Effect of Pretreatment with Acids on the N-Functionalization of Carbon Nanofibers Using Melamine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Synthesis of the Catalyst
2.3. Synthesis, Pretreatment and N-Functionalization of CNFs
2.4. Characterization of the Samples
3. Results and Discussion
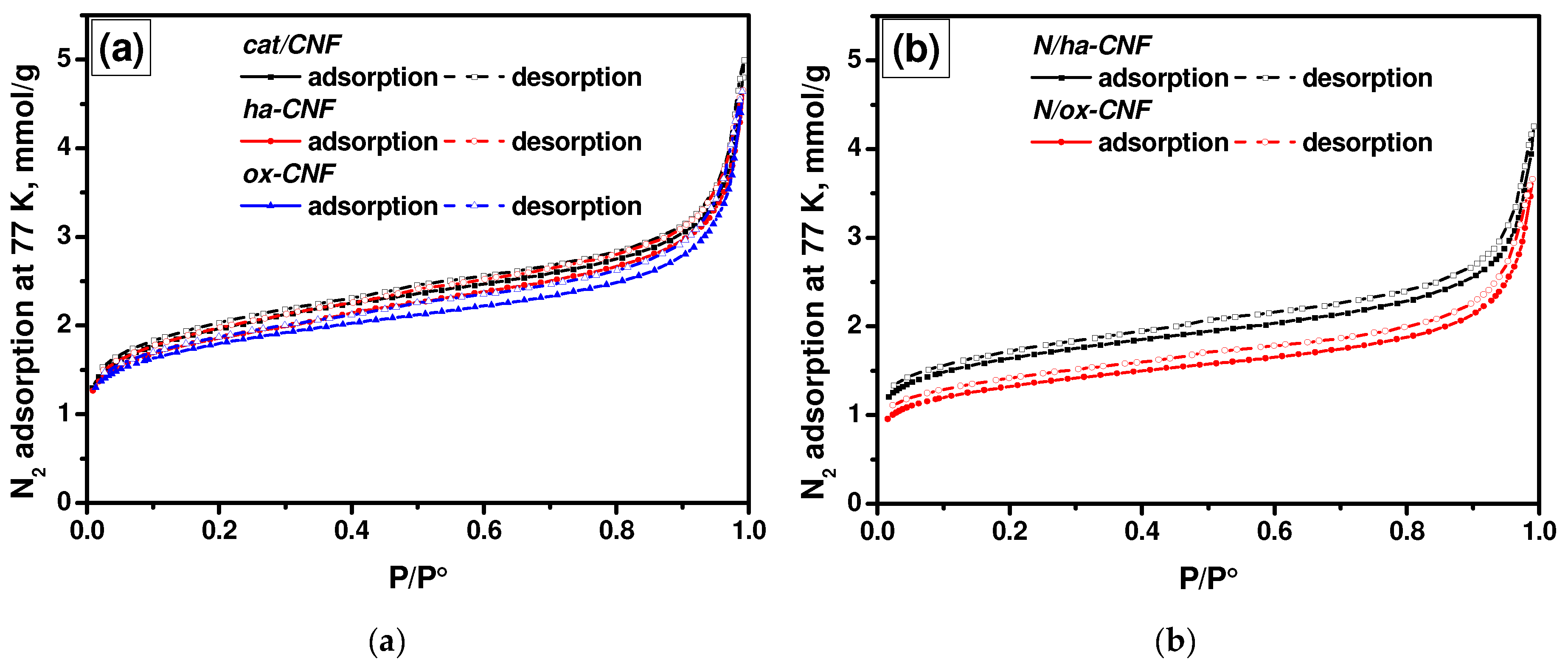
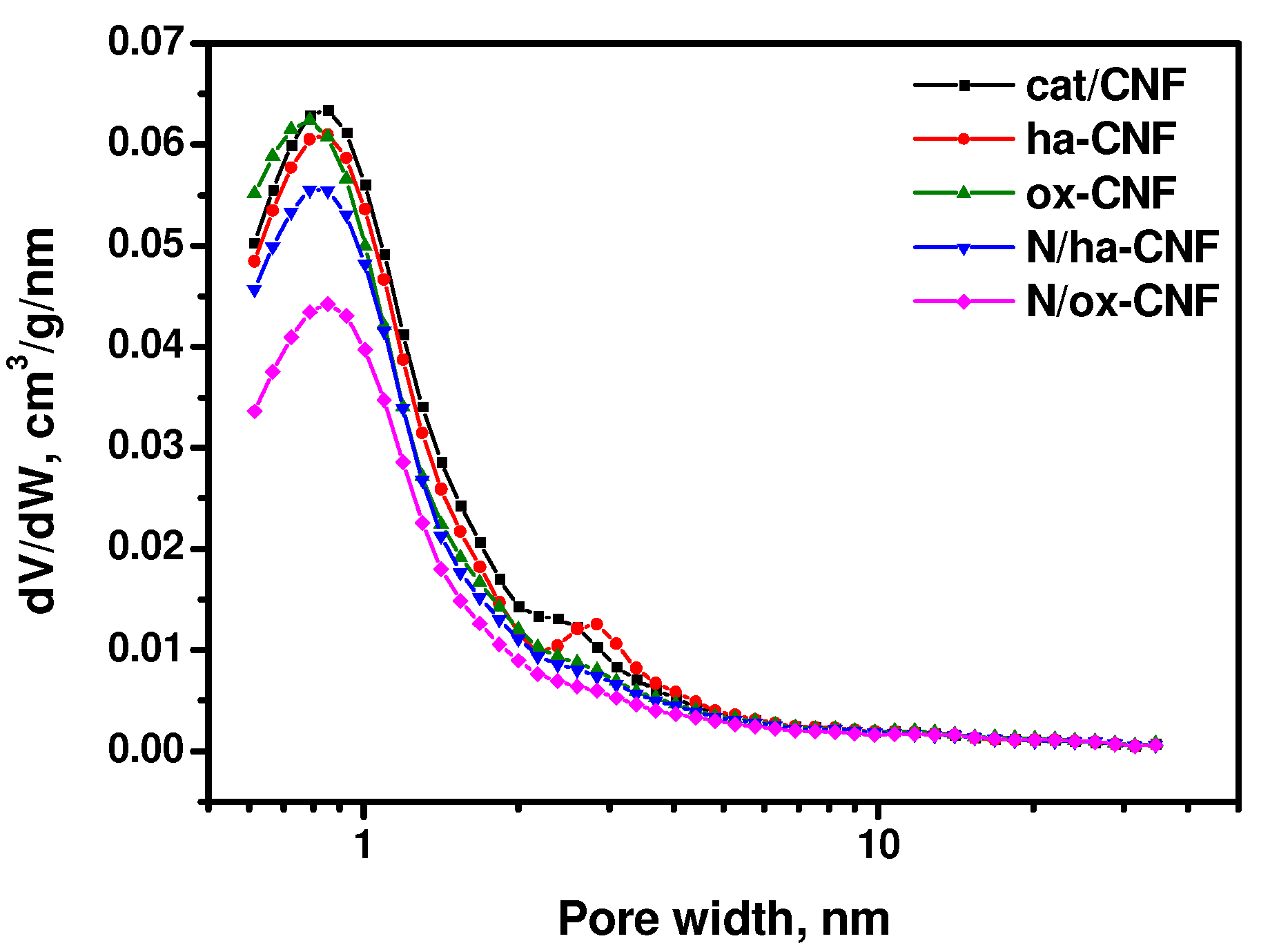
3.1. Textural Properties of CNFs
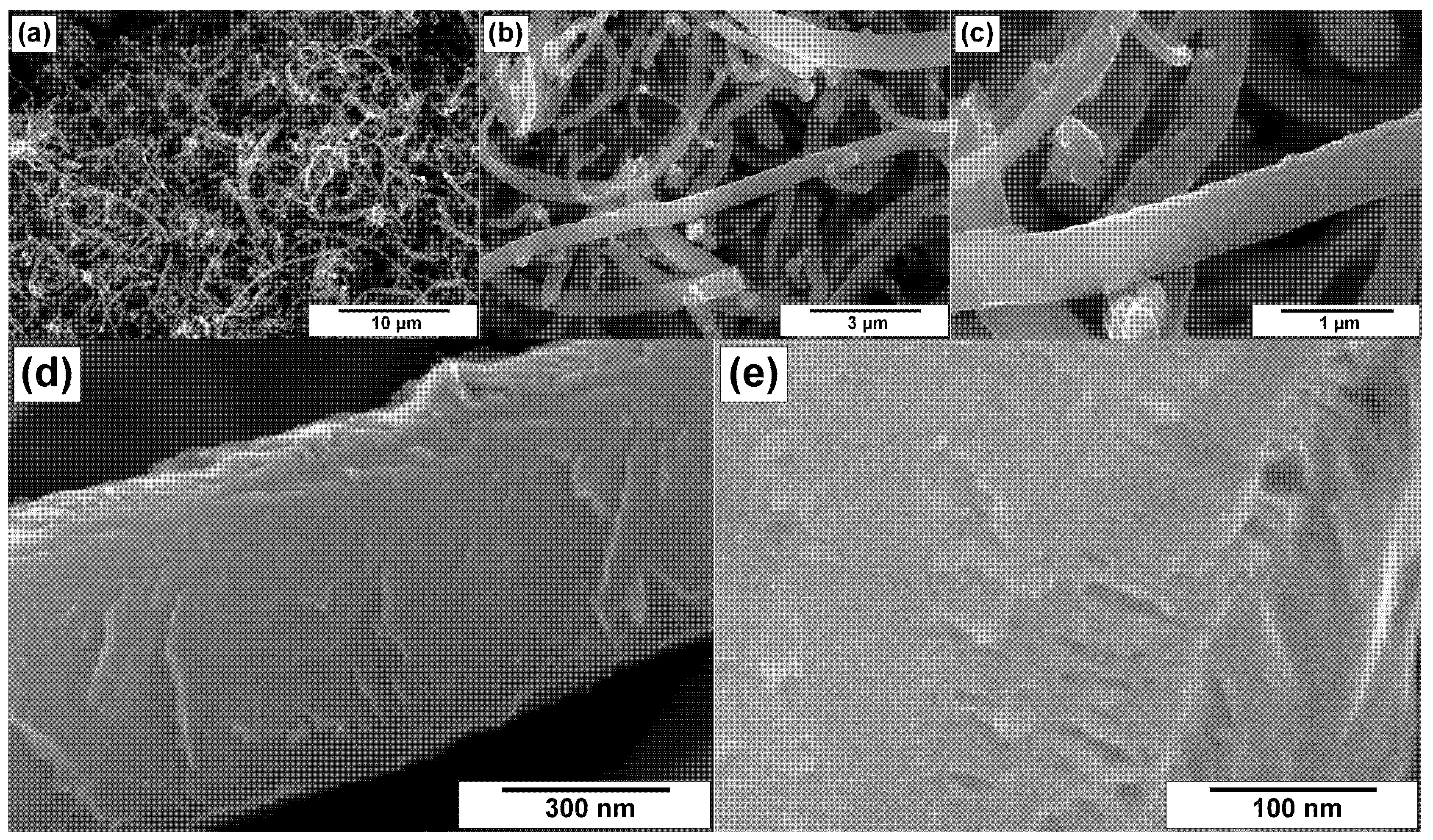
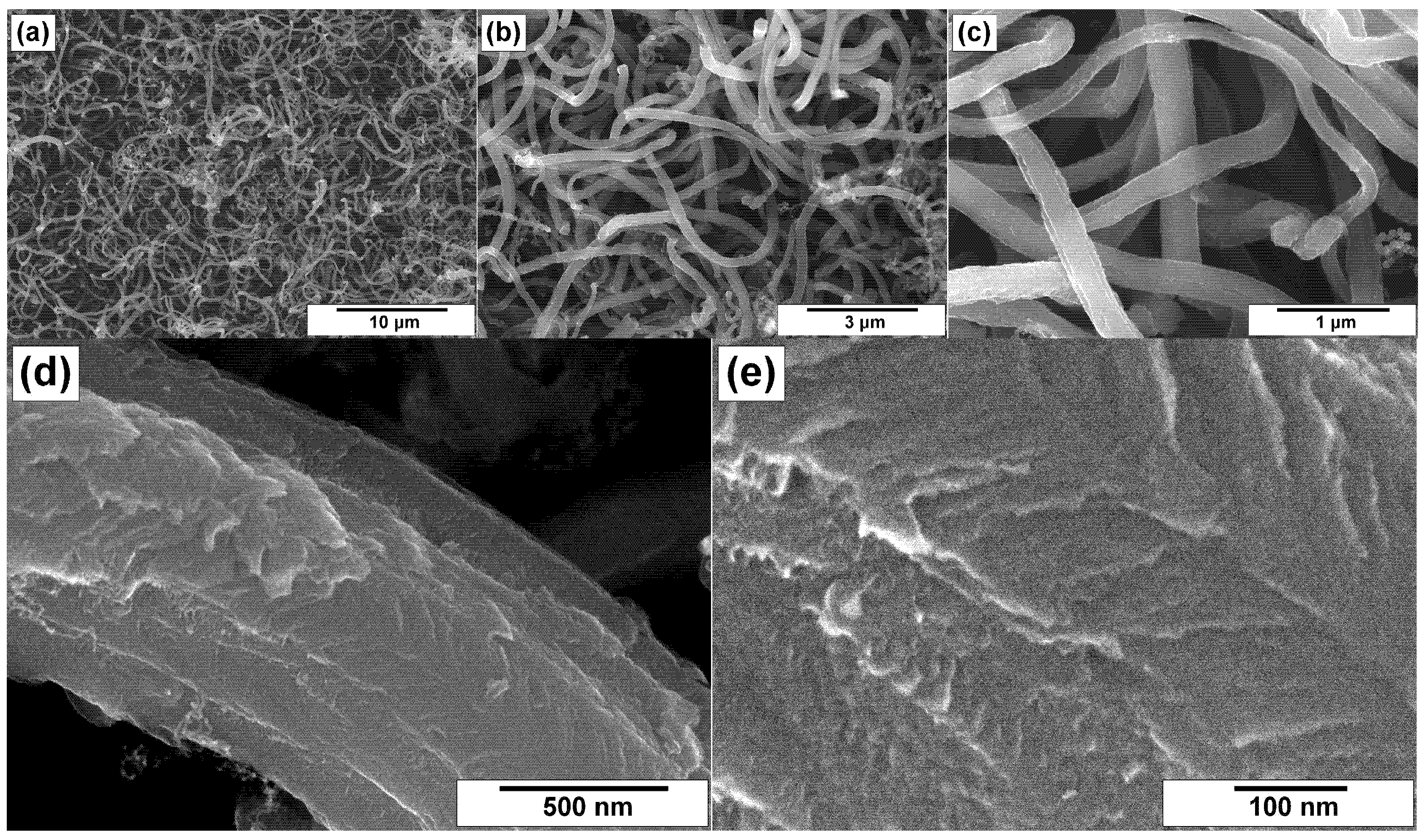
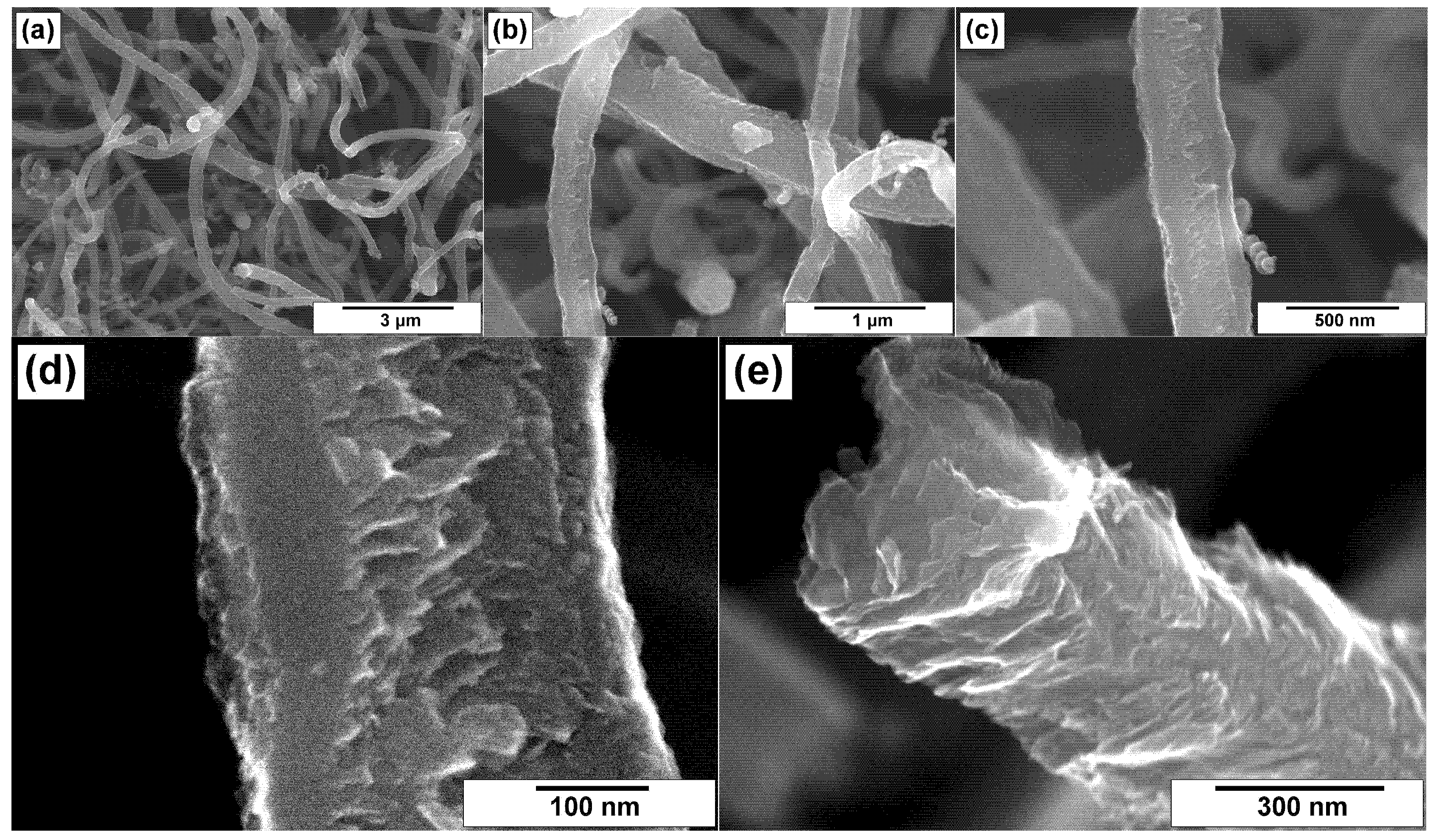
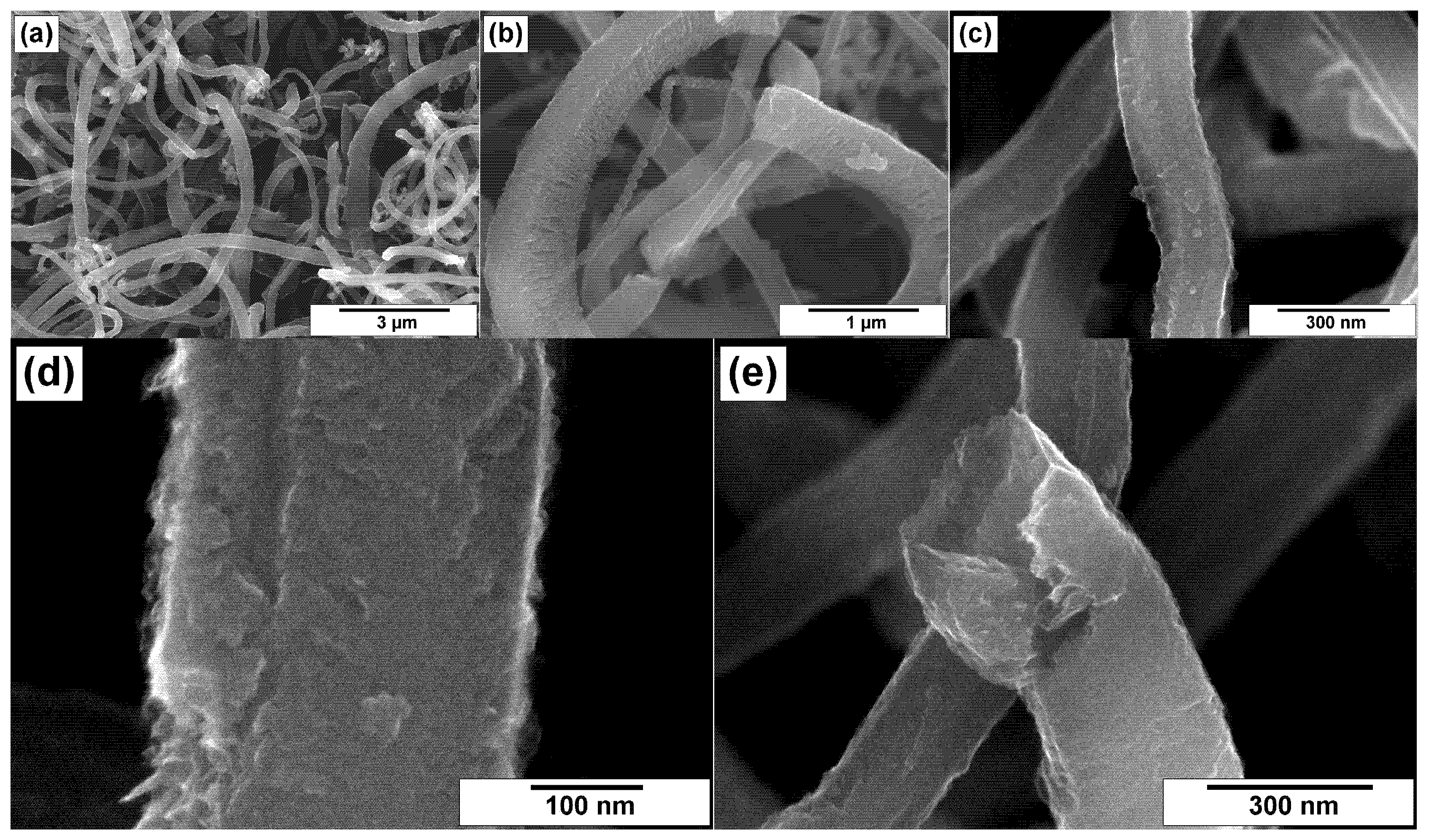
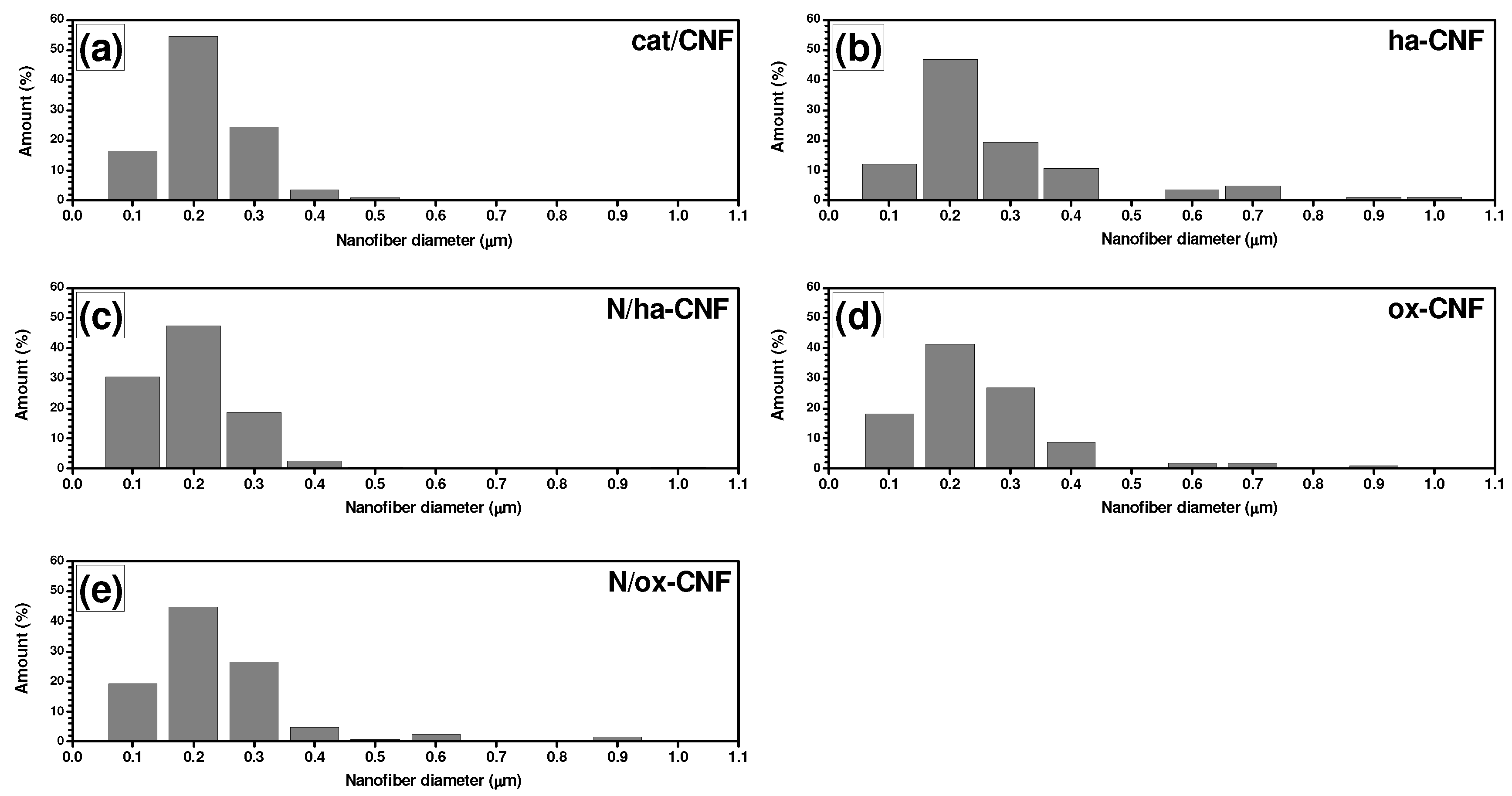
3.2. SEM Characterization of CNFs
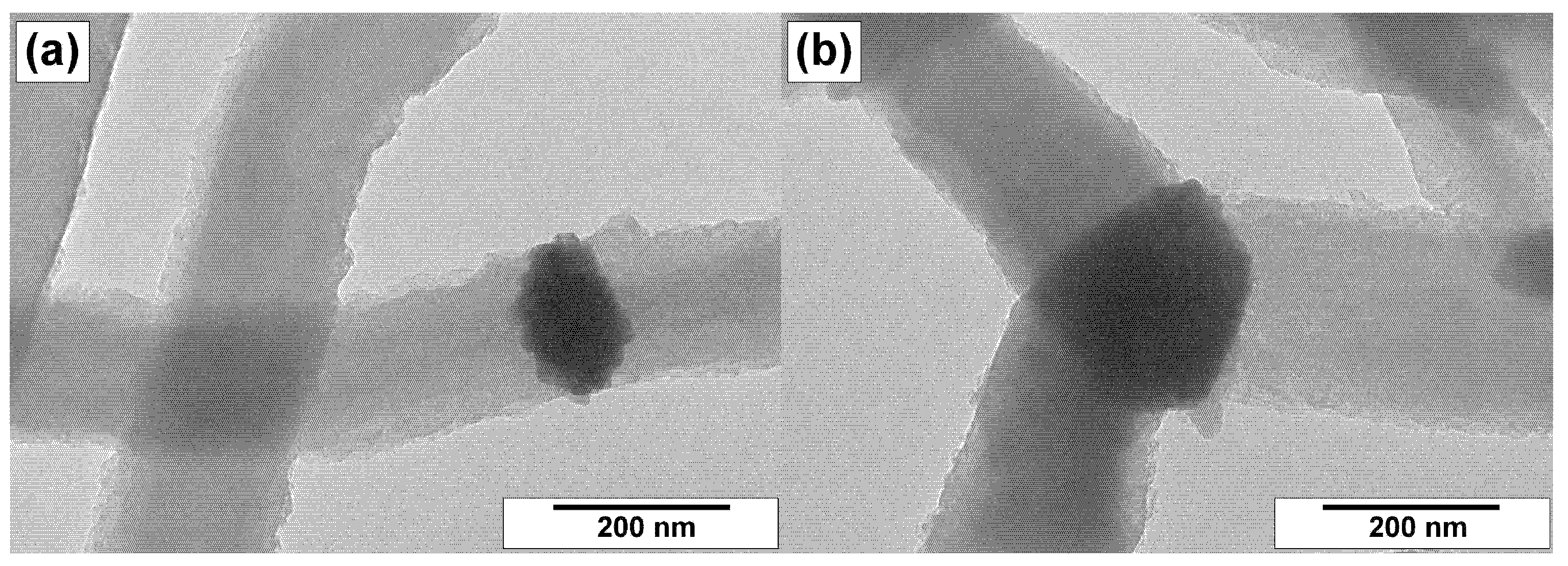
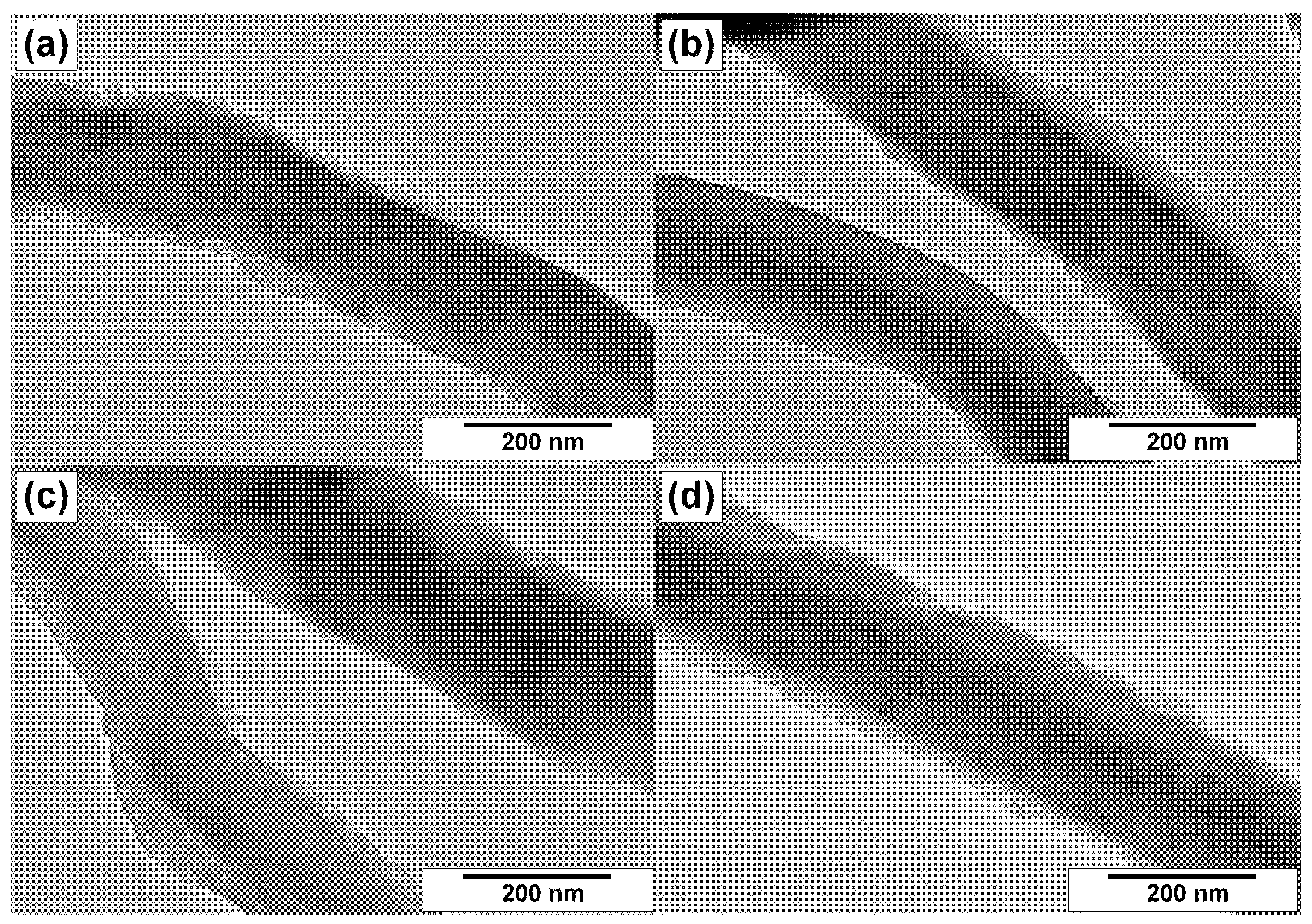
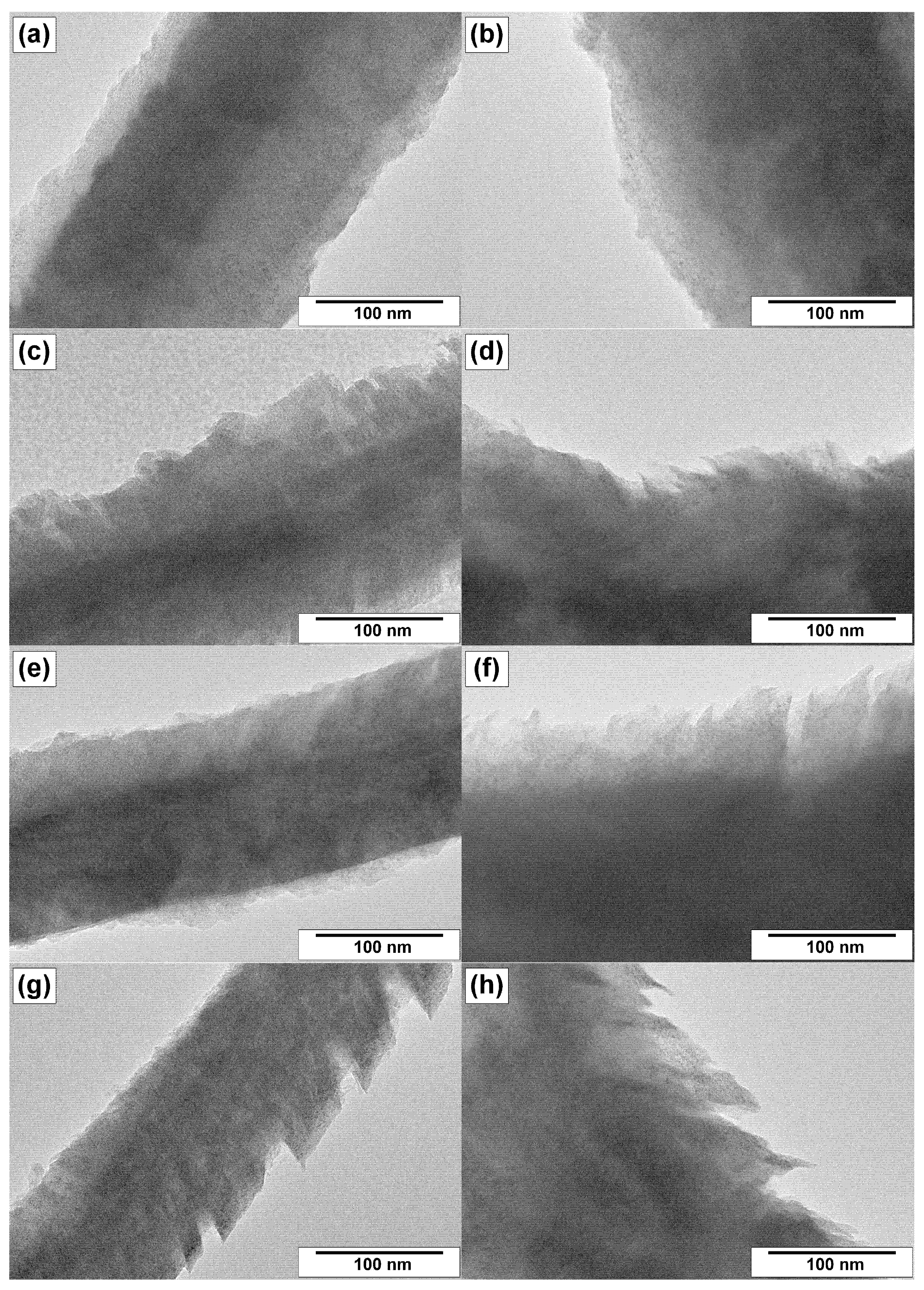
3.3. TEM Characterization of CNFs
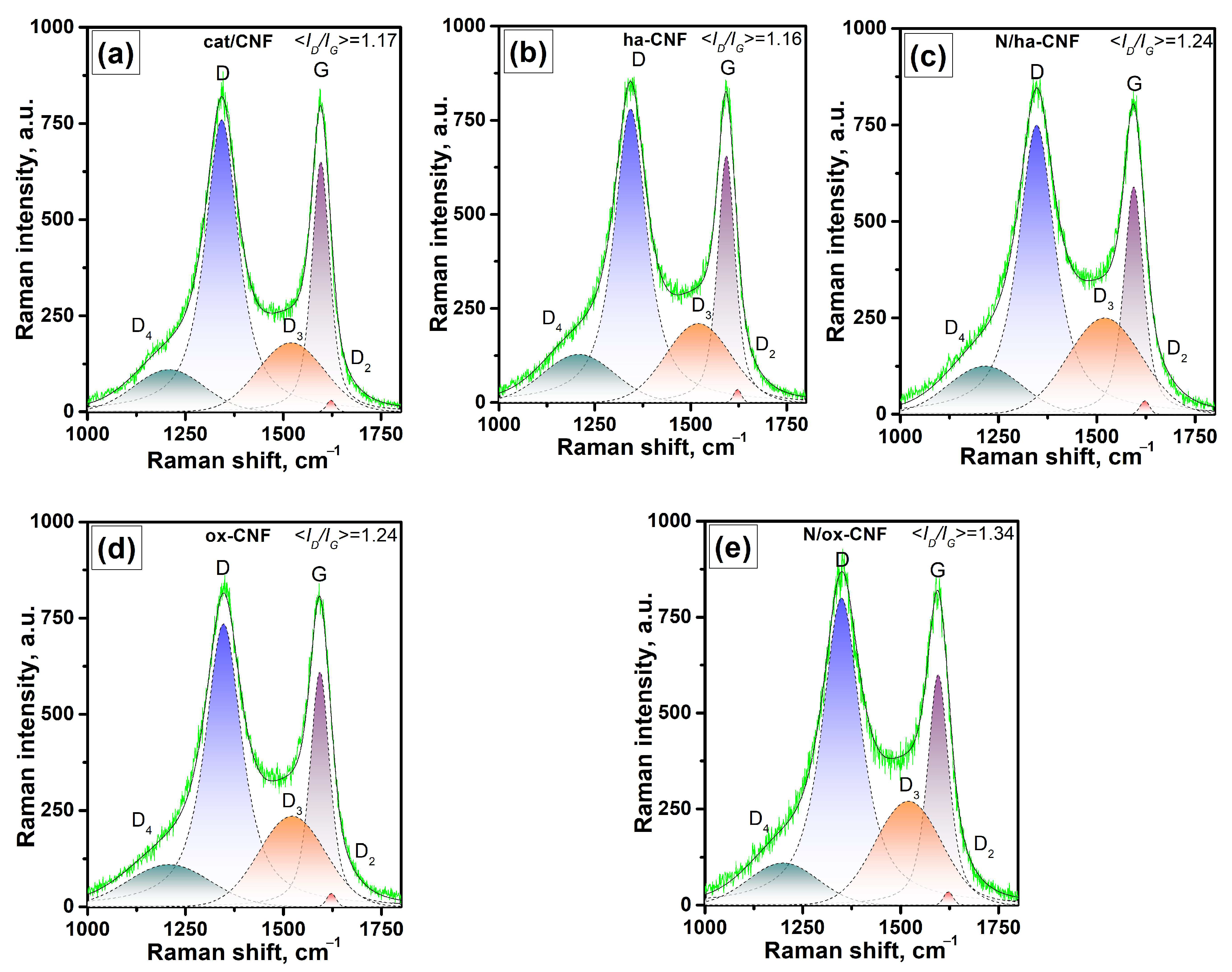
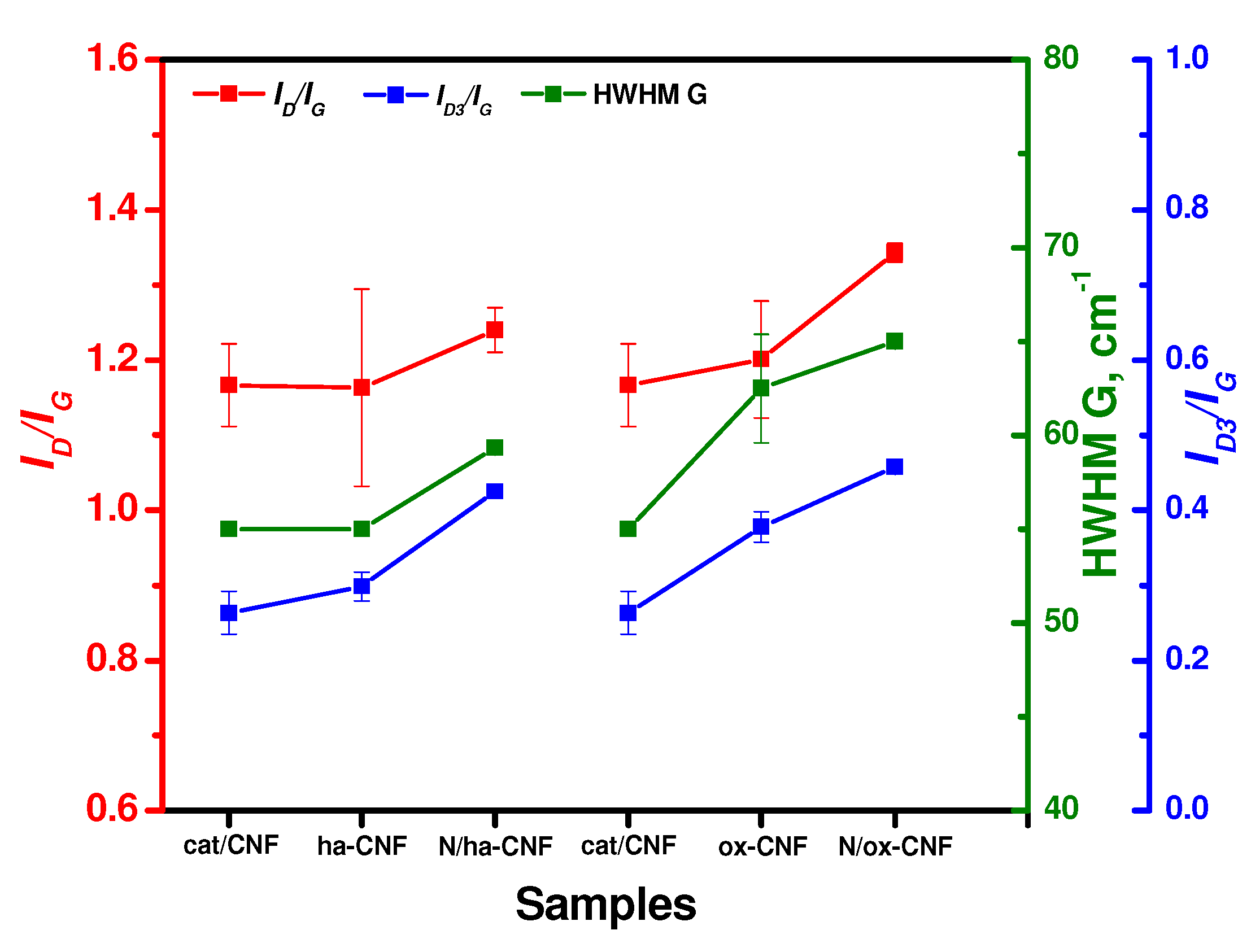
3.4. Raman Spectroscopy Data
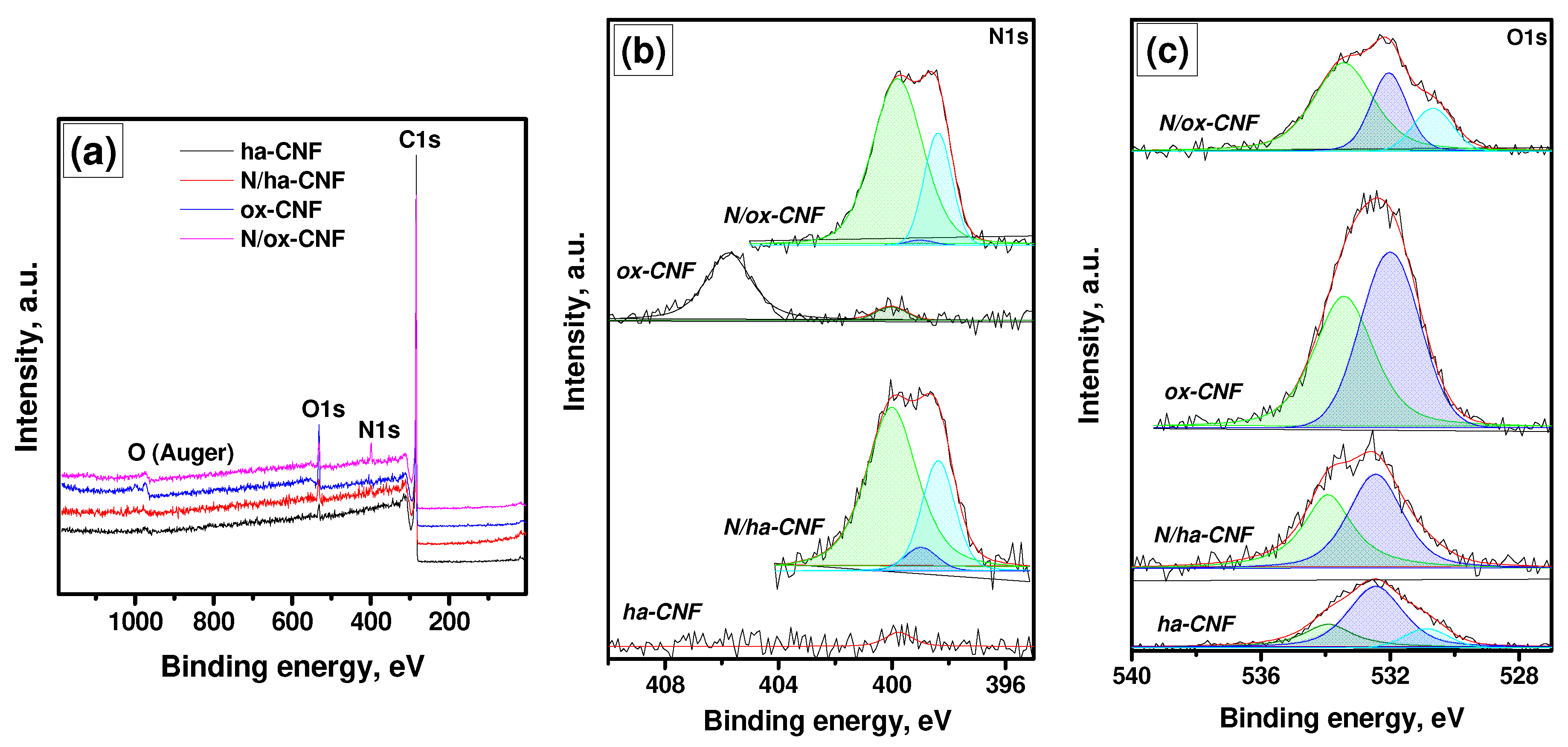
3.5. XPS Study of CNFs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, W.J.; Maiti, U.N.; Lee, J.M.; Lim, J.; Han, T.H.; Kim, S.O. Nitrogen-doped carbon nanotubes and graphene composite structures for energy and catalytic applications. Chem. Commun. 2014, 50, 6818–6830. [Google Scholar] [CrossRef] [PubMed]
- Podyacheva, O.Y.; Ismagilov, Z.R. Nitrogen-doped carbon nanomaterials: To the mechanism of growth, electrical conductivity and application in catalysis. Catal. Today 2015, 249, 12–22. [Google Scholar] [CrossRef]
- Inagaki, M.; Toyoda, M.; Soneda, Y.; Morishita, T. Nitrogen-doped carbon materials. Carbon 2018, 132, 104–140. [Google Scholar] [CrossRef]
- Hulicova-Jurcakova, D.; Seredych, M.; Lu, G.Q.; Bandosz, T.J. Combined effect of nitrogen- and oxygen-containing functional groups of microporous activated carbon on its electrochemical performance in supercapacitors. Adv. Func. Mater. 2009, 19, 438–447. [Google Scholar] [CrossRef]
- Nasini, U.B.; Bairi, V.G.; Ramasahayam, S.K.; Bourdo, S.E.; Viswanathan, T.; Shaikh, A.U. Phosphorous and nitrogen dual heteroatom doped mesoporous carbon synthesized via microwave method for supercapacitor application. J. Power Sources 2014, 250, 257–265. [Google Scholar] [CrossRef]
- Gao, F.; Shao, G.; Qu, J.; Lv, S.; Li, Y.; Wu, M. Tailoring of porous and nitrogen-rich carbons derived from hydrochar for high-performance supercapacitor electrodes. Electrochim. Acta 2015, 155, 201–208. [Google Scholar] [CrossRef]
- Liu, C.; Wang, J.; Li, J.; Zeng, M.; Luo, R.; Shen, J.; Sun, X.; Han, W.; Wang, L. Synthesis of N-doped hollow-structured mesoporous carbon nanospheres for high-performance supercapacitors. ACS Appl. Mater. Interf. 2016, 8, 7194–7204. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, F.; Lee, S.; Li, X.; Zhao, H.; Wang, Y.; Hao, S.; Zhang, X. Facile fabrication of MnOx and N co-doped hierarchically porous carbon microspheres for high-performance supercapacitors. Electrochim. Acta 2016, 191, 1018–1025. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Lim, H.; Jyoung, J.-Y.; Lee, E.-S.; Yi, J.S.; Lee, D. Effects of doping methods and kinetic relevance of N and O atomic co-functionalization on carbon electrode for V(IV)/V(V) redox reactions in vanadium redox flow battery. Electrochim. Acta 2017, 245, 724–733. [Google Scholar] [CrossRef]
