Microstructure and Mechanical Properties of W-Al2O3 Alloy Plates Prepared by a Wet Chemical Method and Rolling Process
Abstract
:1. Introduction
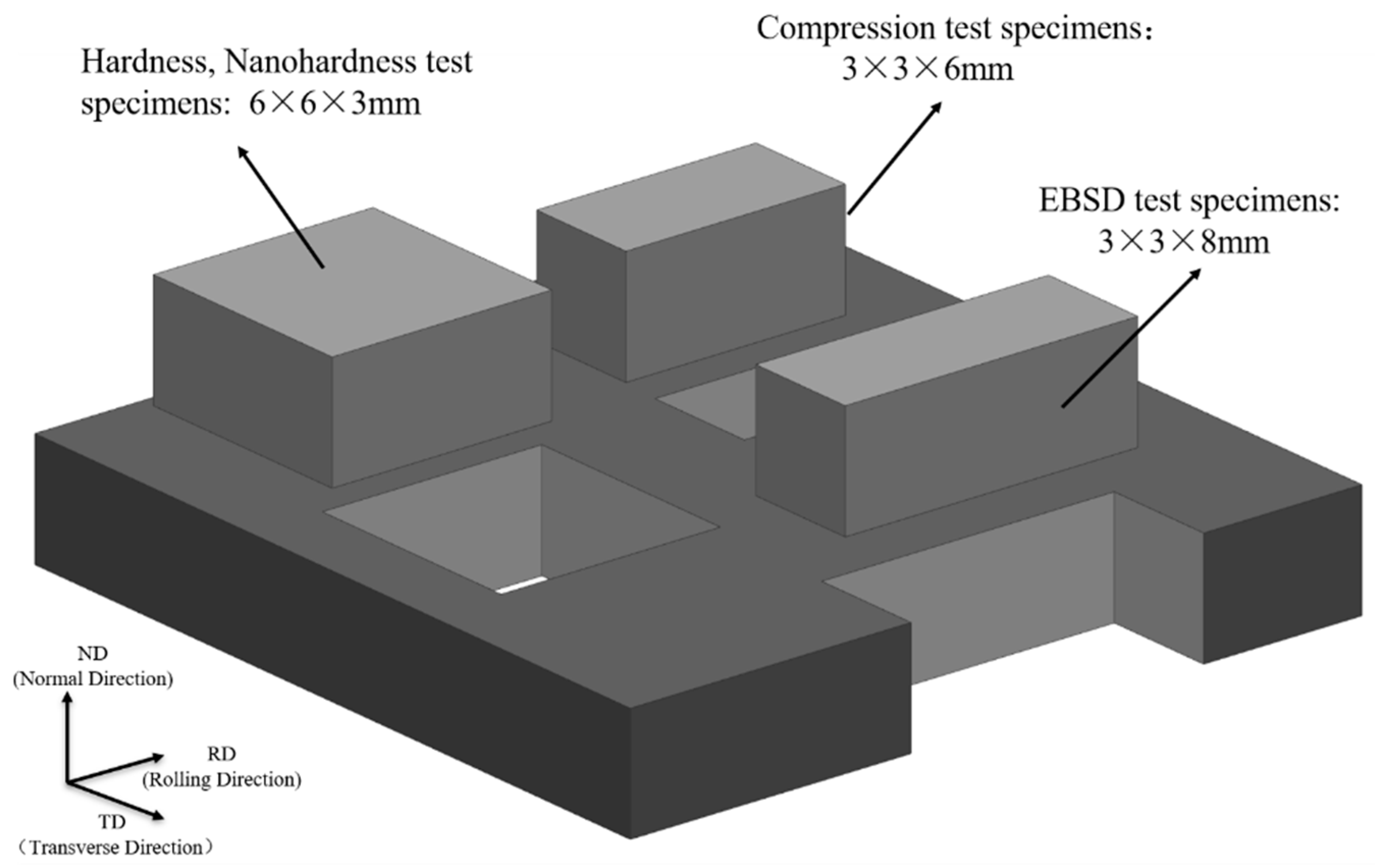
2. Materials and Methods
3. Results and Discussion
3.1. Powder Characterization
3.2. Microstructure of Sintered and Rolled Alloys
3.3. Microstructure and Interface Feature
3.4. Mechanical Properties
4. Conclusions
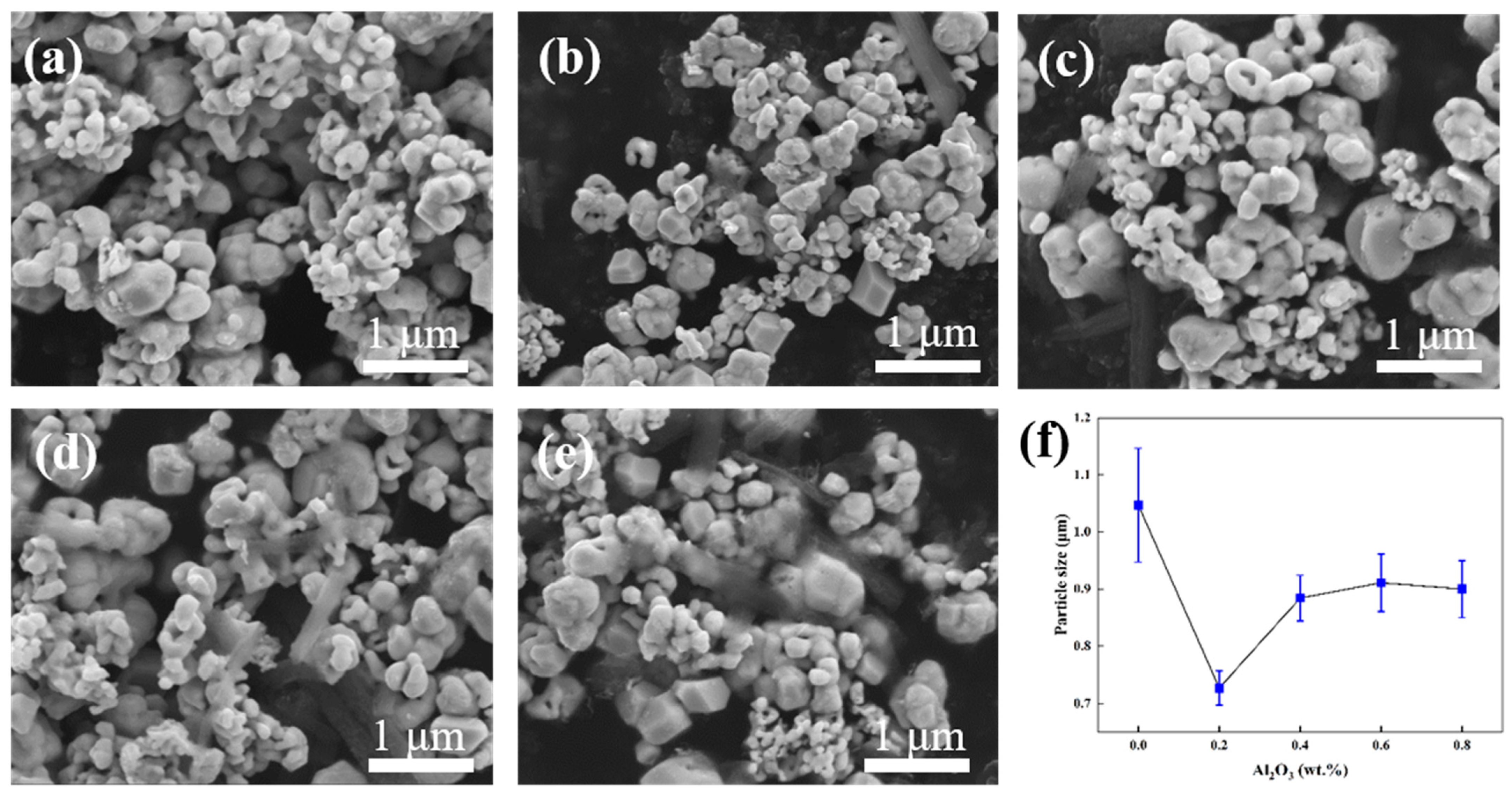
