The Influence of Surface Modification with Biopolymers on the Structure of Melt-Blown and Spun-Bonded Poly(lactic acid) Nonwovens
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
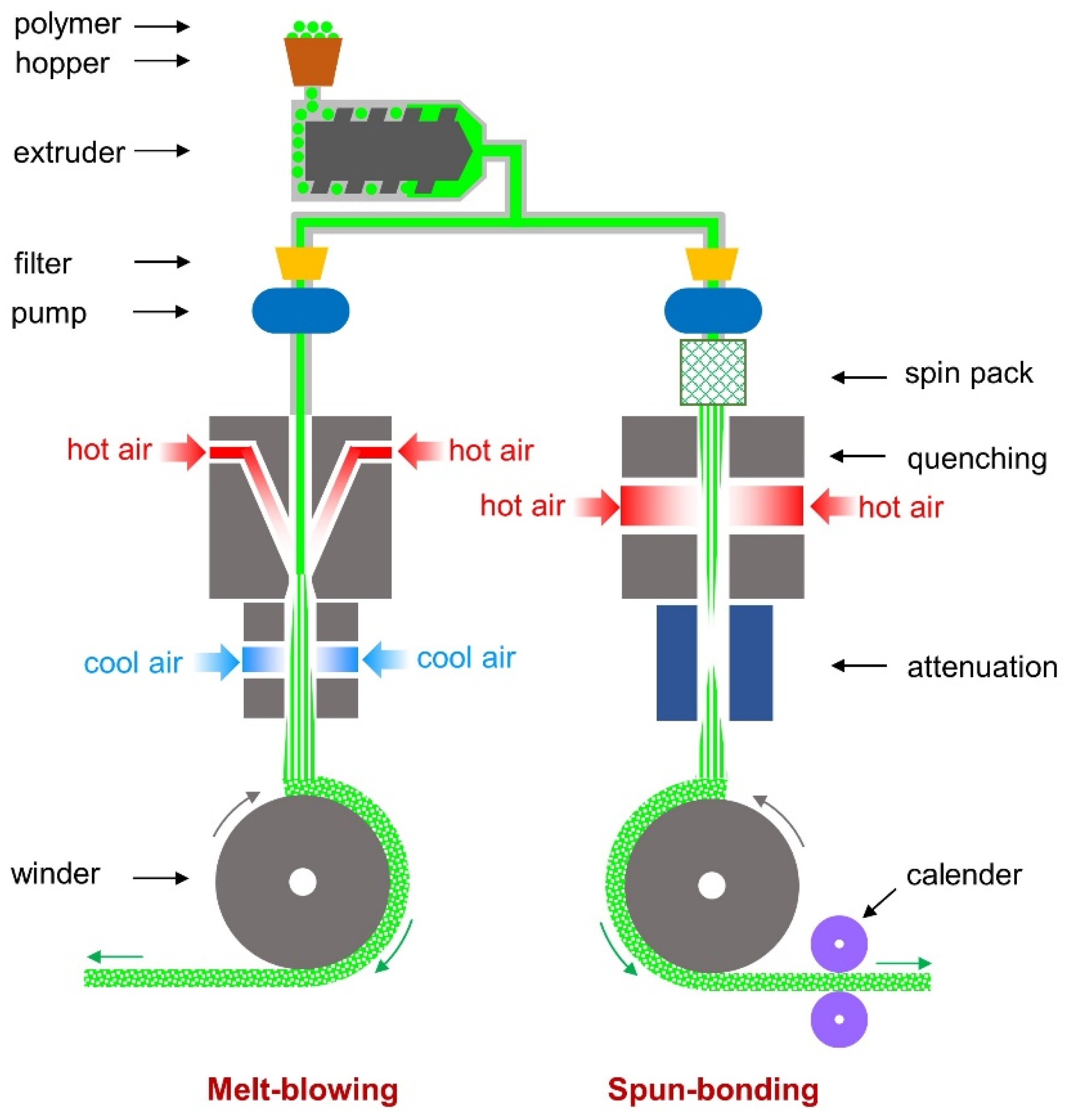
2.2.1. Nonwovens Fabrication
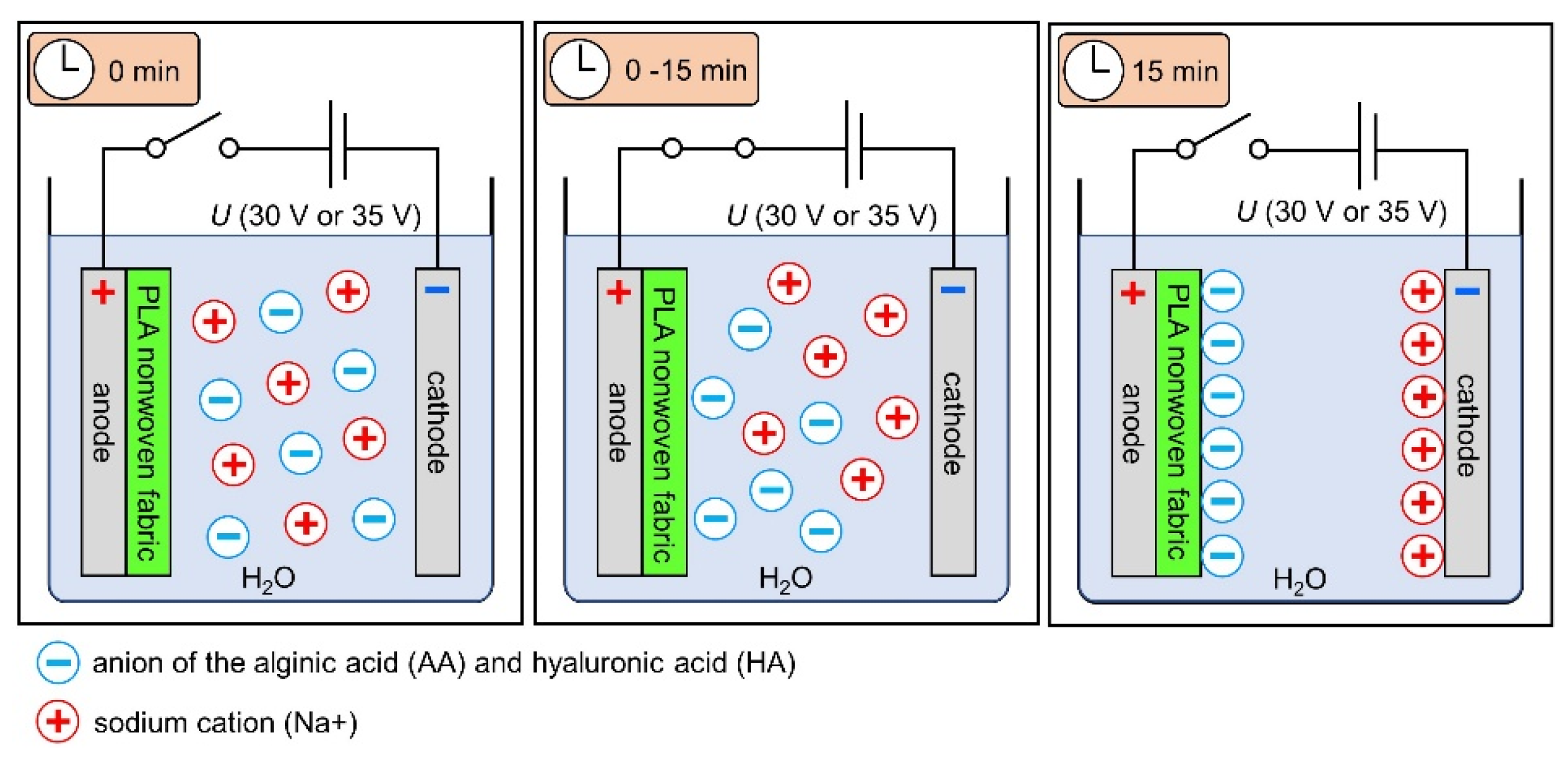
2.2.2. Electrodeposition
2.2.3. Evaluation of the Modified Nonwovens’ Structural Properties
X-ray Micro-Computed Tomography (Micro-CT)
Fourier Transform INFRARED SPECTROSCOPY (FTIR)
Microscopic Analysis
3. Results
3.1. Micro-CT Analysis
3.2. FTIR Analysis
3.3. Microscopic Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| AA; SA | alginic acid; sodium salt of alginic acid, sodium alginate |
| CT; micro-CT | computed tomography; micro-computed tomography |
| EPD | electrophoretic deposition |
| FTIR | Fourier transform infrared spectroscopy |
| HA; SH | hyaluronic acid; sodium hyaluronate |
| MB | melt-blown |
| PLA | poly(lactic acid) |
| SB | spun-bonded |
References
- Pabjanczyk-Wlazlo, E.; Tarzynska, N.; Bednarowicz, A.; Puszkarz, A.K.; Szparaga, G. Polymer-Based Electrophoretic Deposition of Nonwovens for Medical Applications: The Effect of Carrier Structure, Solution, and Process Parameters. Mar. Drugs 2021, 19, 533. [Google Scholar] [CrossRef] [PubMed]
- Ramot, Y.; Haim-Zada, M.; Domb, A.J.; Nyska, A. Biocompatibility and safety of PLA and its copolymers. Adv. Drug Deliv. Rev. 2016, 107, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Młotek, M.; Gadomska-Gajadhur, A.; Sobczak, A.; Kruk, A.; Perron, M.; Krawczyk, K. Modification of PLA Scaffold Surface for Medical Applications. Appl. Sci. 2021, 11, 1815. [Google Scholar] [CrossRef]
- Kan, Z.; Luo, W.; Shi, T.; Wei, C.; Han, B.; Zheng, D.; Liu, S. Facile Preparation of Stereoblock PLA From Ring-Opening Polymerization of rac-Lactide by a Synergetic Binary Catalytic System Containing Ureas and Alkoxides. Front. Chem. 2018, 6, 547. [Google Scholar] [CrossRef] [Green Version]
- Afsi, N.; Othman, S.; Bakir, T.; Costa, L.I.; Sakly, A.; Sheibat-Othman, N. Model predictive control for continuous lactide ring-opening polymerization processes. Asian J. Control 2020, 23, 92–104. [Google Scholar] [CrossRef]
- Riaz, S.; Fatima, N.; Rasheed, A.; Riaz, M.; Anwar, F.; Khatoon, Y. Metabolic Engineered Biocatalyst: A Solution for PLA Based Problems. Int. J. Biomater. 2018, 2018, 1–9. [Google Scholar] [CrossRef]
- Chow, W.S.; Lim, Y.T. Antistatic and Thermal Properties of Poly(Lactic Acid)/Polypropylene/Carbon Nanotube Nanocomposites. J. Eng. Sci. 2020, 16, 57–69. [Google Scholar] [CrossRef]