- Ma, C.; Chen, X.; Long, D.; Wang, J.; Qiao, W.; Ling, L. High-surface-area and high-nitrogen-content carbon microspheres prepared by a pre-oxidation and mild KOH activation for superior supercapacitor. Carbon 2017, 118, 699–708. [Google Scholar] [CrossRef]
- Chen, Y.; Xiao, Z.; Liu, Y.; Fan, L.-Z. A simple strategy toward hierarchically porous graphene/nitrogen-rich carbon foams for high-performance supercapacitors. J. Mater. Chem. A 2017, 5, 24178–24184. [Google Scholar] [CrossRef]
- Sutarsis; Patra, J.; Su, C.-Y.; Li, J.; Bresser, D.; Passerini, S.; Chang, J.-K. Manipulation of nitrogen-heteroatom configuration for enhanced charge-storage performance and reliability of nanoporous carbon electrodes. ACS Appl. Mater. Interf. 2020, 12, 32797–32805. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Lu, Y.; Wang, Q.; Xu, F.; Sun, L.; Wang, Y.; Lao, J.; Liao, L.; Zhang, K.; Zhang, H.; et al. Porous carbon with facial tuning of the heteroatom N by polyvinylidene chloride dehalogenation toward enhanced supercapacitor performance. J. Electrochem. Energy Conv. Stor. 2023, 20, 011004. [Google Scholar] [CrossRef]
- Li, Z.; Liu, J.; Xia, C.; Li, F. Nitrogen-functionalized ordered mesoporous carbons as multifunctional supports of ultrasmall Pd nanoparticles for hydrogenation of phenol. ACS Catal. 2013, 3, 2440–2448. [Google Scholar] [CrossRef]
- Chen, S.; Qi, P.; Chen, J.; Yuan, Y. Platinum nanoparticles supported on N-doped carbon nanotubes for the selective oxidation of glycerol to glyceric acid in a base-free aqueous solution. RSC Adv. 2015, 5, 31566–31574. [Google Scholar] [CrossRef] [Green Version]
- Soares, O.S.G.P.; Rocha, R.P.; Gonçalves, A.G.; Figueiredo, J.L.; Órfão, J.J.M.; Pereira, M.F.R. Highly active N-doped carbon nanotubes prepared by an easy ball milling method for advanced oxidation processes. Appl. Catal. B Environ. 2016, 192, 296–303. [Google Scholar] [CrossRef]
- Ferrero, G.A.; Fuertes, A.B.; Sevilla, M.; Titirici, M.-M. Efficient metal-free N-doped mesoporous carbon catalysts for ORR by a template-free approach. Carbon 2016, 106, 179–187. [Google Scholar] [CrossRef] [Green Version]
- Liang, P.; Zhang, C.; Duan, X.; Sun, H.; Liu, S.; Tade, M.O.; Wang, S. N-doped graphene from metal–organic frameworks for catalytic oxidation of p-hydroxylbenzoic acid: N-functionality and mechanism. ACS Sustain. Chem. Eng. 2017, 5, 2693–2701. [Google Scholar] [CrossRef]
- Mian, M.M.; Liu, G.; Zhou, H. Preparation of N-doped biochar from sewage sludge and melamine for peroxymonosulfate activation: N-functionality and catalytic mechanisms. Sci. Total Environ. 2020, 744, 140862. [Google Scholar] [CrossRef]
