Development and Characterization of PEGDA Microneedles for Localized Drug Delivery of Gemcitabine to Treat Inflammatory Breast Cancer
Abstract
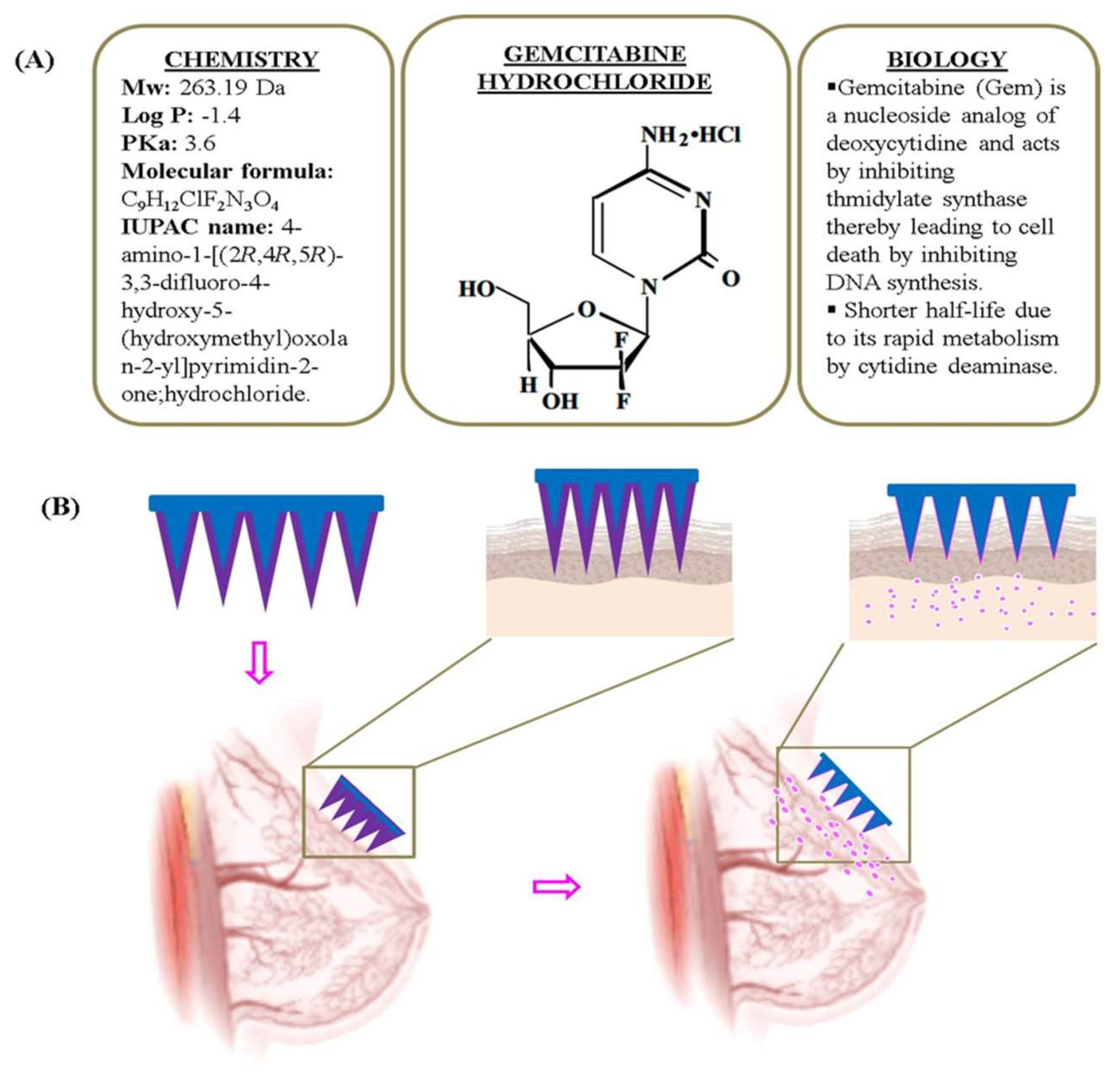
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods-Fabrication of MNPs
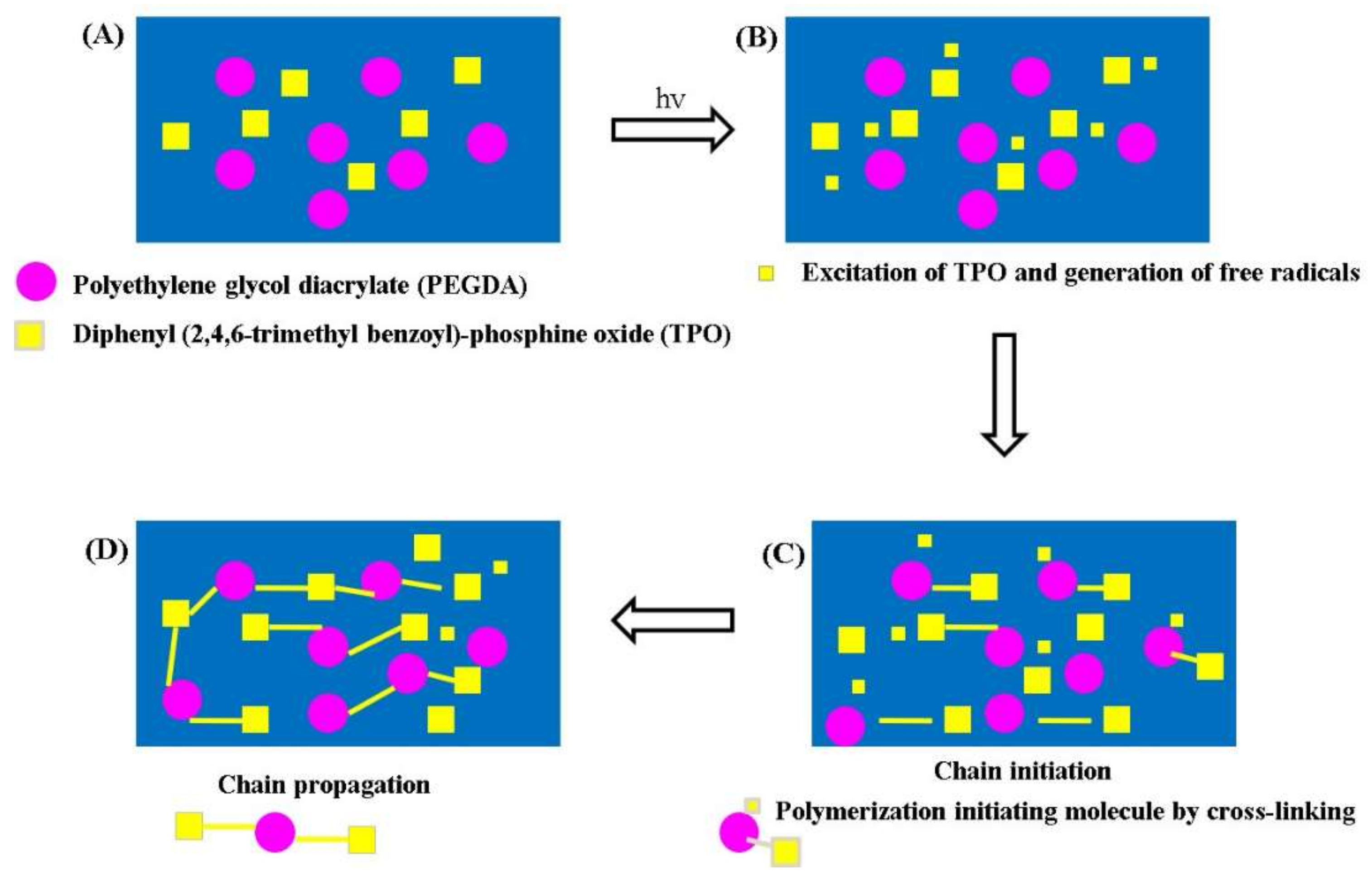