- Puchalski, M.; Sulak, K.; Chrzanowski, M.; Sztajnowski, S.; Ska, I.K.; Krucińska, I. Effect of processing variables on the thermal and physical properties of poly(L-lactide) spun bond fabrics. Text. Res. J. 2014, 85, 535–547. [Google Scholar] [CrossRef]
- Puchalski, M.; Krucińska, I.; Sulak, K.; Chrzanowski, M.; Wrzosek, H. Influence of the calender temperature on the crystallization behaviors of polylactide spun-bonded non-woven fabrics. Text. Res. J. 2013, 83, 1775–1785. [Google Scholar] [CrossRef]
- Salahuddin, N.; Abdelwahab, M.; Gaber, M.; Elneanaey, S. Synthesis and Design of Norfloxacin drug delivery system based on PLA/TiO2 nanocomposites: Antibacterial and antitumor activities. Mater. Sci. Eng. C 2019, 108, 110337. [Google Scholar] [CrossRef]
- da Silva, D.; Kaduri, M.; Poley, M.; Adir, O.; Krinsky, N.; Shainsky-Roitman, J.; Schroeder, A. Biocompatibility, biodegradation and excretion of polylactic acid (PLA) in medical implants and theranostic systems. Chem. Eng. J. 2018, 340, 9–14. [Google Scholar] [CrossRef] [PubMed]
- DeStefano, V.; Khan, S.; Tabada, A. Applications of PLA in modern medicine. Eng. Regen. 2020, 1, 76–87. [Google Scholar] [CrossRef]
- Rankin, T.M.; Giovinco, N.A.; Cucher, D.J.; Watts, G.; Hurwitz, B.; Armstrong, D.G. Three-dimensional printing surgical instruments: Are we there yet? J. Surg. Res. 2014, 189, 193–197. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Jiang, S.; Liu, S.; Chen, S.; Lin, Z.Y.; Pan, G.; He, F.; Li, F.; Fan, C.; Cui, W. Optimization of intrinsic and extrinsic tendon healing through controllable water-soluble mitomycin-C release from electrospun fibers by mediating adhesion-related gene expression. Biomaterials 2015, 61, 61–74. [Google Scholar] [CrossRef]
- Cheng, L.; Sun, X.; Zhao, X.; Wang, L.; Yu, J.; Pan, G.; Li, B.; Yang, H.; Zhang, Y.; Cui, W. Surface biofunctional drug-loaded electrospun fibrous scaffolds for comprehensive repairing hypertrophic scars. Biomaterials 2016, 83, 169–181. [Google Scholar] [CrossRef]
- Wang, H.; Wei, Q.; Wang, X.; Gao, W.; Zhao, X. Antibacterial properties of PLA nonwoven medical dressings coated with nanostructured silver. Fibers Polym. 2008, 9, 556–560. [Google Scholar] [CrossRef]
- Zhu, C.; Jiang, W.; Hu, J.; Sun, P.; Li, A.-M.; Zhang, Q. Polylactic Acid Nonwoven Fabric Surface Modified with Stereocomplex Crystals for Recyclable Use in Oil/Water Separation. ACS Appl. Polym. Mater. 2020, 2, 2509–2516. [Google Scholar] [CrossRef]