- Anfar, Z.; El Fakir, A.A.; Zbair, M.; Hafidi, Z.; Amedlous, A.; Majdoub, M.; Farsad, S.; Amjlef, A.; Jada, A.; El Alem, N. New functionalization approach synthesis of Sulfur doped, Nitrogen doped and Co-doped porous carbon: Superior metal-free Carbocatalyst for the catalytic oxidation of aqueous organics pollutants. Chem. Eng. J. 2021, 405, 126660. [Google Scholar] [CrossRef]
- Lee, B.; Tian, S.; Xiong, G.; Yang, Y.; Zhu, X. Solvothermal synthesis of transition metal (iron/copper) and nitrogen co−doped carbon nanomaterials: Comparing their peroxidase—Like properties. J. Nanopart. Res. 2022, 24, 85. [Google Scholar] [CrossRef]
- Prabakaran, E.; Pillay, K. Synthesis and characterization of fluorescent N-CDs/ZnONPs nanocomposite for latent fingerprint detection by using powder brushing method. Arabian J. Chem. 2020, 13, 3817–3835. [Google Scholar] [CrossRef]
- Pérez-Cadenas, M.; Moreno-Castilla, C.; Carrasco-Marín, F.; Pérez-Cadenas, A.F. Surface chemistry, porous texture, and morphology of N-doped carbon xerogels. Langmuir 2009, 25, 466–470. [Google Scholar] [CrossRef] [PubMed]
- Maboya, W.K.; Coville, N.J.; Mhlanga, S.D. Fabrication of chlorine nitrogen co-doped carbon nanomaterials by an injection catalytic vapor deposition method. Mater. Res. Express 2021, 8, 015007. [Google Scholar] [CrossRef]
- Kenzhin, R.M.; Bauman, Y.I.; Volodin, A.M.; Mishakov, I.V.; Vedyagin, A.A. One-step synthesis of nitrogen-doped carbon nanofibers from melamine over nickel alloy in a closed system. Chem. Phys. Lett. 2017, 685, 259–262. [Google Scholar] [CrossRef]
- Kenzhin, R.M.; Bauman, Y.I.; Volodin, A.M.; Mishakov, I.V.; Vedyagin, A.A. Interaction of heteroatom-containing organic compounds with bulk nickel alloy in a closed reactor system. Juniper Online J. Mater. Sci. 2018, 4, 555633. [Google Scholar] [CrossRef]
- Potylitsyna, A.R.; Mishakov, I.V.; Bauman, Y.I.; Kibis, L.S.; Shubin, Y.V.; Volochaev, M.N.; Melgunov, M.S.; Vedyagin, A.A. Metal dusting as a key route to produce functionalized carbon nanofibers. Reac. Kinet. Mech. Catal. 2022, 135, 1387–1404. [Google Scholar] [CrossRef]
- Brzhezinskaya, M.; Mishakov, I.V.; Bauman, Y.I.; Shubin, Y.V.; Maksimova, T.A.; Stoyanovskii, V.O.; Gerasimov, E.Y.; Vedyagin, A.A. One-pot functionalization of catalytically derived carbon nanostructures with heteroatoms for toxic-free environment. Appl. Surf. Sci. 2022, 590, 153055. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, J.; Qin, Y.-H.; Niu, D.-F.; Zhang, X.-S.; Niu, L.; Zhou, X.-G.; Lu, T.-H.; Yuan, W.-K. Ultrasonic synthesis of nitrogen-doped carbon nanofibers as platinum catalyst support for oxygen reduction. J. Power Sources 2011, 196, 9356–9360. [Google Scholar] [CrossRef]
- Yin, J.; Qiu, Y.; Yu, J.; Zhou, X.; Wu, W. Enhancement of electrocatalytic activity for oxygen reduction reaction in alkaline and acid media from electrospun nitrogen-doped carbon nanofibers by surface modification. RSC Adv. 2013, 3, 15655–15663. [Google Scholar] [CrossRef]