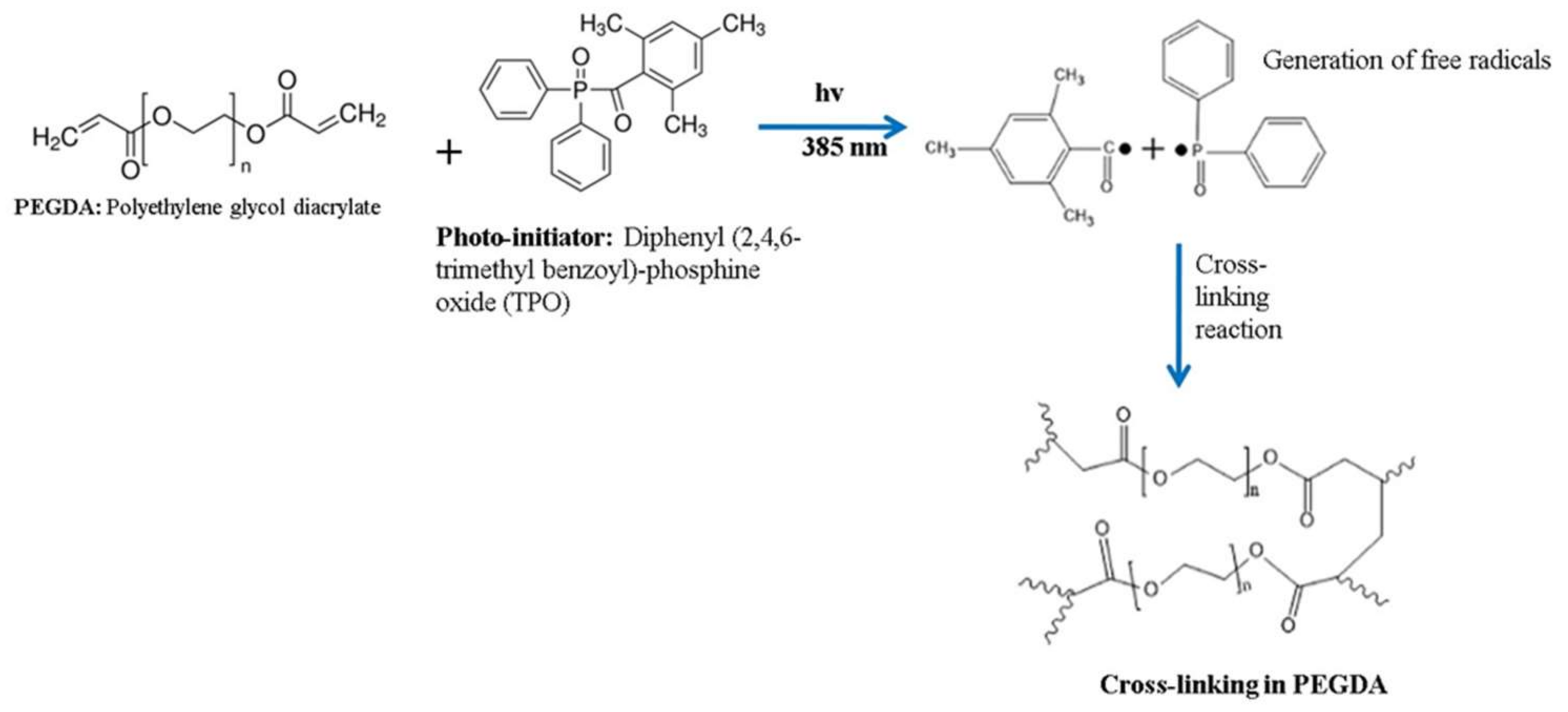
2.2.1. Preparation of Polymer Solution
2.2.2. Loading Modelled MNPs
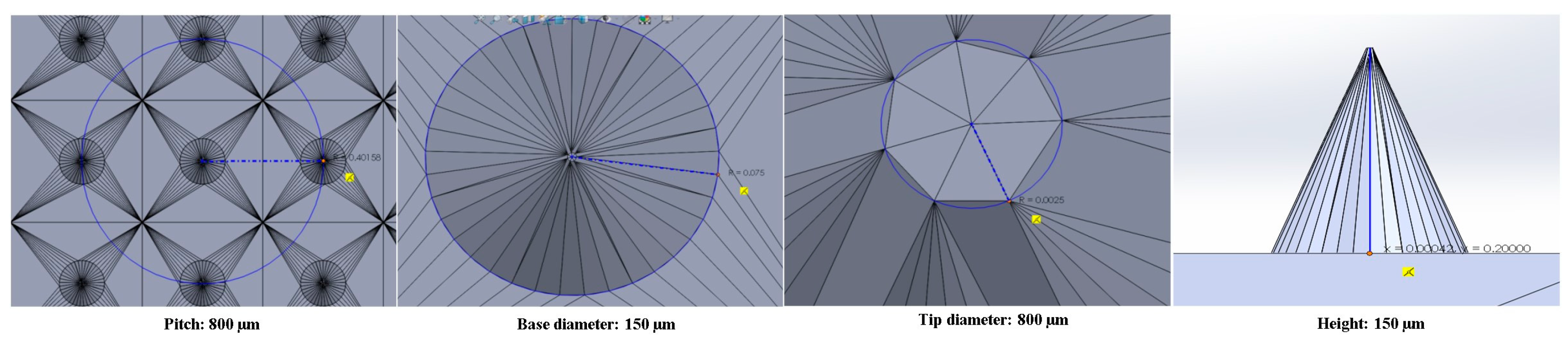
2.2.3. Additive Printing by PµSL
2.3. Characterization of MNPs
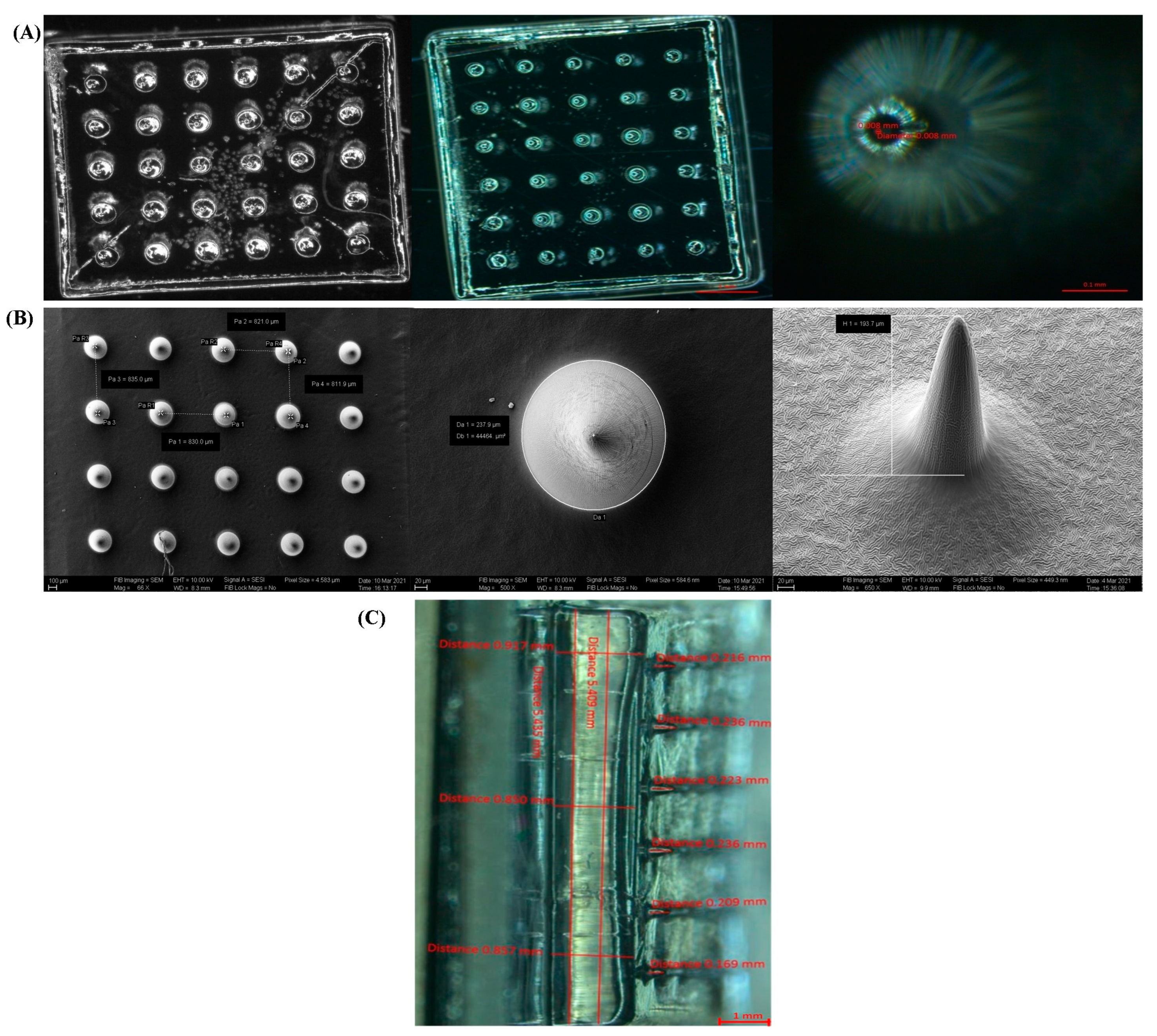
2.3.1. Stereomicroscopy and Scanning Electron Microscopy