- Shi, J.; Zhang, L.; Xiao, P.; Huang, Y.; Chen, P.; Wang, X.; Gu, J.; Zhang, J.; Chen, T. Biodegradable PLA Nonwoven Fabric with Controllable Wettability for Efficient Water Purification and Photocatalysis Degradation. ACS Sustain. Chem. Eng. 2018, 6, 2445–2452. [Google Scholar] [CrossRef]
- Kudzin, M.H.; Mrozińska, Z. Biofunctionalization of Textile Materials. 2. Antimicrobial Modification of Poly(lactide) (PLA) Nonwoven Fabricsby Fosfomycin. Polymers 2020, 12, 768. [Google Scholar] [CrossRef] [Green Version]
- Sequeira, J.; Rao, B.H.S.; Kedia, P.R. Efficacy of Sodium Hyaluronate for Temporomandibular Joint Disorder by Single-Puncture Arthrocentesis. J. Maxillofac. Oral Surg. 2018, 18, 88–92. [Google Scholar] [CrossRef]
- Shuborna, N.S.; Chaiyasamut, T.; Sakdajeyont, W.; Vorakulpipat, C.; Rojvanakarn, M.; Wongsirichat, N. Generation of novel hyaluronic acid biomaterials for study of pain in third molar intervention: A review. J. Dent. Anesthesia Pain Med. 2019, 19, 11–19. [Google Scholar] [CrossRef]
- Chen, L.H.; Xue, J.F.; Zheng, Z.Y.; Shuhaidi, M.; Thu, H.E.; Hussain, Z. Hyaluronic acid, an efficient biomacromolecule for treatment of inflammatory skin and joint diseases: A review of recent developments and critical appraisal of preclinical and clinical investigations. Int. J. Biol. Macromol. 2018, 116, 572–584. [Google Scholar] [CrossRef] [PubMed]
- Abatangelo, G.; Vindigni, V.; Avruscio, G.; Pandis, L.; Brun, P. Hyaluronic Acid: Redefining Its Role. Cells 2020, 9, 1743. [Google Scholar] [CrossRef] [PubMed]
- Manca, M.; Castangia, I.; Zaru, M.; Nácher, A.; Valenti, D.; Fernàndez-Busquets, X.; Fadda, A.; Manconi, M. Development of curcumin loaded sodium hyaluronate immobilized vesicles (hyalurosomes) and their potential on skin inflammation and wound restoring. Biomaterials 2015, 71, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.; Yoo, C.Y.; Park, S.N. Improved stability and skin permeability of sodium hyaluronate-chitosan multilayered liposomes by Layer-by-Layer electrostatic deposition for quercetin delivery. Colloids Surfaces B Biointerfaces 2015, 129, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Rao, K.M.; Nagappan, S.; Seo, D.J.; Ha, C.-S. pH sensitive halloysite-sodium hyaluronate/poly(hydroxyethyl methacrylate) nanocomposites for colon cancer drug delivery. Appl. Clay Sci. 2014, 97–98, 33–42. [Google Scholar] [CrossRef]