- Sevilla, M.; Yu, L.; Zhao, L.; Ania, C.O.; Titiricic, M.-M. Surface modification of CNTs with N-doped carbon: An effective way of enhancing their performance in supercapacitors. ACS Sustain. Chem. Eng. 2014, 2, 1049–1055. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Zhang, X.; Zhou, Y.; Guo, S.; Wang, K.; Liang, Z.; Xu, Q. Nitrogen-doped hierarchical porous carbon nanowhisker ensembles on carbon nanofiber for high-performance supercapacitors. ACS Sustain. Chem. Eng. 2014, 2, 1525–1533. [Google Scholar] [CrossRef]
- Kerdi, F.; Ait Rass, H.; Pinel, C.; Besson, M.; Peru, G.; Leger, B.; Rio, S.; Monflier, E.; Ponchel, A. Evaluation of surface properties and pore structure of carbon on the activity of supported Ru catalysts in the aqueous-phase aerobic oxidation of HMF to FDCA. Appl. Catal. A Gen. 2015, 506, 206–219. [Google Scholar] [CrossRef]
- Wang, T.; Chen, Z.-X.; Chen, Y.-G.; Yang, L.-J.; Yang, X.-D.; Ye, J.-Y.; Xia, H.-P.; Zhou, Z.-Y.; Sun, S.-G. Identifying the active site of N-doped graphene for oxygen reduction by selective chemical modification. ACS Energy Lett. 2018, 3, 986–991. [Google Scholar] [CrossRef]
- Butsyk, O.; Olejnik, P.; Romero, E.; Plonska-Brzezinska, M.E. Postsynthetic treatment of carbon nano-onions: Surface modification by heteroatoms to enhance their capacitive and electrocatalytic properties. Carbon 2019, 147, 90–104. [Google Scholar] [CrossRef]
- Ruiz-Garcia, C.; Heras, F.; Calvo, L.; Alonso-Morales, N.; Rodriguez, J.J.; Gilarranz, M.A. Improving the activity in hydrodechlorination of Pd/C catalysts by nitrogen doping of activated carbon supports. J. Environ. Chem. Eng. 2020, 8, 103689. [Google Scholar] [CrossRef]
- Golub, F.S.; Beloshapkin, S.; Gusel’nikov, A.V.; Bolotov, V.A.; Parmon, V.N.; Bulushev, D.A. Boosting hydrogen production from formic acid over Pd catalysts by deposition of N-containing precursors on the carbon support. Energies 2019, 12, 3885. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Bian, F.; Wu, X.; Sun, L.; Yang, H.; Meng, X.; Qin, G. Microstructure evolution and its correlation with performance in nitrogen-containing porous carbon prepared by polypyrrole carbonization: Insights from hybrid calculations. Materials 2022, 15, 3705. [Google Scholar] [CrossRef]
- Mishakov, I.V.; Vedyagin, A.A.; Bauman, Y.I.; Shubin, Y.V.; Buyanov, R.A. Synthesis of carbon nanofibers via catalytic chemical vapor deposition of halogenated hydrocarbons. In Carbon Nanofibers: Synthesis, Applications and Performance; Chang-Seop, L., Ed.; Nova Science Publishers: Hauppauge, NY, USA, 2018; pp. 77–181. [Google Scholar]
- Mishakov, I.V.; Afonnikova, S.D.; Bauman, Y.I.; Shubin, Y.V.; Trenikhin, M.V.; Serkova, A.N.; Vedyagin, A.A. Carbon erosion of a bulk nickel–copper alloy as an effective tool to synthesize carbon nanofibers from hydrocarbons. Kinet. Catal. 2022, 63, 97–107. [Google Scholar] [CrossRef]
- Atwater, M.A.; Guevara, L.N.; Knauss, S.J. Multifunctional porous catalyst produced by mechanical alloying. Mater. Res. Lett. 2019, 7, 131–136. [Google Scholar] [CrossRef]