- Among the alloy powders of different compositions, the average grain size of the W-0.2 wt.% Al2O3 powder was only 727 nm, while the grain size of the rest of the powders was about 1 μm. The XRD pattern showed no diffraction peaks of Al2O3 due to the low Al2O3 content.
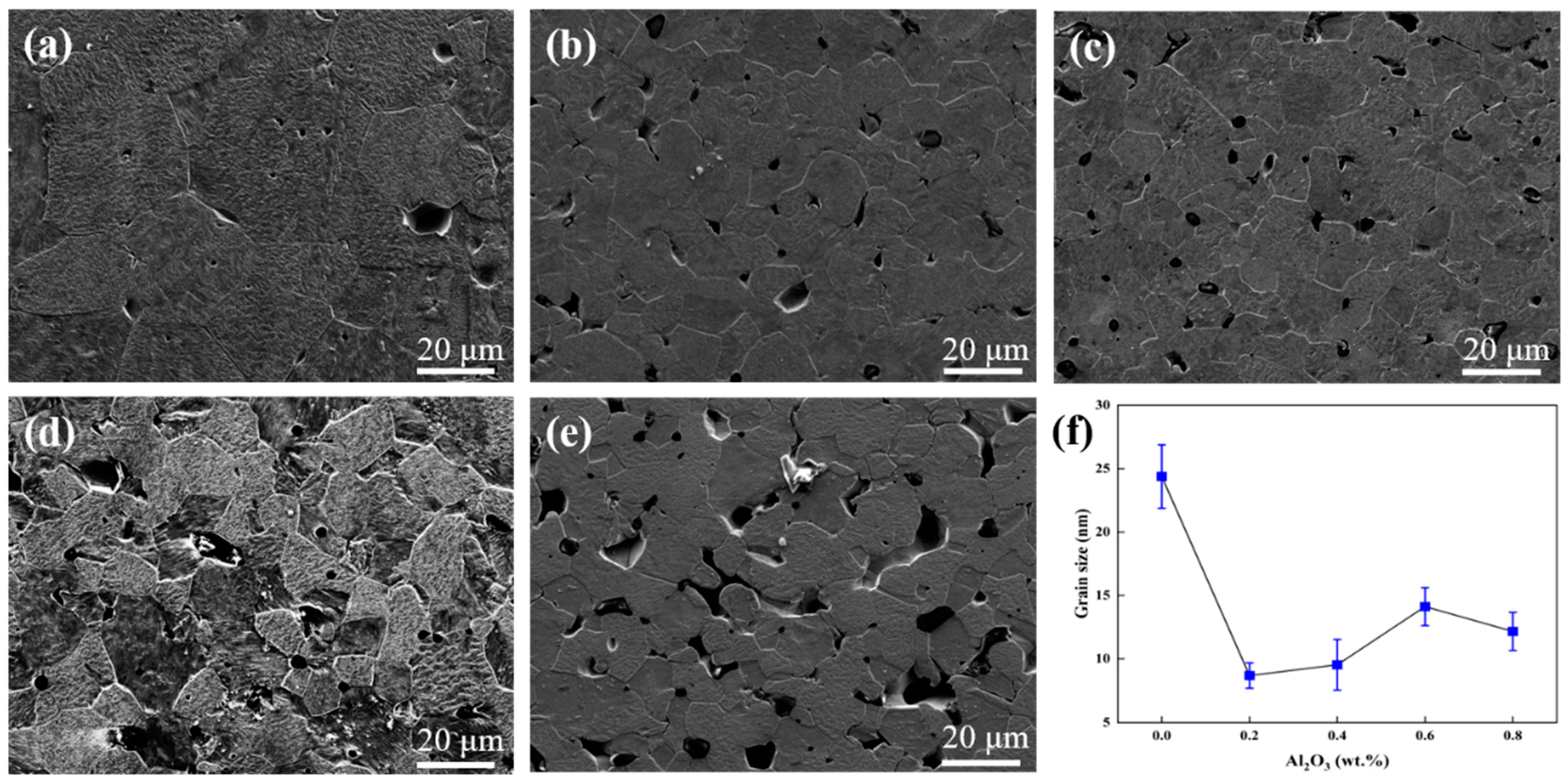
- In the sintered specimens, the average grain size of pure tungsten was 24 μm, and that of W-0.2 wt.% Al2O3 was only 9 m. The addition of Al2O3 was effective in refining the grains.