- Liu, S.; Jin, M.-N.; Quan, Y.-S.; Kamiyama, F.; Katsumi, H.; Sakane, T.; Yamamoto, A. The development and characteristics of novel microneedle arrays fabricated from hyaluronic acid, and their application in the transdermal delivery of insulin. J. Control. Release 2012, 161, 933–941. [Google Scholar] [CrossRef]
- Hirama, H.; Ishikura, Y.; Kano, S.; Hayase, M.; Mekaru, H. Monodispersed sodium hyaluronate microcapsules for transdermal drug delivery systems. Mater. Adv. 2021, 2, 7007–7016. [Google Scholar] [CrossRef]
- Damiano, R.; Cicione, A. The role of sodium hyaluronate and sodium chondroitin sulphate in the management of bladder disease. Ther. Adv. Urol. 2011, 3, 223–232. [Google Scholar] [CrossRef] [Green Version]
- Hecht, H.; Srebnik, S. Structural Characterization of Sodium Alginate and Calcium Alginate. Biomacromolecules 2016, 17, 2160–2167. [Google Scholar] [CrossRef]
- Jain, D.; Bar-Shalom, D. Alginate drug delivery systems: Application in context of pharmaceutical and biomedical research. Drug Dev. Ind. Pharm. 2014, 40, 1576–1584. [Google Scholar] [CrossRef] [PubMed]
- Hariyadi, D.M.; Islam, N. Current Status of Alginate in Drug Delivery. Adv. Pharmacol. Pharm. Sci. 2020, 2020, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, K.; Ganguly, K.; More, U.A.; Reddy, K.R.; Dugge, T.; Naik, B.; Aminabhavi, T.M.; Noolvi, M.N. Sodium alginate in drug delivery and biomedical areas. In Natural Polysaccharides in Drug Delivery and Biomedical Applications; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 59–100. [Google Scholar] [CrossRef]
- Solovieva, E.V.; Fedotov, A.Y.; Mamonov, V.E.; Komlev, V.S.; Panteleyev, A.A. Fibrinogen-modified sodium alginate as a scaffold material for skin tissue engineering. Biomed. Mater. 2017, 13, 025007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahoo, D.R.; Biswal, T. Alginate and its application to tissue engineering. SN Appl. Sci. 2021, 3, 1–19. [Google Scholar] [CrossRef]
- Mihailović, D.; Šaponjić, Z.; Radoičić, M.; Radetić, T.; Jovančić, P.; Nedeljković, J.; Radetić, M. Functionalization of polyester fabrics with alginates and TiO2 nanoparticles. Carbohydr. Polym. 2010, 79, 526–532. [Google Scholar] [CrossRef]
- Marković, D.; Radoičić, M.; Barudžija, T.; Radetić, M. Modification of PET and PA fabrics with alginate and copper oxides nanoparticles. Compos. Interfaces 2021, 28, 1171–1187. [Google Scholar] [CrossRef]
- Enescu, D. Use of chitosan in surface modification of textile materials. Rom. Biotechnol. Lett. 2008, 13, 4037–4048. [Google Scholar]