- Mishakov, I.V.; Bauman, Y.I.; Korneev, D.V.; Vedyagin, A.A. Metal dusting as a route to produce active catalyst for processing chlorinated hydrocarbons into carbon nanomaterials. Top. Catal. 2013, 56, 1026–1032. [Google Scholar] [CrossRef]
- Zhu, Q.-L.; Xu, Q. Immobilization of ultrafine metal nanoparticles to high-surface-area materials and their catalytic applications. Chem 2016, 1, 220–245. [Google Scholar] [CrossRef]
- Saadun, A.J.; Ruiz–Ferrando, A.; Büchele, S.; Faust Akl, D.; López, N.; Pérez–Ramírez, J. Structure sensitivity of nitrogen–doped carbon–supported metal catalysts in dihalomethane hydrodehalogenation. J. Catal. 2021, 404, 291–305. [Google Scholar] [CrossRef]
- Liu, S.; Otero, J.A.; Martin-Martinez, M.; Rodriguez-Franco, D.; Rodriguez, J.J.; Gómez-Sainero, L.M. Understanding hydrodechlorination of chloromethanes. Past and future of the technology. Catalysts 2020, 10, 1462. [Google Scholar] [CrossRef]
- Chesnokov, V.V.; Kriventsov, V.V.; Malykhin, S.E.; Svintsitskiy, D.A.; Podyacheva, O.Y.; Lisitsyn, A.S.; Richards, R.M. Nature of active palladium sites on nitrogen doped carbon nanofibers in selective hydrogenation of acetylene. Diam. Relat. Mater. 2018, 89, 67–73. [Google Scholar] [CrossRef]
- Hu, F.; Leng, L.; Zhang, M.; Chen, W.; Yu, Y.; Wang, J.; Horton, J.H.; Li, Z. Direct synthesis of atomically dispersed palladium atoms supported on graphitic carbon nitride for efficient selective hydrogenation reactions. ACS Appl. Mater. Interf. 2020, 12, 54146–54154. [Google Scholar] [CrossRef]
- Glyzdova, D.V.; Vedyagin, A.A.; Tsapina, A.M.; Kaichev, V.V.; Trigub, A.L.; Trenikhin, M.V.; Shlyapin, D.A.; Tsyrulnikov, P.G.; Lavrenov, A.V. A study on structural features of bimetallic Pd-M/C (M: Zn, Ga, Ag) catalysts for liquid-phase selective hydrogenation of acetylene. Appl. Catal. A-Gen. 2018, 563, 18–27. [Google Scholar] [CrossRef]
- Bulushev, D.A.; Bulusheva, L.G. Catalysts with single metal atoms for the hydrogen production from formic acid. Catal. Rev. 2022, 64, 835–874. [Google Scholar] [CrossRef]
- Suboch, A.N.; Podyacheva, O.Y. Pd Catalysts supported on bamboo-like nitrogen-doped carbon nanotubes for hydrogen production. Energies 2021, 14, 1501. [Google Scholar] [CrossRef]
- Kwak, Y.; Kirk, J.; Moon, S.; Ohm, T.; Lee, Y.-J.; Jang, M.; Park, L.-H.; Ahn, C.-i.; Jeong, H.; Sohn, H.; et al. Hydrogen production from homocyclic liquid organic hydrogen carriers (LOHCs): Benchmarking studies and energy-economic analyses. Energ. Conv. Manag. 2021, 239, 114124. [Google Scholar] [CrossRef]
- Abdin, Z.; Tang, C.; Liu, Y.; Catchpole, K. Large-scale stationary hydrogen storage via liquid organic hydrogen carriers. iScience 2021, 24, 102966. [Google Scholar] [CrossRef] [PubMed]
- Niermann, M.; Drünert, S.; Kaltschmitt, M.; Bonhoff, K. Liquid organic hydrogen carriers (LOHCs)—Techno-economic analysis of LOHCs in a defined process chain. Energy Environ. Sci. 2019, 12, 290–307. [Google Scholar] [CrossRef]