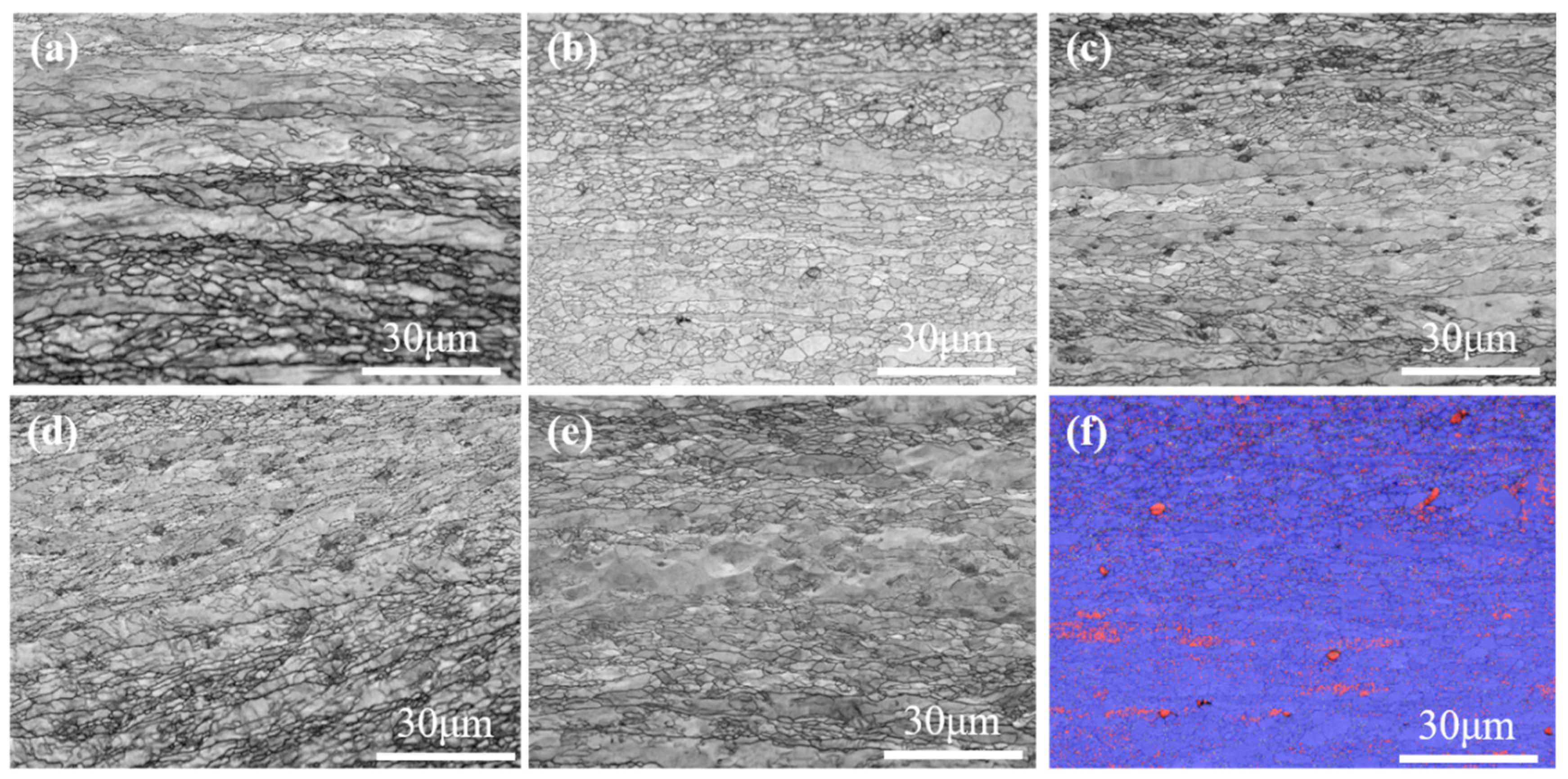
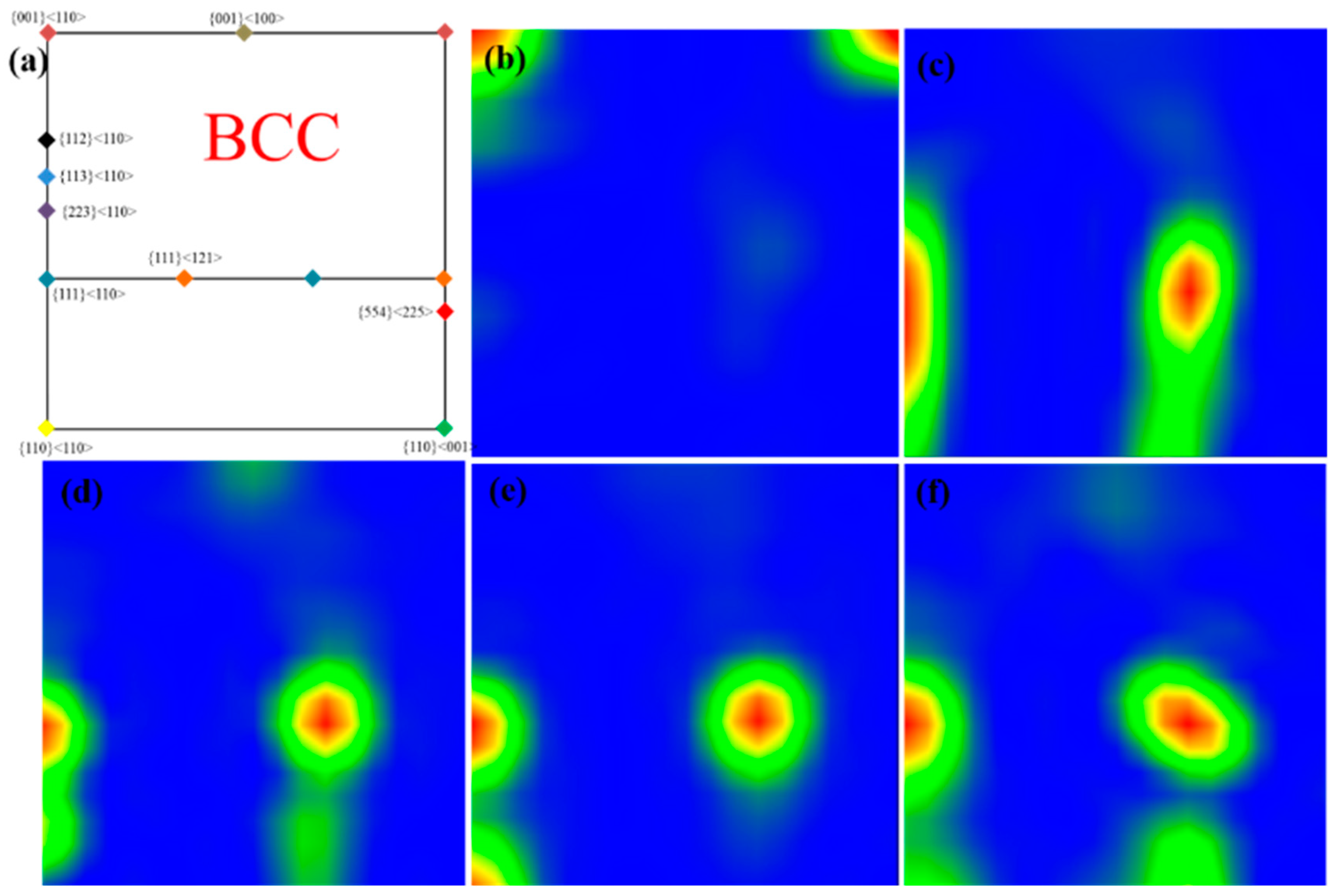
- The maximum Ferret diameter is 10 μm for pure tungsten and 6 μm for W-0.2 wt.% Al2O3 plates, with the same size trend as in the sintered state, due to dislocation cleavage of the grains during the rolling process. The texture of the plates changes from {001} <110> to {111} <110> after the addition of Al2O3.