- Islam, S.U.; Butola, B. Recent advances in chitosan polysaccharide and its derivatives in antimicrobial modification of textile materials. Int. J. Biol. Macromol. 2018, 121, 905–912. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, Y.; Zhu, J.; Huang, J.; Zhang, H. Inhibition of bacterial adhesion and biofilm formation of sulfonated chitosan against Pseudomonas aeruginosa. Carbohydr. Polym. 2019, 206, 412–419. [Google Scholar] [CrossRef]
- Shin, Y.; Yoo, D.; Min, K. Antimicrobial finishing of polypropylene nonwoven fabric by treatment with chitosan oligomer. J. Appl. Polym. Sci. 2000, 9, 43–45. [Google Scholar] [CrossRef]
- Bračič, M.; Perez, L.; Martinez-Pardo, R.I.; Kogej, K.; Hribernik, S.; Šauperl, O.; Zemljič, L.F. A novel synergistic formulation between a cationic surfactant from lysine and hyaluronic acid as an antimicrobial coating for advanced cellulose materials. Cellulose 2014, 21, 2647–2663. [Google Scholar] [CrossRef]
- Voncina, B.; Fras, L.Z.; Ristic, T. Active textile dressings for wound healing. In Advances in Smart Medical Textiles. Treatments and Health Monitoring; Elsevier: Amsterdam, The Netherlands, 2016; pp. 73–92. [Google Scholar] [CrossRef]
- Pielka, S.; Paluch, D.; Staniszewska-Kuś, J.; Żywicka, B.; Solski, L.; Szosland, L.; Czarny, A.; Zaczyńska, E. Wound Healing Acceleration by a Textile Dressing Containing Dibutyrylchitin and Chitin. Fibres Text. East. Eur. 2003, 2, 79–84. [Google Scholar]
- Lin, X.; Wang, W.; Zhang, W.; Zhang, Z.; Zhou, G.; Cao, Y.; Liu, W. Hyaluronic Acid Coating Enhances Biocompatibility of Nonwoven PGA Scaffold and Cartilage Formation. Tissue Eng. Part C Methods 2017, 23, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, H.; Aly, A.; Abou-Okeil, A. A non-woven fabric wound dressing containing layer–by–layer deposited hyaluronic acid and chitosan. Int. J. Biol. Macromol. 2018, 114, 929–934. [Google Scholar] [CrossRef]
- Latańska, I.; Kolesińska, B.; Draczyński, Z.; Sujka, W. The use of chitin and chitosan in manufacturing dressing materials. Prog. Chem. Appl. Chitin Its Deriv. 2020, XXV, 16–36. [Google Scholar] [CrossRef]
- Kubíčková, J.; Medek, T.; Husby, J.; Matonohová, J.; Vágnerová, H.; Marholdová, L.; Velebný, V.; Chmelař, J. Nonwoven Textiles from Hyaluronan for Wound Healing Applications. Biomolecules 2021, 12, 16. [Google Scholar] [CrossRef]
- Montaser, A.; Rehan, M.; El-Senousy, W.; Zaghloul, S. Designing strategy for coating cotton gauze fabrics and its application in wound healing. Carbohydr. Polym. 2020, 244, 116479. [Google Scholar] [CrossRef]