- Gómez, S.; Rendtorff, N.M.; Aglietti, E.F.; Sakka, Y.; Suárez, G. Surface modification of multiwall carbon nanotubes by sulfonitric treatment. Appl. Surf. Sci. 2016, 379, 264–269. [Google Scholar] [CrossRef]
- Turan, K.; Kaur, P.; Manhas, D.; Sharma, J.; Verma, G. Novel insights into the dispersed and acid-mediated surface modification of the carbon nanofibers. Mater. Chem. Phys. 2020, 239, 121978. [Google Scholar] [CrossRef]
- Ud Din, I.; Shaharun, M.S.; Subbarao, D.; Naeem, A. Surface modification of carbon nanofibers by HNO3 treatment. Ceram. Int. 2016, 42, 966–970. [Google Scholar] [CrossRef]
- Afonnikova, S.D.; Mishakov, I.V.; Bauman, Y.I.; Trenikhin, M.V.; Shubin, Y.V.; Serkova, A.N.; Vedyagin, A.A. Preparation of Ni-Cu Catalyst for carbon nanofiber production by the mechanochemical route. Top. Catal. 2022. [Google Scholar] [CrossRef]
- Mel’gunov, M.S.; Ayupov, A.B. Direct method for evaluation of BET adsorbed monolayer capacity. Micropor. Mesopor. Mat. 2017, 243, 147–153. [Google Scholar] [CrossRef]
- Sing, K. The use of nitrogen adsorption for the characterisation of porous materials. Colloids Surf. A 2001, 187–188, 3–9. [Google Scholar] [CrossRef]
- Gor, G.Y.; Thommes, M.; Cychosz, K.A.; Neimark, A.V. Quenched solid density functional theory method for characterization of mesoporous carbons by nitrogen adsorption. Carbon 2012, 50, 1583–1590. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stickle, W.F.; Sobol, W.M.; Bomben, K.D. (Eds.) Handbook of X-Ray Photoelectron Spectroscopy; Perkin-Elmer Corporation Physical Electronics Division: Eden Prairie, MN, USA, 1992; p. 261. [Google Scholar]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Shubin, Y.V.; Bauman, Y.I.; Plyusnin, P.E.; Mishakov, I.V.; Tarasenko, M.S.; Mel’gunov, M.S.; Stoyanovskii, V.O.; Vedyagin, A.A. Facile synthesis of triple Ni-Mo-W alloys and their catalytic properties in chemical vapor deposition of chlorinated hydrocarbons. J. Alloys Compnd. 2021, 866, 158778. [Google Scholar] [CrossRef]
- Mishakov, I.V.; Buyanov, R.A.; Zaikovskii, V.I.; Strel’tsov, I.A.; Vedyagin, A.A. Catalytic synthesis of nanosized feathery carbon structures via the carbide cycle mechanism. Kinet. Catal. 2008, 49, 868–872. [Google Scholar] [CrossRef]
- Monthioux, M.; Noé, L.; Dussault, L.; Dupin, J.C.; Latorre, N.; Ubieto, T.; Romeo, E.; Royo, C.; Monzón, A.; Guimon, C. Texturising and structurising mechanisms of carbon nanofilaments during growth. J. Mater. Chem. 2007, 17, 4611–4618. [Google Scholar] [CrossRef]
- Chesnokov, V.V.; Buyanov, R.A. The formation of carbon filaments upon decomposition of hydrocarbons catalysed by iron subgroup metals and their alloys. Russ. Chem. Rev. 2000, 69, 623–638. [Google Scholar] [CrossRef]
- Nemanich, R.J.; Solin, S.A. First- and second-order Raman scattering from finite-size crystals of graphite. Phys. Rev. B 1979, 20, 392–401. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef] [Green Version]