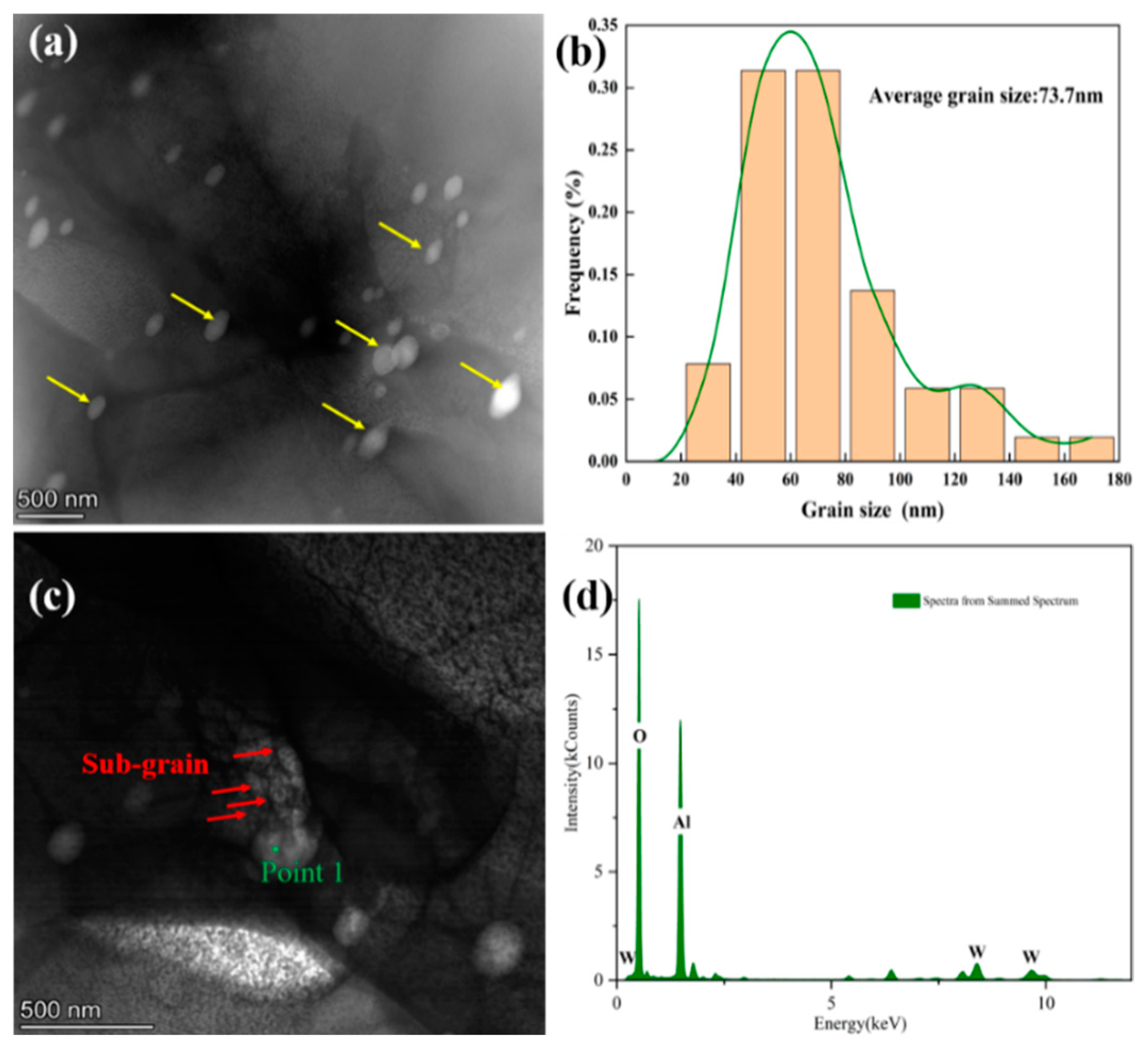
- As can be observed in the TEM image, the average grain size of the Al2O3 in the plate is approximately 100 nm, with a transition layer at the interface between the two phases and a non-co-grained interface relationship.