- Risbud, M.V.; Karamuk, E.; Mayer, J. Designing hydrogel coated textile scaffolds for tissue engineering: Effect of casting conditions and degradation behavior studied at microstructure level. J. Mater. Sci. Lett. 2002, 21, 1191–1194. [Google Scholar] [CrossRef]
- Risbud, M.V.; Karamuk, E.; Schlosser, V.; Mayer, J. Hydrogel-coated textile scaffolds as candidate in liver tissue engineering: II. Evaluation of spheroid formation and viability of hepatocytes. J. Biomater. Sci. Polym. Ed. 2003, 14, 719–731. [Google Scholar] [CrossRef]
- Zapotocky, V.; Pospisilova, M.; Janouchova, K.; Svadlak, D.; Batova, J.; Sogorkova, J.; Cepa, M.; Betak, J.; Stepankova, V.; Sulakova, R.; et al. Fabrication of biodegradable textile scaffold based on hydrophobized hyaluronic acid. Int. J. Biol. Macromol. 2017, 95, 903–909. [Google Scholar] [CrossRef]
- Rajzer, I.; Menaszek, E.; Kwiatkowski, R.; Planell, J.A.; Castano, O. Electrospun gelatin/poly(ε-caprolactone) fibrous scaffold modified with calcium phosphate for bone tissue engineering. Mater. Sci. Eng. C 2014, 44, 183–190. [Google Scholar] [CrossRef] [PubMed]
- PN-EN 29073-1:1994; Textiles-Fabrics-Determination of the Surface Mass. Polish Committee for Standardization: Warsaw, Poland, 1994.
- PN EN 12127:2000; Textiles-Fabrics-Determination of Mass Per Unit Area Using Small Samples. Polish Committee for Standardization: Warsaw, Poland, 2013.
- Schladitz, K. Quantitative micro-CT. J. Microsc. 2011, 243, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Verma, P.; Shofner, M.L.; Lin, A.; Wagner, K.B.; Griffin, A.C. Inducing out-of-plane auxetic behavior in needle-punched nonwovens. Phys. Status Solidi (b) 2015, 252, 1455–1464. [Google Scholar] [CrossRef]
- Verma, P.; Shofner, M.L.; Lin, A.; Wagner, K.B.; Griffin, A.C. Induction of auxetic response in needle-punched nonwovens: Effects of temperature, pressure, and time. Phys. Status Solidi (b) 2016, 253, 1270–1278. [Google Scholar] [CrossRef]
- Hanif, M.I. Letter to the editor on respiratory mask using a combination of spunbond, meltblown, and activated carbon materials for reducing exposure to CO: An in vivo study. Environ. Sci. Pollut. Res. 2020, 28, 3748. [Google Scholar] [CrossRef]
- Law, V.J.; Dowling, D.P. MGS decontamination of N95-like respirators: Dielectric considerations. Glob. J. Res. Eng. Comput. Sci. 2021, 1, 6–21. [Google Scholar]