2.3.2. Mechanical Testing
2.4. Fabrication of Drug-Coated MNPs by Stencil Plate/Screen Method
2.5. Characterization of Drug-Coated MNPs
2.5.1. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
2.5.2. In Vitro Permeation Studies
2.6. Statistical Analysis
3. Results and Discussion
3.1. Fabrication of MNPs
3.2. Characterization of MNPs
3.2.1. Stereomicroscopy and Scanning Electron Microscopy
3.2.2. Mechanical Testing
3.3. Fabrication of Drug-Coated MNs by Stencil Plate/Screen Method
3.4. Characterization of Drug-Coated MNPs
3.4.1. FTIR Analysis
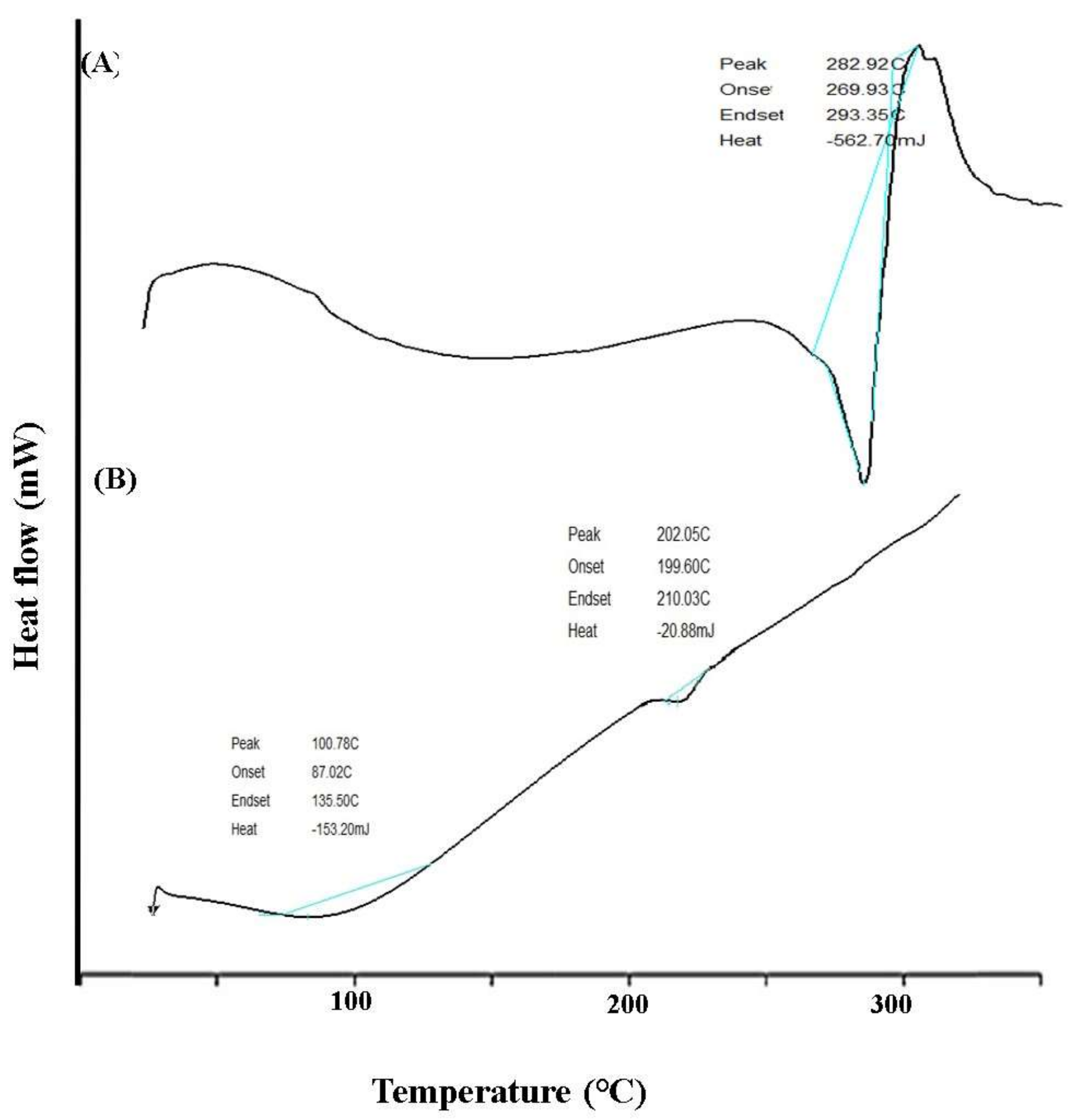
3.4.2. DSC Analysis
3.4.3. In Vitro Permeation Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Top 10 Emerging Technologies of 2020. Special Report. November 2020. Available online: http://www3.weforum.org/docs/WEF_Top_10_Emerging_Technologies_2020.pdf (accessed on 25 April 2021).