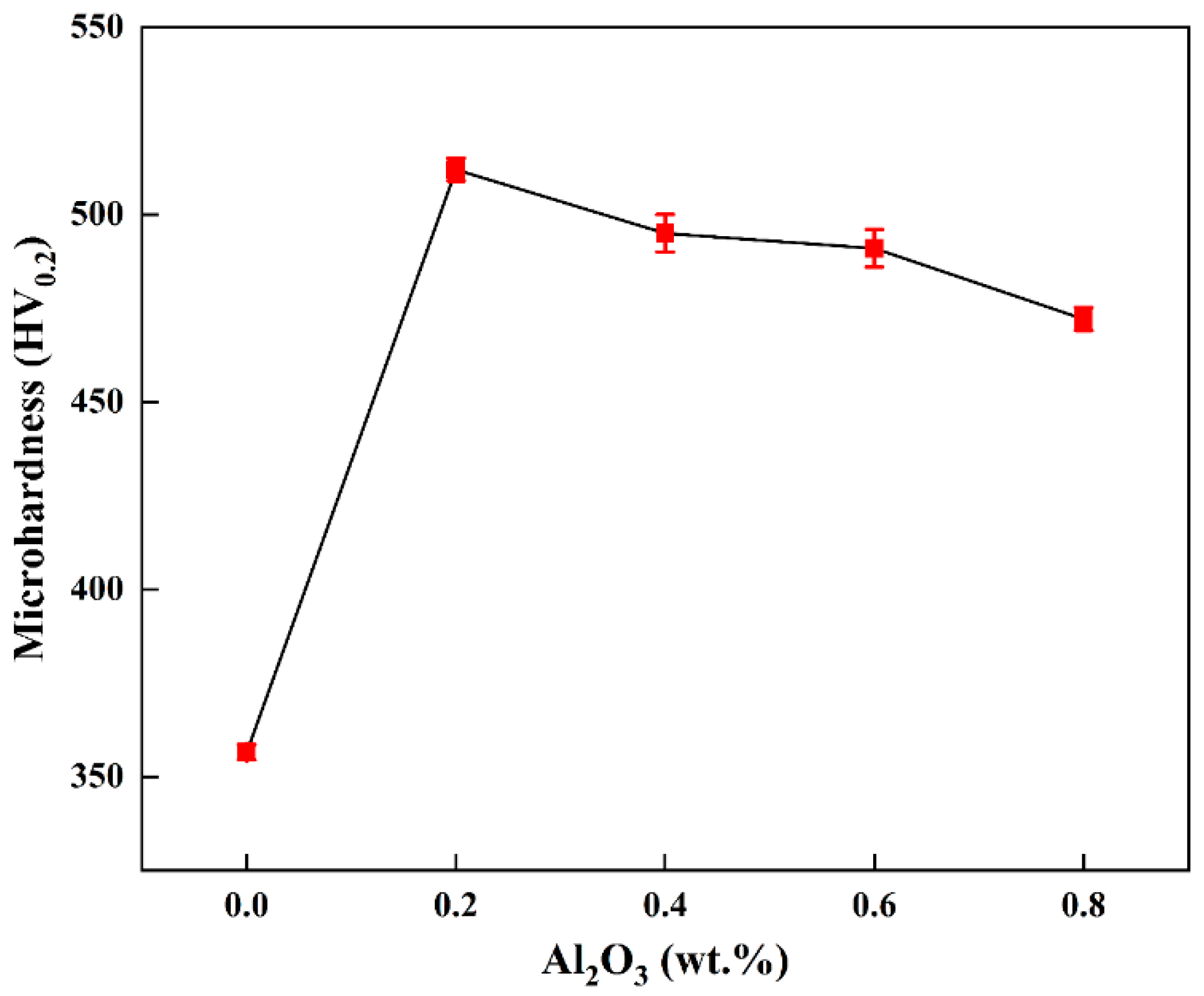
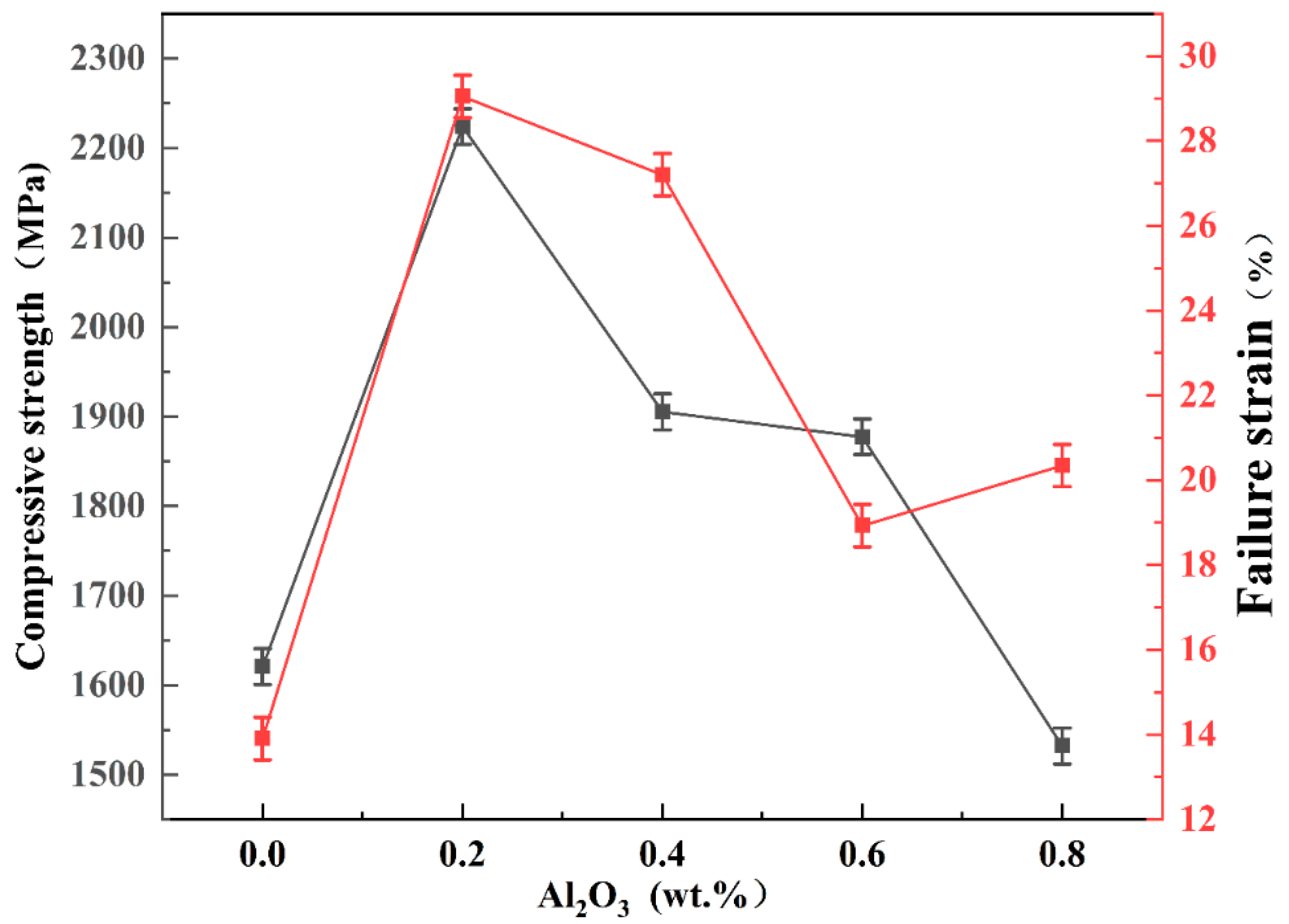
- Comparing the mechanical properties of pure tungsten plates with those of tungsten alloy plates of different compositions, the microhardness has increased by up to 156 HV0.2, an increase of 43%, while the nano-hardness increased by 2.75 GPa, an increase of 24.1%. The compressive strength of pure tungsten plates was 1620 MPa, but the addition of Al2O3 increased the compressive strength by up to 600 MPa, an increase of 37%. Pure tungsten plates fractured at a compression deflection of 13.9%, and at an Al2O3 content of 0.2 wt.%, after which the plates fractured only at a deflection of 29%. This proves that the doping of Al2O3 has enhanced the strength and toughness of the alloy plates.