- Walczak, R.; Neumann, P. Improving the Quality of Nonwoven Materials Manufacturing based on Analysis of its Water Permeability. Found. Manag. 2016, 8, 35–42. [Google Scholar] [CrossRef] [Green Version]
- Midha, V.; Dakuri, A.; Midha, V. Studies on the properties of nonwoven surgical gowns. J. Ind. Text. 2012, 43, 174–190. [Google Scholar] [CrossRef]
- Öztürk, M.K.; Venkataraman, M.; Mishra, R. Influence of structural parameters on thermal performance of polypropylene nonwovens. Polym. Adv. Technol. 2018, 29, 3027–3034. [Google Scholar] [CrossRef]
- Mao, N. Nonwoven fabric filters. Fibrous Filter Media; Brown, P.J., Cox, C.L., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; Volume 2013, pp. 133–171. [Google Scholar] [CrossRef]
- Niu, X.; Rouabhia, M.; Chiffot, N.; King, M.W.; Zhang, Z. An electrically conductive 3D scaffold based on a nonwoven web of poly(l-lactic acid) and conductive poly(3,4-ethylenedioxythiophene). J. Biomed. Mater. Res. Part A 2015, 103, 2635–2644. [Google Scholar] [CrossRef]
- VanGordon, S.B.; Voronov, R.S.; Blue, T.B.; Shambaugh, R.L.; Papavassiliou, D.V.; Sikavitsas, V.I. Effects of Scaffold Architecture on Preosteoblastic Cultures under Continuous Fluid Shear. Ind. Eng. Chem. Res. 2010, 50, 620–629. [Google Scholar] [CrossRef]
- Sefat, F.; Mozafari, M.; Atala, A. Introduction to Tissue Engineering Scaffolds; Elsevier Ltd.: Amsterdam, The Netherlands, 2019; pp. 3–22. [Google Scholar] [CrossRef]
- Shirwaiker, R.A.; Fisher, M.B.; Anderson, B.; Schuchard, K.G.; Warren, P.B.; Maze, B.; Grondin, P.; Ligler, F.S.; Pourdeyhimi, B. High-Throughput Manufacture of 3D Fiber Scaffolds for Regenerative Medicine. Tissue Eng. Part C Methods 2020, 26, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Trevisol, T.C.; Langbehn, R.K.; Battiston, S.; Immich, A.P.S. Nonwoven membranes for tissue engineering: An overview of cartilage, epithelium, and bone regeneration. J. Biomater. Sci. Polym. Ed. 2019, 30, 1026–1049. [Google Scholar] [CrossRef] [PubMed]
- A Tuin, S.; Pourdeyhimi, B.; Loboa, E.G. Creating tissues from textiles: Scalable nonwoven manufacturing techniques for fabrication of tissue engineering scaffolds. Biomed. Mater. 2016, 11, 015017. [Google Scholar] [CrossRef]

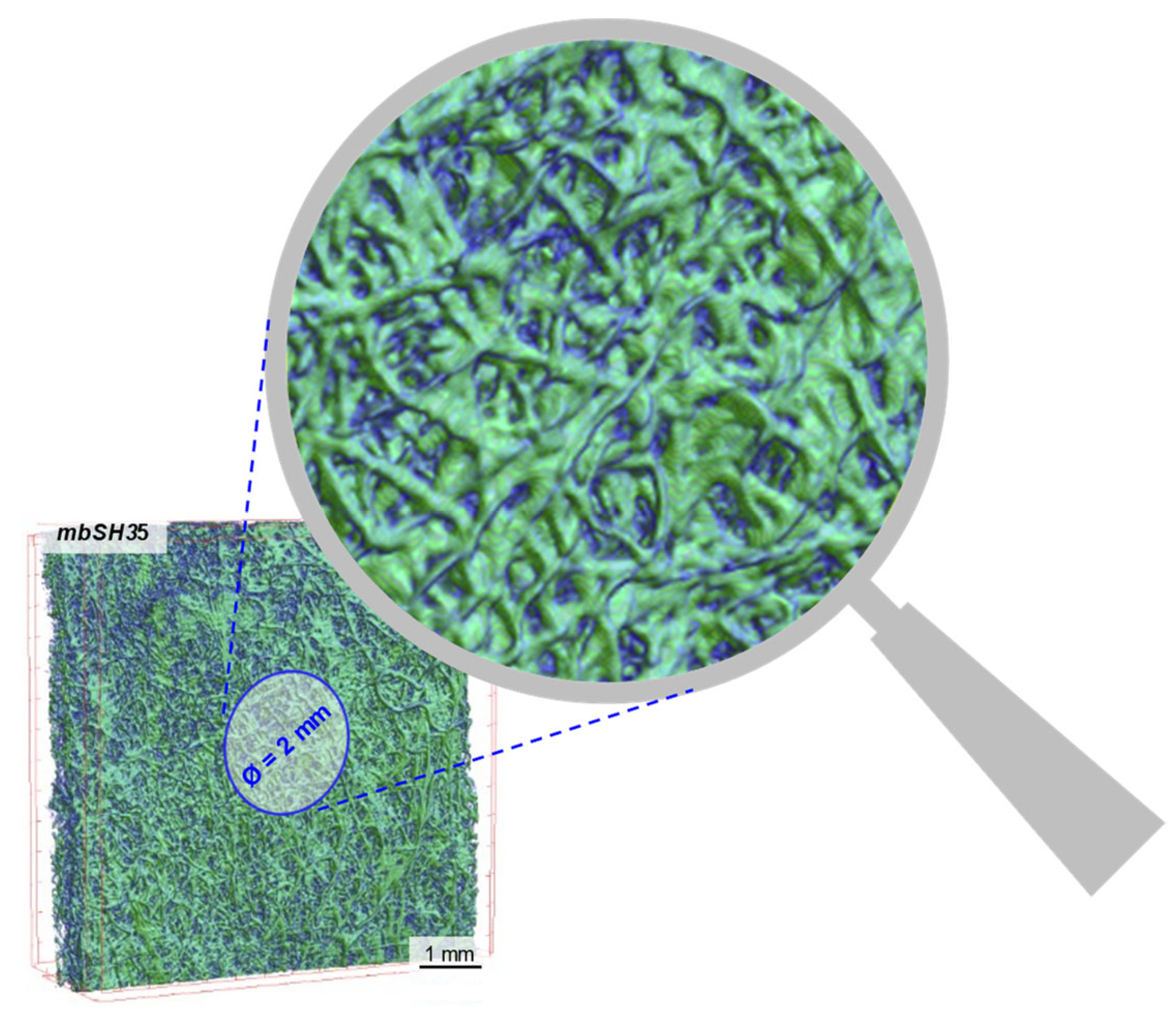
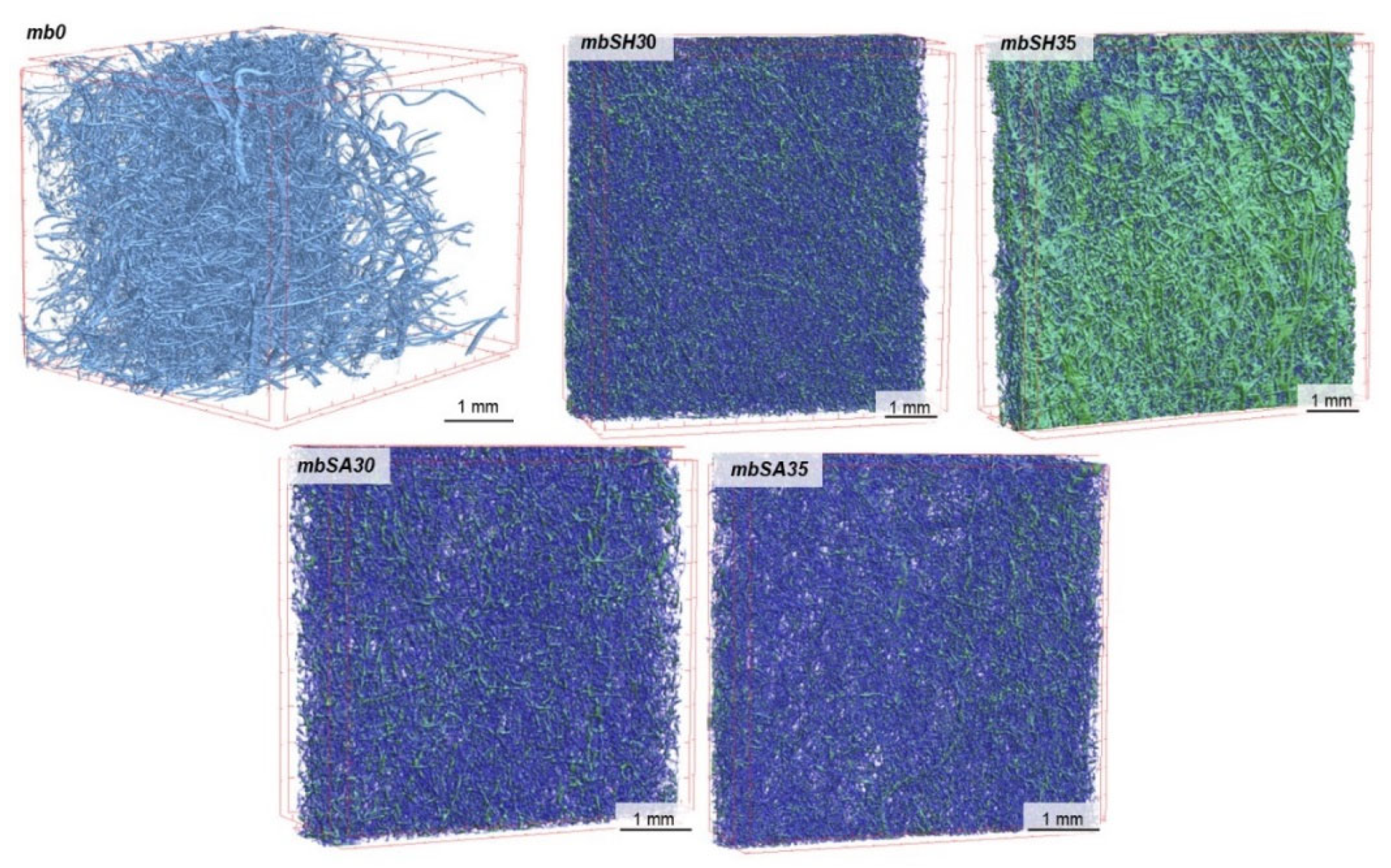



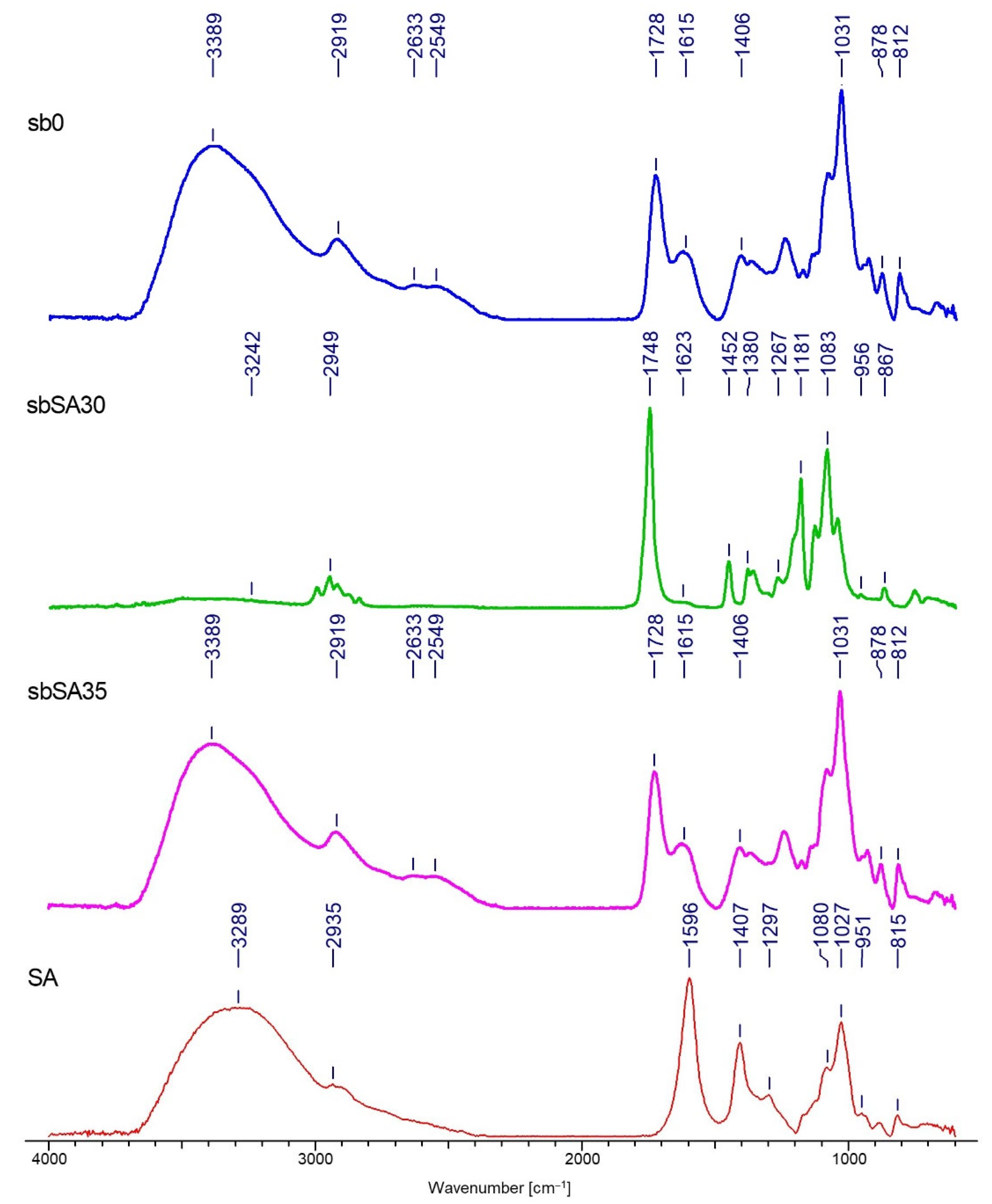

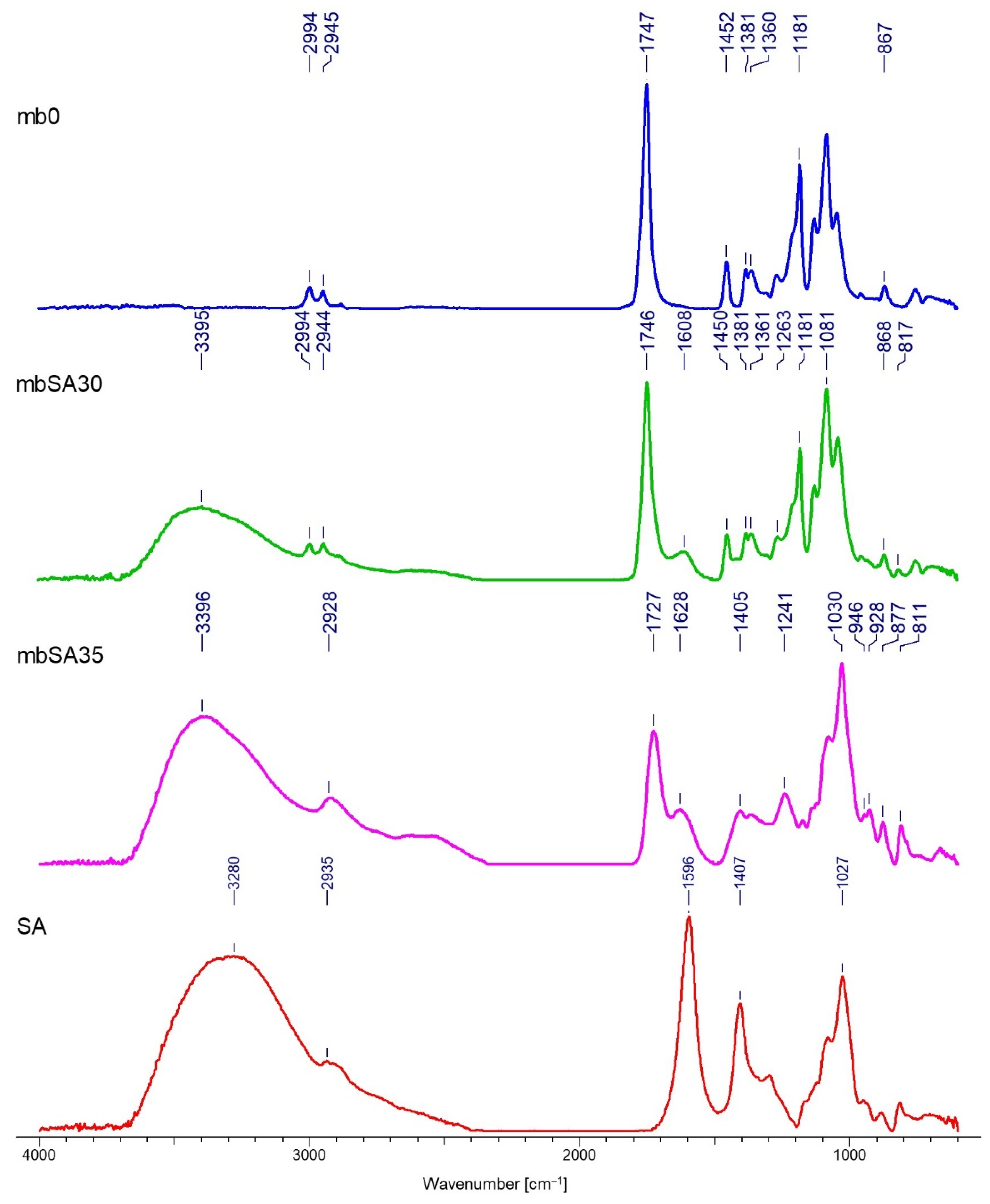

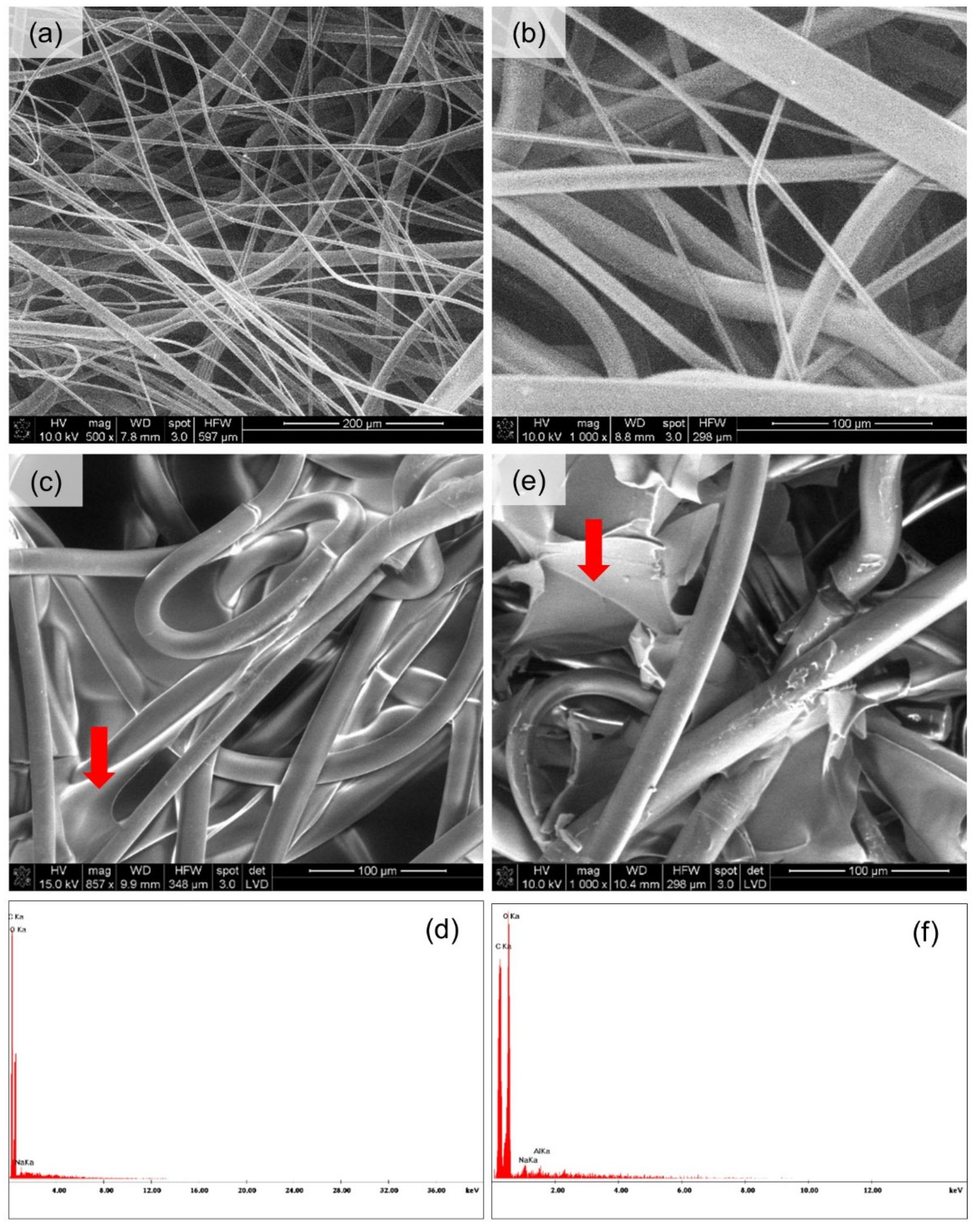
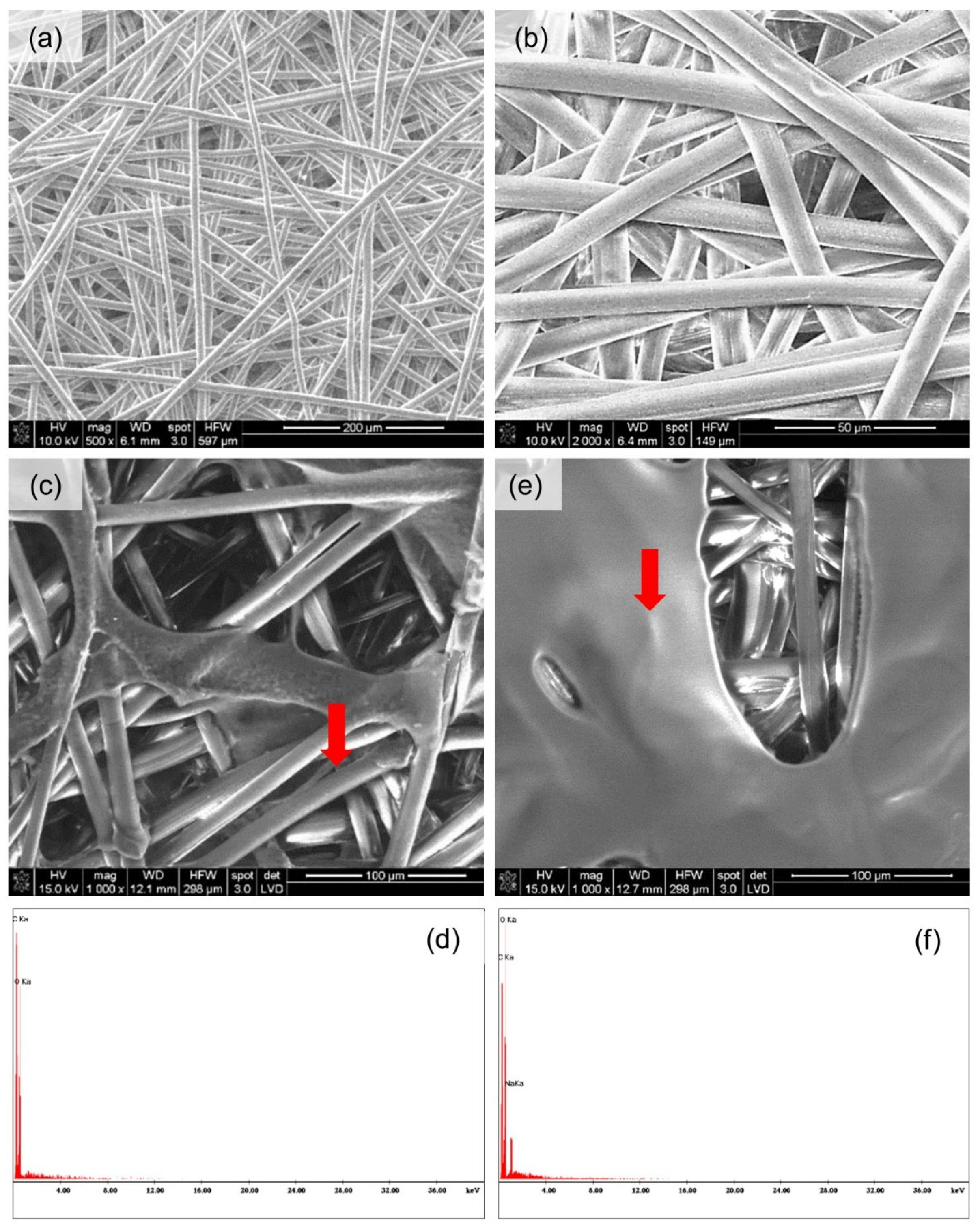