- Tuinstra, F.; Koenig, J.L. Raman Spectrum of Graphite. J. Chem. Phys. 1970, 53, 1126–1130. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Alsmeyer, D.C.; McCreery, R.L. Raman spectroscopy of carbon materials: Structural basis of observed spectra. Chem. Mater. 1990, 2, 557–563. [Google Scholar] [CrossRef]
- Sadezky, A.; Muckenhuber, H.; Grothe, H.; Niessner, R.; Pöschl, U. Raman microspectroscopy of soot and related carbonaceous materials: Spectral analysis and structural information. Carbon 2005, 43, 1731–1742. [Google Scholar] [CrossRef]
- Liu, C.; Sun, C.; Gao, Y.; Lan, W.; Chen, S. Improving the electrochemical properties of carbon paper as cathodes for microfluidic fuel cells by the electrochemical activation in different solutions. ACS Omega 2021, 6, 19153–19161. [Google Scholar] [CrossRef]
- Fu, W.Y.; Wang, K.F.; Lv, X.S.; Fu, H.L.; Dong, X.G.; Chen, L.; Zhang, X.M.; Jiang, G.M. Palladium nanoparticles assembled on titanium nitride for enhanced electrochemical hydrodechlorination of 2,4-dichlorophenol in water. Chin. J. Catal. 2018, 39, 693–700. [Google Scholar] [CrossRef]
- Lindberg, B.J.; Hedman, J. Molecular spectroscopy by means of ESCA. Chem. Scripta 1975, 7, 155–166. [Google Scholar]
- Dementjev, A.P.; de Graaf, A.; van de Sanden, M.C.M.; Maslakov, K.I.; Naumkin, A.V.; Serov, A.A. X-Ray photoelectron spectroscopy reference data for identification of the C3N4 phase in carbon–nitrogen films. Diam. Relat. Mater. 2000, 9, 1904–1907. [Google Scholar] [CrossRef]
- Kuntumalla, M.K.; Attrash, M.; Akhvlediani, R.; Michaelson, S.; Hoffman, A. Nitrogen bonding, work function and thermal stability of nitrided graphite surface: An in situ XPS, UPS and HREELS study. Appl. Surf. Sci. 2020, 525, 146562. [Google Scholar] [CrossRef]
- Ayiania, M.; Smith, M.; Hensley, A.J.R.; Scudiero, L.; McEwen, J.-S.; Garcia-Perez, M. Deconvoluting the XPS spectra for nitrogen-doped chars: An analysis from first principles. Carbon 2020, 162, 528–544. [Google Scholar] [CrossRef]
- Xu, Y.; Mo, Y.; Tian, J.; Wang, P.; Yu, H.; Yu, J. The synergistic effect of graphitic N and pyrrolic N for the enhanced photocatalytic performance of nitrogen-doped graphene/TiO2 nanocomposites. Appl. Catal. B Environ. 2016, 181, 810–817. [Google Scholar] [CrossRef]
- Chen, C.-M.; Zhang, Q.; Yang, M.-G.; Huang, C.-H.; Yang, Y.-G.; Wang, M.-Z. Structural evolution during annealing of thermally reduced graphene nanosheets for application in supercapacitors. Carbon 2012, 50, 3572–3584. [Google Scholar] [CrossRef]
- Oh, Y.J.; Yoo, J.J.; Kim, Y.I.; Yoon, J.K.; Yoon, H.N.; Kim, J.-H.; Park, S.B. Oxygen functional groups and electrochemical capacitive behavior of incompletely reduced graphene oxides as a thin-film electrode of supercapacitor. Electrochim. Acta 2014, 116, 118–128. [Google Scholar] [CrossRef] [Green Version]
- Folkesson, B.; Sundberg, P. A Reinvestigation of the binding energy versus atomic charge relation for oxygen from X-ray photoelectron spectroscopy. Spectroscopy Lett. 1987, 20, 193–200. [Google Scholar] [CrossRef]