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Talignani, A.; Seede, R.; Whitt, A.; Zheng, S.; Ye, J.; Karaman, I.; Kirka, M.M.; Katoh, Y.; Wang, Y.M. A review on additive manufacturing of refractory tungsten and tungsten alloys. Addit. Manuf. 2022, 58, 103009. [Google Scholar] [CrossRef]
- Zhou, H.-B.; Liu, Y.-L.; Jin, S.; Zhang, Y.; Luo, G.N.; Lu, G.-H. Towards suppressing H blistering by investigating the physical origin of the H–He interaction in W. Nuclear Fusion 2010, 50, 115010. [Google Scholar] [CrossRef]
- Xu, Q.; Sato, K.; Cao, X.Z.; Zhang, P.; Wang, B.Y.; Yoshiie, T.; Watanabe, H.; Yoshida, N. Interaction of deuterium with vacancies induced by ion irradiation in W. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2013, 315, 146–148. [Google Scholar] [CrossRef]
- Wang, H.; Gao, Y.; Fu, E.; Yang, T.; Xue, J.; Yan, S.; Chu, P.K.; Wang, Y. Irradiation effects on multilayered W/ZrO2 film under 4MeV Au ions. J. Nucl. Mater. 2014, 455, 86–90. [Google Scholar] [CrossRef]
- Miao, S.; Xie, Z.M.; Zhang, T.; Wang, X.P.; Fang, Q.F.; Liu, C.S.; Luo, G.N.; Liu, X.; Lian, Y.Y. Mechanical properties and thermal stability of rolled W-0.5wt% TiC alloys. Mater. Sci. Eng. A 2016, 671, 87–95. [Google Scholar] [CrossRef]
- Battabyal, M.; Schäublin, R.; Spätig, P.; Baluc, N. W–2wt.%Y2O3 composite: Microstructure and mechanical properties. Mater. Sci. Eng. A 2012, 538, 53–57. [Google Scholar] [CrossRef]
- Chakraborty, S.P.; Banerjee, S.; Sanyal, G.; Bhave, V.S.; Paul, B.; Sharma, I.G.; Suri, A.K. Studies on the synthesis of a Mo–30wt% W alloy by non-conventional approaches. J. Alloys Compd. 2010, 501, 211–217. [Google Scholar] [CrossRef]
- Veleva, L.; Oksiuta, Z.; Vogt, U.; Baluc, N. Sintering and characterization of W–Y and W–Y2O3 materials. Fusion Eng. Des. 2009, 84, 1920–1924. [Google Scholar] [CrossRef]
- Ryu, H.J.; Hong, S.H. Fabrication and properties of mechanically alloyed oxide-dispersed tungsten heavy alloys. Mater. Sci. Eng. A 2003, 363, 179–184. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, K.H.; Kim, E.-P.; Cheong, D.-I.; Hong, S.H. Fabrication of high temperature oxides dispersion strengthened tungsten composites by spark plasma sintering process. Int. J. Refract. Met. Hard Mater. 2009, 27, 842–846. [Google Scholar] [CrossRef]
- Kim, Y.; Hong, M.-H.; Lee, S.H.; Kim, E.-P.; Lee, S.; Noh, J.-W. The effect of yttrium oxide on the sintering behavior and hardness of tungsten. Met. Mater. Int. 2006, 12, 245–248. [Google Scholar] [CrossRef]
- Mabuchi, M.; Okamoto, K.; Saito, N.; Asahina, T.; Igarashi, T. Deformation behavior and strengthening mechanisms at intermediate temperatures in W-La2O3. Mater. Sci. Eng. A 1997, 237, 241–249. [Google Scholar] [CrossRef]
- Zhao, M.; Zhou, Z.; Zhong, M.; Tan, J.; Lian, Y.; Liu, X. Thermal shock behavior of fine grained W–Y2O3 materials fabricated via two different manufacturing technologies. J. Nucl. Mater. 2016, 470, 236–243. [Google Scholar] [CrossRef]
- Li, Z.; Xu, L.; Wei, S.; Chen, C.; Xiao, F. Fabrication and mechanical properties of tungsten alloys reinforced with c-ZrO2 particles. J. Alloys Compd. 2018, 769, 694–705. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, L.; Wei, S.; Pan, K.; Wu, X.; Li, Q. Preparation, microstructure, and constitutive equation of W-0.25 wt% Al2O3 alloy. Mater. Sci. Eng. A 2019, 744, 79–85. [Google Scholar] [CrossRef]
- Zhou, Y.; Gao, Y.; Wei, S.; Pan, K.; Hu, Y. Preparation and characterization of Mo/Al2O3 composites. Int. J. Refract. Met. Hard Mater. 2016, 54, 186–195. [Google Scholar] [CrossRef]