| Nr | Textile Type | Textile Name | Composition | Thickness a [mm] | Surface Mass b [g·m−2] | Total Porosity a [%] |
|---|---|---|---|---|---|---|
| 1 | Melt-blown nonwoven fabric | mb0 | Polylactide (PLA) | 4.900 | 169.44 | 83 |
| 2 | Spun-bonded nonwoven fabric | sb0 | Polylactide (PLA) | 0.258 | 48.5 | 58 |
| Nr | Textile Type | Textile Name | Modification Parameters | Thickness a [mm] | Total Porosity a [%] | Surface Mass b [g·m−2] | Volume of the Nonwoven Occupied by the Deposited Material a [%] | Deposited Layer Thickness a [μm] | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Solution | Voltage [V] | Duration [min] | ||||||||
| 1 | Melt-blown PLA nonwoven fabric | mb0 | none | none | none | 4.910 | 83 | 169.44 | 0 | none |
| 2 | mbSH30 | 1.5% SH | 30 | 3 | 1.745 | 63 | 13 | none | ||
| 3 | mbSH35 | 35 | 1.130 | 51 | 16 | 112 * | ||||
| 4 | mbSA30 | 1.5% SA | 30 | 1.340 | 49 | 8 | none | |||
| 5 | mbSA35 | 35 | 1.625 | 53 | 10 | none | ||||
| 1 | Spun-bonded PLA nonwoven fabric | sb0 | none | none | none | 0.260 | 58 | 48.5 | 0 | none |
| 2 | sbSH30 | 1.5% SH | 30 | 3 | 0.320 | 60 | 10 | none | ||
| 3 | sbSH35 | 35 | 0.280 | 35 | 30 | 51 * | ||||
| 4 | sbSA30 | 1.5% SA | 30 | 0.350 | 57 | 11 | none | |||
| 5 | sbSA35 | 35 | 0.290 | 66 | 15 | none | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pabjańczyk-Wlazło, E.K.; Puszkarz, A.K.; Bednarowicz, A.; Tarzyńska, N.; Sztajnowski, S. The Influence of Surface Modification with Biopolymers on the Structure of Melt-Blown and Spun-Bonded Poly(lactic acid) Nonwovens. Materials 2022, 15, 7097. https://doi.org/10.3390/ma15207097
Pabjańczyk-Wlazło EK, Puszkarz AK, Bednarowicz A, Tarzyńska N, Sztajnowski S. The Influence of Surface Modification with Biopolymers on the Structure of Melt-Blown and Spun-Bonded Poly(lactic acid) Nonwovens. Materials. 2022; 15(20):7097. https://doi.org/10.3390/ma15207097
Chicago/Turabian StylePabjańczyk-Wlazło, Ewelina K., Adam K. Puszkarz, Anna Bednarowicz, Nina Tarzyńska, and Sławomir Sztajnowski. 2022. "The Influence of Surface Modification with Biopolymers on the Structure of Melt-Blown and Spun-Bonded Poly(lactic acid) Nonwovens" Materials 15, no. 20: 7097. https://doi.org/10.3390/ma15207097
APA StylePabjańczyk-Wlazło, E. K., Puszkarz, A. K., Bednarowicz, A., Tarzyńska, N., & Sztajnowski, S. (2022). The Influence of Surface Modification with Biopolymers on the Structure of Melt-Blown and Spun-Bonded Poly(lactic acid) Nonwovens. Materials, 15(20), 7097. https://doi.org/10.3390/ma15207097