| # | Sample Designation | Description |
|---|---|---|
| 1 | cat/CNF | As-prepared CNFs obtained via CCVD of ethylene at 550 °C over Ni–Cu alloy catalyst |
| 2 | ha-CNF | cat/CNF sample pretreated in hydrochloric acid (elimination of metal particles) |
| 3 | ox-CNF | cat/CNF sample pretreated in a mixture of sulfur and nitric acids (elimination of metal particles, oxidation of the CNF’s surface) |
| 4 | N/ha-CNF | ha-CNF sample post-functionalized with melamine |
| 5 | N/ox-CNF | ox-CNF sample post-functionalized with melamine |
| Sample | SSA, m2/g | Pore Volume, cm3/g | Average Pore Diameter (4V/A), nm |
|---|---|---|---|
| cat/CNF | 161.9 | 0.173 | 4.3 |
| ha-CNF | 154.3 | 0.161 | 4.2 |
| ox-CNF | 147.4 | 0.161 | 4.4 |
| N/ha-CNF | 137.1 | 0.147 | 4.3 |
| N/ox-CNF | 110.2 | 0.127 | 4.6 |
| Sample | SSA of the 1st Adsorption layer *, m2/g | Volume of Micropores in CNFs *, cm3/g | Outer SSA of CNFs *, m2/g | Volume of Micropores < 2 nm †, cm3/g | Outer SSA of Pores > 2 nm †, m2/g |
|---|---|---|---|---|---|
| cat/CNF | 155.6 | 0.059 | 48.5 | 0.057 | 20.4 |
| ha-CNF | 150.3 | 0.055 | 38.5 | 0.053 | 20.7 |
| ox-CNF | 137.7 | 0.051 | 36.4 | 0.053 | 17.2 |
| N/ha-CNF | 134.5 | 0.046 | 34.2 | 0.048 | 16.4 |
| N/ox-CNF | 109.5 | 0.035 | 31.7 | 0.038 | 14.1 |
| Sample | N/C | O/C |
|---|---|---|
| ha-CNF | 0.0007 | 0.025 |
| N/ha-CNF | 0.014 | 0.046 |
| ox-CNF | 0.014 | 0.118 |
| N/ox-CNF | 0.043 | 0.064 |
| BE, eV | Sample | Species | Ref. | |||
|---|---|---|---|---|---|---|
| ha-CNF | N/ha-CNF | ox-CNF | N/ox-CNF | |||
| 398.4 | - | 0.0036 | - | 0.011 | C-NH2 | [75,76] |
| C≡N | [61,75] | |||||
| 399.0 | - | 0.00078 | - | 0.00048 | C=N-C | [75,77] |
| 399.8 | - | - | - | 0.032 | Pyrrolic N | [76,77,78] |
| 400.0 | - | 0.0098 | - | - | ||
| ~400.0 | 0.0007 | - | 0.0013 | - | Not identified | |
| 405.8 | - | - | 0.013 | - | C-NO2 | [74] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maksimova, T.A.; Mishakov, I.V.; Bauman, Y.I.; Ayupov, A.B.; Mel’gunov, M.S.; Dmitrachkov, A.M.; Nartova, A.V.; Stoyanovskii, V.O.; Vedyagin, A.A. Effect of Pretreatment with Acids on the N-Functionalization of Carbon Nanofibers Using Melamine. Materials 2022, 15, 8239. https://doi.org/10.3390/ma15228239
Maksimova TA, Mishakov IV, Bauman YI, Ayupov AB, Mel’gunov MS, Dmitrachkov AM, Nartova AV, Stoyanovskii VO, Vedyagin AA. Effect of Pretreatment with Acids on the N-Functionalization of Carbon Nanofibers Using Melamine. Materials. 2022; 15(22):8239. https://doi.org/10.3390/ma15228239
Chicago/Turabian StyleMaksimova, Tatyana A., Ilya V. Mishakov, Yury I. Bauman, Artem B. Ayupov, Maksim S. Mel’gunov, Aleksey M. Dmitrachkov, Anna V. Nartova, Vladimir O. Stoyanovskii, and Aleksey A. Vedyagin. 2022. "Effect of Pretreatment with Acids on the N-Functionalization of Carbon Nanofibers Using Melamine" Materials 15, no. 22: 8239. https://doi.org/10.3390/ma15228239