- Zabihi, M.; Toroghinejad, M.R.; Shafyei, A. Application of powder metallurgy and hot rolling processes for manufacturing aluminum/alumina composite strips. Mater. Sci. Eng. A 2013, 560, 567–574. [Google Scholar] [CrossRef]
- Tian, B.; Liu, P.; Song, K.; Li, Y.; Liu, Y.; Ren, F.; Su, J. Microstructure and properties at elevated temperature of a nano-Al2O3 particles dispersion-strengthened copper base composite. Mater. Sci. Eng. A 2006, 435–436, 705–710. [Google Scholar] [CrossRef]
- Rajkovic, V.; Bozic, D.; Jovanovic, M.T. Effects of copper and Al2O3 particles on characteristics of Cu–Al2O3 composites. Mater. Des. 2010, 31, 1962–1970. [Google Scholar] [CrossRef]
- Wang, J.; Gan, X.; Li, Z.; Zhou, K. Microstructure and gas sensing property of porous spherical In2O3 particles prepared by hydrothermal method. Powder Technol. 2016, 303, 138–146. [Google Scholar] [CrossRef]
- Visa, M. Synthesis and characterization of new zeolite materials obtained from fly ash for heavy metals removal in advanced wastewater treatment. Powder Technol. 2016, 294, 338–347. [Google Scholar] [CrossRef]
- Selvarajan, S.; Suganthi, A.; Rajarajan, M.; Arunprasath, K. Highly efficient BiVO4/WO3 nanocomposite towards superior photocatalytic performance. Powder Technol. 2017, 307, 203–212. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, L.; Pan, K.; Wei, S.; Wu, X.; Li, Q. Effect of Al2O3 content and swaging on microstructure and mechanical properties of Al2O3/W alloys. Int. J. Refract. Met. Hard Mater. 2020, 86, 105082. [Google Scholar] [CrossRef]
- Sudharshan Phani, P.; Oliver, W.C. A critical assessment of the effect of indentation spacing on the measurement of hardness and modulus using instrumented indentation testing. Mater. Des. 2019, 164, 107563. [Google Scholar] [CrossRef]
- Debata, M.; Acharya, T.S.; Sengupta, P.; Acharya, P.P.; Bajpai, S.; Jayasankar, K. Effect of high energy ball milling on structure and properties of 95W-3.5Ni-1.5Fe heavy alloys. Int. J. Refract. Met. Hard Mater. 2017, 69, 170–179. [Google Scholar] [CrossRef]
- Zong, L.; Xu, L.; Luo, C.; Li, Z.; Zhao, Y.; Xu, Z.; Zhu, C.; Wei, S. Fabrication of nano-ZrO2 strengthened WMoNbTaV refractory high-entropy alloy by spark plasma sintering. Mater. Sci. Eng. A 2022, 843, 143113. [Google Scholar] [CrossRef]
- Senkov, O.N.; Wilks, G.B.; Scott, J.M.; Miracle, D.B. Mechanical properties of Nb25Mo25Ta25W25 and V20Nb20Mo20Ta20W20 refractory high entropy alloys. Intermetallics 2011, 19, 698–706. [Google Scholar] [CrossRef]
- Han, Z.D.; Luan, H.W.; Liu, X.; Chen, N.; Li, X.Y.; Shao, Y.; Yao, K.F. Microstructures and mechanical properties of TixNbMoTaW refractory high-entropy alloys. Mater. Sci. Eng. A 2018, 712, 380–385. [Google Scholar] [CrossRef]
- Han, Z.D.; Chen, N.; Zhao, S.F.; Fan, L.W.; Yang, G.N.; Shao, Y.; Yao, K.F. Effect of Ti additions on mechanical properties of NbMoTaW and VNbMoTaW refractory high entropy alloys. Intermetallics 2017, 84, 153–157. [Google Scholar] [CrossRef]
- Yao, H.W.; Qiao, J.W.; Gao, M.C.; Hawk, J.A.; Ma, S.G.; Zhou, H.F.; Zhang, Y. NbTaV-(Ti,W) refractory high-entropy alloys: Experiments and modeling. Mater. Sci. Eng. A 2016, 674, 203–211. [Google Scholar] [CrossRef]
- Li, Z.; Chen, Y.; Wei, S.; Pan, K.; Shen, H.; Xu, L. Effect of rotary swaging and subsequent annealing on microstructure and mechanical properties of W-1.5ZrO2 alloys. J. Alloy. Compd. 2021, 875, 160041. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, L.; Wei, S.; Pan, K.; Wu, X.; Li, Q. Effect of ZrO2 content on microstructure and mechanical properties of W alloys fabricated by spark plasma sintering. Int. J. Refract. Met. Hard Mater. 2019, 79, 79–89. [Google Scholar] [CrossRef]