- Seetharam, A.A.; Choudhry, H.; Bakhrebah, M.A.; Abdulaal, W.H.; Gupta, M.S.; Rizvi, S.M.D.; Alam, Q.; Gowda, D.V.; Moin, A. Microneedles drug delivery systems for treatment of cancer: A recent update. Pharmaceutics 2020, 12, 1101. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-C.; Park, J.-H.; Prausnitz, M.R. Microneedles for drug and vaccine delivery. Adv. Drug Deliv. Rev. 2012, 64, 1547–1568. [Google Scholar] [CrossRef] [PubMed]
- Widera, G.; Johnson, J.; Kim, L.; Libiran, L.; Nyam, K.; Daddona, P.E.; Cormier, M. Effect of delivery parameters on immunization to ovalbumin following intracutaneous administration by a coated microneedle array patch system. Vaccine 2006, 24, 1653–1664. [Google Scholar] [CrossRef] [PubMed]
- Bediz, B.; Korkmaz, E.; Khilwani, R.; Donahue, C.; Erdos, G.; Falo, L.D.; Ozdoganlar, O.B. Dissolvable microneedle arrays for intradermal delivery of biologics: Fabrication and application. Pharm. Res. 2014, 31, 117–135. [Google Scholar] [CrossRef]
- McGrath, M.G.; Vrdoljak, A.; O’Mahony, C.; Oliveira, J.C.; Moore, A.C.; Crean, A.M. Determination of parameters for successful spray coating of silicon microneedle arrays. Int. J. Pharm. 2011, 415, 140–149. [Google Scholar] [CrossRef]
- Park, J.-H.; Choi, S.-O.; Seo, S.; Choy, Y.B.; Prausnitz, M.R. A microneedle roller for transdermal drug delivery. Eur. J. Pharm. Biopharm. 2010, 76, 282–289. [Google Scholar] [CrossRef]
- Lee, J.W.; Park, J.-H.; Prausnitz, M.R. Dissolving microneedles for transdermal drug delivery. Biomaterials 2008, 29, 2113–2124. [Google Scholar] [CrossRef]
- McCrudden, M.T.C.; Alkilani, A.Z.; McCrudden, C.M.; McAlister, E.; McCarthy, H.O.; Woolfson, A.D.; Donnelly, R.F. Design and physicochemical characterisation of novel dissolving polymeric microneedle arrays for transdermal delivery of high dose, low molecular weight drugs. J. Control. Release 2014, 180, 71–80. [Google Scholar] [CrossRef]
- Sullivan, S.P.; Koutsonanos, D.G.; del Pilar Martin, M.; Lee, J.W.; Zarnitsyn, V.; Choi, S.-O.; Murthy, N.; Compans, R.W.; Skountzou, I.; Prausnitz, M.R. Dissolving polymer microneedle patches for influenza vaccination. Nat. Med. 2010, 16, 915–920. [Google Scholar] [CrossRef]
- Chen, M.-C.; Ling, M.-H.; Lai, K.-Y.; Pramudityo, E. Chitosan microneedle patches for sustained transdermal delivery of macromolecules. Biomacromolecules 2012, 13, 4022–4031. [Google Scholar] [CrossRef]
- Park, J.-H.; Davis, S.; Yoon, Y.-K.; Prausnitz, M.R.; Allen, M.G. Micromachined biodegradable microstructures. In Proceedings of the Sixteenth Annual International Conference on Micro Electro Mechanical Systems, MEMS-03, Kyoto, Japan, 19–23 January 2003; IEEE: Piscataway, NJ, USA, 2003; pp. 371–374. [Google Scholar]
- Park, J.-H.; Allen, M.G.; Prausnitz, M.R. Biodegradable polymer microneedles: Fabrication, mechanics and transdermal drug delivery. J. Control. Release 2005, 104, 51–66. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zeng, M.; Shan, H.; Tong, C. Microneedle patches as drug and vaccine delivery platform. Curr. Med. Chem. 2017, 24, 2413–2422. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Chávez, J.J.; Bonilla-Martinez, D.; Angélica, M.; Molina-Trinidad, E.; Casas-Alancaster, N.; Revilla-Vázquez, A.L. Microneedles: A valuable physical enhancer to increase transdermal drug delivery. J. Clin. Pharmacol. 2011, 51, 964–977. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Choi, S.