., Meng, F., & Ju, G. (2022). Mechanical Properties, Crystallization Behaviors and Phase Morphologies of PLA/GTR Blends by Reactive Compatibilization. Materials, 15(20), 7095. https://doi.org/10.3390/ma15207095