- Xiao, F.; Xu, L.; Zhou, Y.; Pan, K.; Li, J.; Liu, W.; Wei, S. A hybrid microstructure design strategy achieving W-ZrO2(Y) alloy with high compressive strength and critical failure strain. J. Alloys Compd. 2017, 708, 202–212. [Google Scholar] [CrossRef]
- Zong, H.T.; Ma, M.Z.; Liu, L.; Zhang, X.Y.; Bai, B.W.; Yu, P.F.; Qi, L.; Jing, Q.; Li, G.; Liu, R.P. Wf/Zr41.2Ti13.8Cu12.5Ni10Be22.5 bulk metallic glass composites prepared by a new melt infiltrating method. J. Alloys Compd. 2010, 504, S106–S109. [Google Scholar] [CrossRef]
- Duan, X.; Huang, Y.; Liu, W.; Cai, Q.; Liu, W.; Ma, Y. Effect of Ta on the microstructure and mechanical properties of WTa alloys prepared by arc melting. Mater. Charact. 2022, 188, 111823. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, S.; Zhu, J.; Kang, K.; Luo, G.; Wu, C.; Shen, Q.; Zhang, L. Microstructure and Compression Strength of W/HfC Composites Synthesized by Plasma Activated Sintering. Met. Mater. Int. 2018, 25, 416–424. [Google Scholar] [CrossRef]
- Liu, W.; Huang, Y.; Wang, Y.; Zhang, Y.; Duan, X.; Liu, W.; Ma, Y. Microstructure and mechanical properties of W-10 wt-%Ta alloys prepared by spark plasma sintering. Mater. Sci. Technol. 2022, 38, 159–168. [Google Scholar] [CrossRef]





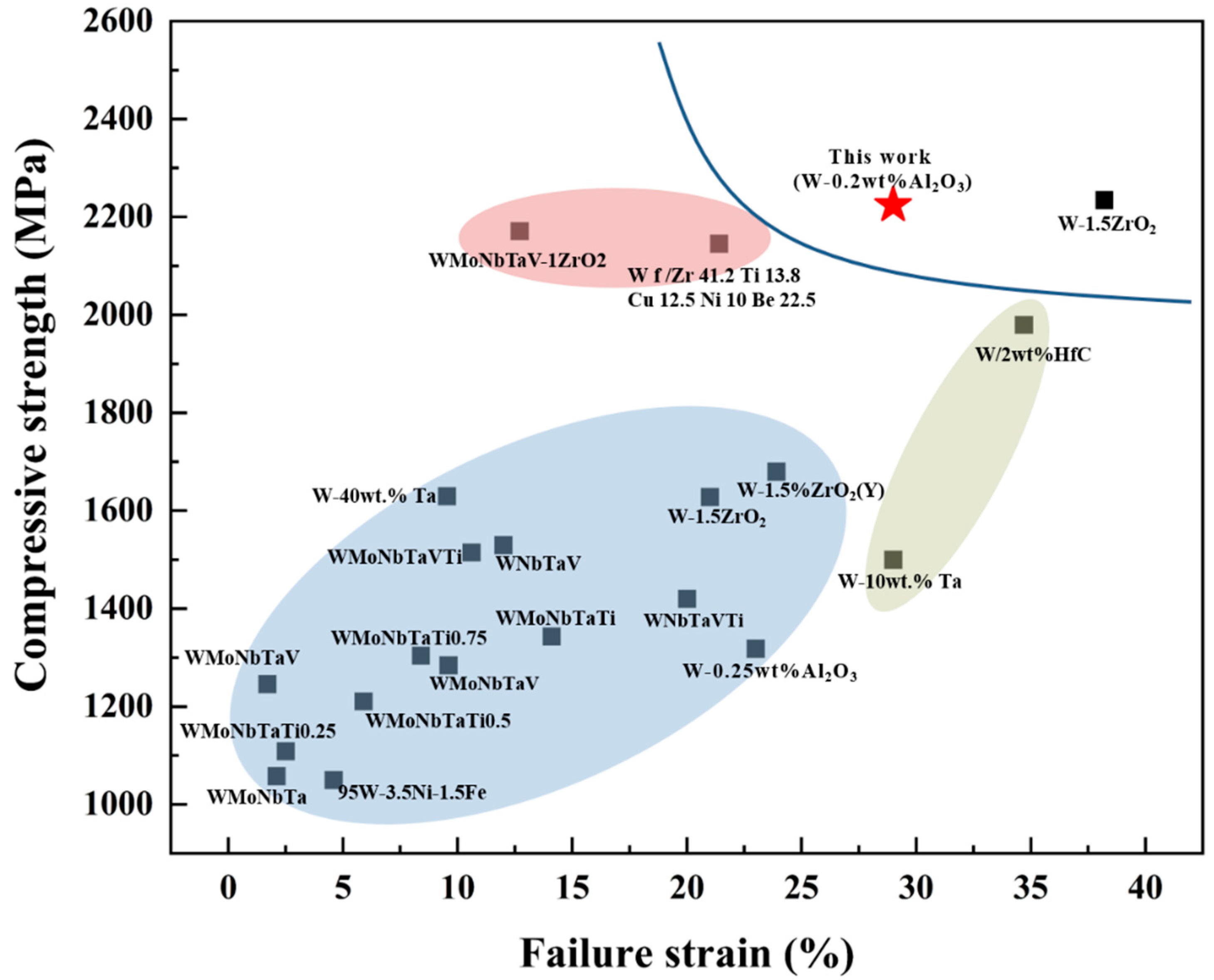
| Alloy | State | Compressive Strength | Elongation | References |
|---|---|---|---|---|
| 95W-3.5Ni-1.5Fe | Swaged | 1434 MPa | 4.6% | [25] |
| WMoNbTaV | Sintered | 1284.6 MPa | 9.6% | [26] |
| WMoNbTaV- 1ZrO2 | Sintered | 2171.1 MPa | 12.7% | [26] |
| WMoNbTaV | Sintered | 1246 MPa | 1.7% | [27] |
| WMoNbTa | Sintered | 1058 MPa | 2.1% | [27] |
| WMoNbTaTi0.25 | Sintered | 1109 MPa | 2.5% | [28] |
| WMoNbTaTi0.5 | Sintered | 1211 MPa | 5.9% | [28] |
| WMoNbTaTi0.75 | Sintered | 1304 MPa | 8.4% | [28] |
| WMoNbTaTi | Sintered | 1343 MPa | 14.1% | [29] |
| WMoNbTaVTi | Sintered | 1515 MPa | 10.6% | [29] |
| WNbTaV | Sintered | 1530 MPa | 12% | [30] |
| WNbTaVTi | Sintered | 1420 MPa | 20% | [30] |
| W-1.5 ZrO2 | Swaged | 2235 MPa | 38.2% | [31] |
| W-1.5 ZrO2 | Sintered | 1628 MPa | 21% | [32] |
| W-1.5% ZrO2(Y) | Sintered | 1680 MPa | 23.9 | [33] |
| Wf/Zr41.2Ti13.8Cu12.5Ni10Be22.5 | Sintered | 2146 MPa | 21.4 | [34] |
| W-40 wt.% Ta | Sintered | 1630 MPa | 9.54% | [35] |
| W/2 wt.% HfC | Sintered | 1980 MPa | 34.7% | [36] |
| W-10 wt.% Ta | Sintered | 1500 MPa | 29% | [37] |
| W-0.25 wt.% Al2O3 | Sintered | 1318 MPa | 23% | [15] |
| W-0.2 wt.% Al2O3 | Rolled | 2224 MPa | 29% | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Dong, X.; Liu, Y.; Wei, S.; Pan, K.; Zhang, C.; Xiong, M.; Mao, F.; Jiang, T.; Yu, H.; et al. Microstructure and Mechanical Properties of W-Al2O3 Alloy Plates Prepared by a Wet Chemical Method and Rolling Process. Materials 2022, 15, 7910. https://doi.org/10.3390/ma15227910
Wang C, Dong X, Liu Y, Wei S, Pan K, Zhang C, Xiong M, Mao F, Jiang T, Yu H, et al. Microstructure and Mechanical Properties of W-Al2O3 Alloy Plates Prepared by a Wet Chemical Method and Rolling Process. Materials. 2022; 15(22):7910. https://doi.org/10.3390/ma15227910
Chicago/Turabian StyleWang, Changji, Xiaonan Dong, Yao Liu, Shizhong Wei, Kunming Pan, Cheng Zhang, Mei Xiong, Feng Mao, Tao Jiang, Hua Yu, and et al. 2022. "Microstructure and Mechanical Properties of W-Al2O3 Alloy Plates Prepared by a Wet Chemical Method and Rolling Process" Materials 15, no. 22: 7910. https://doi.org/10.3390/ma15227910