-O.; Felner, E.I.; Prausnitz, M.R. Dissolving microneedle patch for transdermal delivery of human growth hormone. Small 2011, 7, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Sobin, L.H.; Compton, C.C. TNM seventh edition: What’s new, what’s changed: Communication from the International Union Against Cancer and the American Joint Committee on Cancer. Cancer 2010, 116, 5336–5339. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Yu, G.; Huang, F. Supramolecular chemotherapy based on host–guest molecular recognition: A novel strategy in the battle against cancer with a bright future. Chem. Soc. Rev. 2017, 46, 7021–7053. [Google Scholar] [CrossRef]
- Zhou, J.; Rao, L.; Yu, G.; Cook, T.R.; Chen, X.; Huang, F. Supramolecular cancer nanotheranostics. Chem. Soc. Rev. 2021, 50, 2839–2891. [Google Scholar] [CrossRef]
- Edge, S.B.; Compton, C.C. The American Joint Committee on Cancer: The 7th edition of the AJCC cancer staging manual and the future of TNM. Ann. Surg. Oncol. 2010, 17, 1471–1474. [Google Scholar] [CrossRef]
- Blackstein, M.; Vogel, C.L.; Ambinder, R.; Cowan, J.; Iglesias, J.; Melemed, A. Gemcitabine as first-line therapy in patients with metastatic breast cancer: A phase II trial. Oncology 2002, 62, 2–8. [Google Scholar] [CrossRef]
- Paroha, S.; Verma, J.; Dubey, R.D.; Dewangan, R.P.; Molugulu, N.; Bapat, R.A.; Sahoo, P.K.; Kesharwani, P. Recent advances and prospects in gemcitabine drug delivery systems. Int. J. Pharm. 2021, 592, 120043. [Google Scholar] [CrossRef]
- Moog, R.; Burger, A.; Brandl, M.; Schüler, J.; Schubert, R.; Unger, C.; Fiebig, H.; Massing, U. Change in pharmacokinetic and pharmacodynamic behavior of gemcitabine in human tumor xenografts upon entrapment in vesicular phospholipid gels. Cancer Chemother. Pharmacol. 2002, 49, 356–366. [Google Scholar] [PubMed]
- Reid, J.M.; Qu, W.; Safgren, S.L.; Ames, M.M.; Krailo, M.D.; Seibel, N.L.; Kuttesch, J.; Holcenberg, J. Phase I trial and pharmacokinetics of gemcitabine in children with advanced solid tumors. J. Clin. Oncol. 2004, 22, 2445–2451. [Google Scholar] [CrossRef] [PubMed]
- Ueno, H.; Kiyosawa, K.; Kaniwa, N. Pharmacogenomics of gemcitabine: Can genetic studies lead to tailor-made therapy? Br. J. Cancer 2007, 97, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Dasanu, C.A. Gemcitabine: Vascular toxicity and prothrombotic potential. Expert Opin. Drug Saf. 2008, 7, 703–716. [Google Scholar] [CrossRef]
- Cheng, Y.C.; Shi, Y.; Zhang, M.-J.; Brazauskas, R.; Hemmer, M.T.; Bishop, M.R.; Nieto, Y.; Stadtmauer, E.; Ayash, L.; Gale, R.P.; et al. Long-term outcome of inflammatory breast cancer compared to non-inflammatory breast cancer in the setting of high-dose chemotherapy with autologous hematopoietic cell transplantation. J. Cancer 2017, 8, 1009. [Google Scholar] [CrossRef]
- Thota, H.S.; Bidwai, V.R.; Vinod, P.; Natchimuthu, B. Fabrication of dimensionally accurate 3D micro components by projection micro-stereolithography technique. Int. J. Addit. Subtractive Mater. Manuf. 2020, 2. [Google Scholar] [CrossRef]
- Larrañeta, E.; Moore, J.; Vicente-Pérez, E.M.; González-Vázquez, P.; Lutton, R.; Woolfson, A.D.; Donnelly, R.F. A proposed model membrane and test method for microneedle insertion studies. Int. J. Pharm. 2014, 472, 65–73. [Google Scholar] [CrossRef]
- Lutton, R.E.M.; Larrañeta, E.; Kearney, M.-C.; Boyd, P.; Woolfson, A.D.; Donnelly, R.F. A novel scalable manufacturing process for the production of hydrogel-forming microneedle arrays. Int. J. Pharm. 2015, 494, 417–429. [Google Scholar] [CrossRef]
- Zhu, J. Bioactive modification of poly (ethylene glycol) hydrogels for tissue engineering. Biomaterials 2010, 31, 4639–4656. [Google Scholar] [CrossRef]
- Donnelly, R.F.; Garland, M.J.; Alkilani, A.Z. Microneedle-iontophoresis combinations for enhanced transdermal drug delivery. In Drug Delivery System; Springer: Berlin/Heidelberg, Germany, 2014; pp. 121–132. [Google Scholar]
- Demartis, S.; Anjani, Q.K.; Volpe-Zanutto, F.; Paredes, A.J.; Jahan, S.A.; Vora, L.K.; Donnelly, R.F.; Gavini, E. Trilayer dissolving polymeric microneedle array loading Rose Bengal transfersomes as a novel adjuvant in early-stage cutaneous melanoma management. Int. J. Pharm. 2022, 627, 122217. [Google Scholar] [CrossRef]
- Xueliang, X.; Guangzhi, G.; Yong, L.; Fengsen, M. Drug delivery with dissolving microneedles: Skin puncture, its influencing factors and improvement strategies. J. Drug Deliv. Sci. Technol. 2022, 76, 103653. [Google Scholar]
- Ramöller, I.K.; Tekko, I.A.; McCarthy, H.O.; Donnelly, R.F. Rapidly dissolving bilayer microneedle arrays--a minimally invasive transdermal drug delivery system for vitamin B12. Int. J. Pharm. 2019, 566, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-S.; Park, J.-H. 3-Dimensional coating polymer microneedles for economical and efficient transdermal drug delivery. Polymer 2014, 38, 391–396. [Google Scholar]



| Item | Details |
|---|---|
| Mobile phase | Phosphate buffer pH7.4 and methanol in 90:10 ratio |
| Injection volume | 40 µL |
| Flowrate | 1 mL/min |
| Absorbance wavelength | 275 nm (UV-Visible detector) |
| Column temperature | 10 °C |
| Column | C18(250X4.6mmi.d., 5 µm, Phenomenex, Torrance, CA, USA) |
| Sl. No. | Item | Target Dimensions (µm) | Average of Obtained Dimensions (µm) |
|---|---|---|---|
| 1 | Base diameter | 150 | 158 |
| 2 | Tip diameter | 5 | 8 |
| 3 | Height | 200 | 193 |
| 4 | Pitch | 800 | 824 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alafnan, A.; Seetharam, A.A.; Hussain, T.; Gupta, M.S.; Rizvi, S.M.D.; Moin, A.; Alamri, A.; Unnisa, A.; Awadelkareem, A.M.; Elkhalifa, A.O.; et al. Development and Characterization of PEGDA Microneedles for Localized Drug Delivery of Gemcitabine to Treat Inflammatory Breast Cancer. Materials 2022, 15, 7693. https://doi.org/10.3390/ma15217693
Alafnan A, Seetharam AA, Hussain T, Gupta MS, Rizvi SMD, Moin A, Alamri A, Unnisa A, Awadelkareem AM, Elkhalifa AO, et al. Development and Characterization of PEGDA Microneedles for Localized Drug Delivery of Gemcitabine to Treat Inflammatory Breast Cancer. Materials. 2022; 15(21):7693. https://doi.org/10.3390/ma15217693
Chicago/Turabian StyleAlafnan, Ahmed, Aravindram Attiguppe Seetharam, Talib Hussain, Maram Suresh Gupta, Syed Mohd Danish Rizvi, Afrasim Moin, Abdulwahab Alamri, Aziz Unnisa, Amir Mahgoub Awadelkareem, AbdElmoneim O. Elkhalifa, and et al. 2022. "Development and Characterization of PEGDA Microneedles for Localized Drug Delivery of Gemcitabine to Treat Inflammatory Breast Cancer" Materials 15, no. 21: 7693. https://doi.org/10.3390/ma15217693
APA StyleAlafnan, A., Seetharam, A. A., Hussain, T., Gupta, M. S., Rizvi, S. M. D., Moin, A., Alamri, A., Unnisa, A., Awadelkareem, A. M., Elkhalifa, A. O., Jayahanumaiah, P., Khalid, M., & Balashanmugam, N. (2022). Development and Characterization of PEGDA Microneedles for Localized Drug Delivery of Gemcitabine to Treat Inflammatory Breast Cancer. Materials, 15(21), 7693. https://doi.org/10.3390/ma15217693









