Computational and Experimental Investigation of the Selective Adsorption of Indium/Iron Ions by the Epigallocatechin Gallate Monomer
Abstract
1. Introduction
2. Materials and Methods
2.1. Computational Methods
2.2. Batch Adsorption Experiment
2.3. The Pseudo-First- and Pseudo-Second-Order Models
2.4. The Langmuir and Freundlich Models
2.5. Thermodynamic Analysis
2.6. Materials and Characterization
3. Results and Discussion
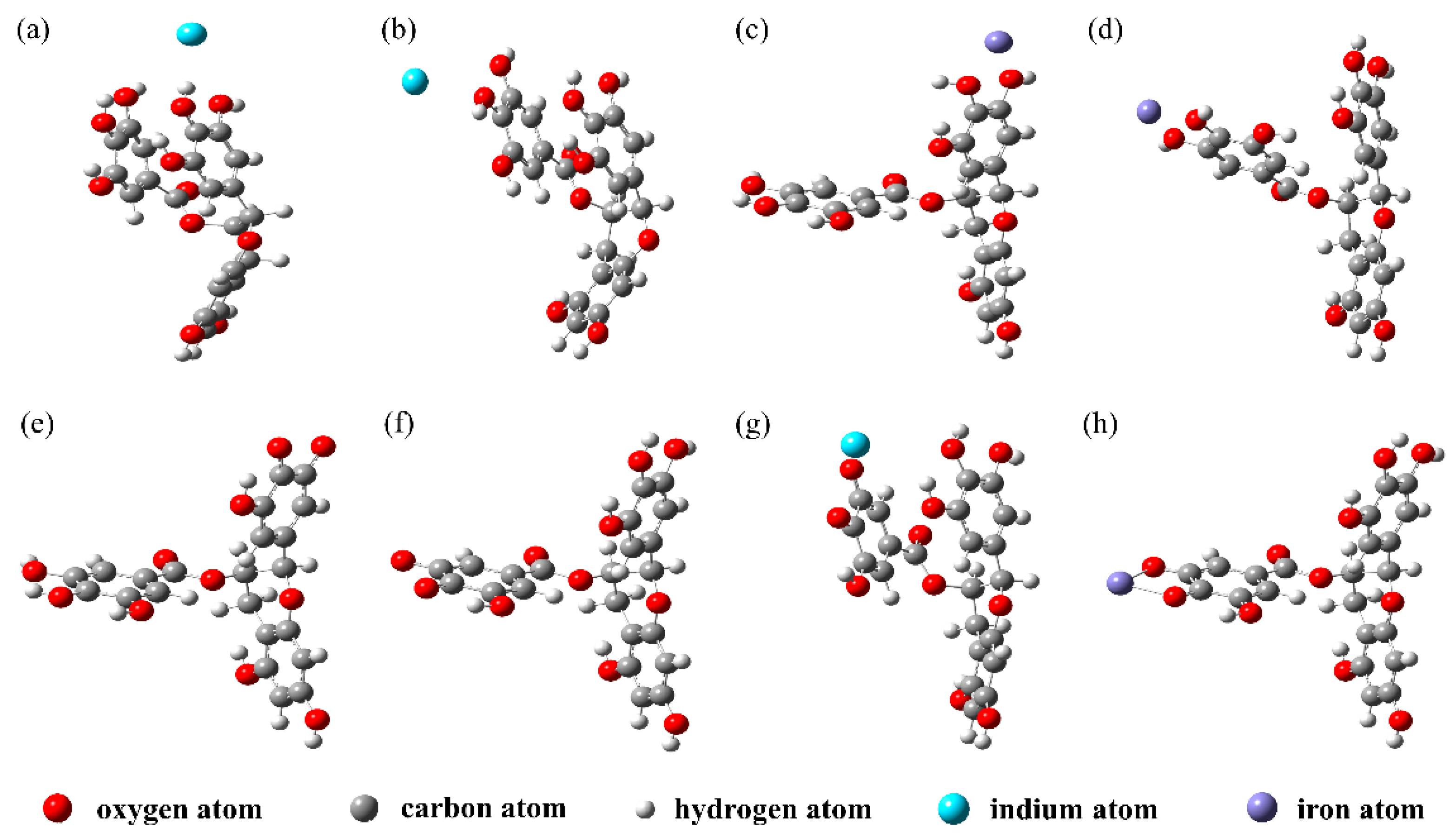
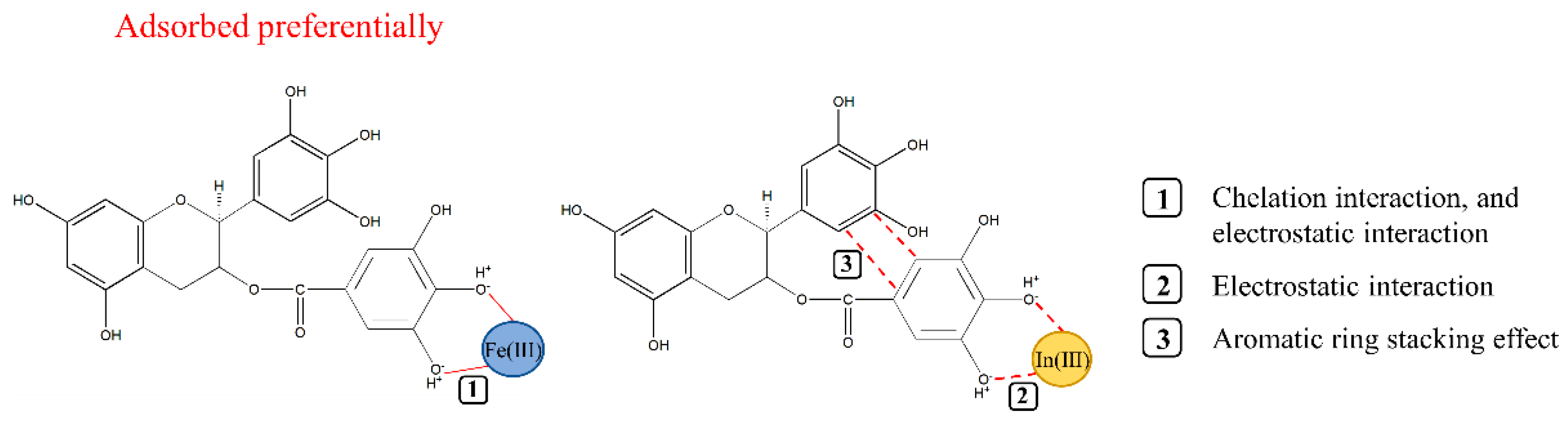
3.1. Construction of the EGCG–Cation Complex
3.2. Bond Length and Bond Order of EGCG–Metal Complexes
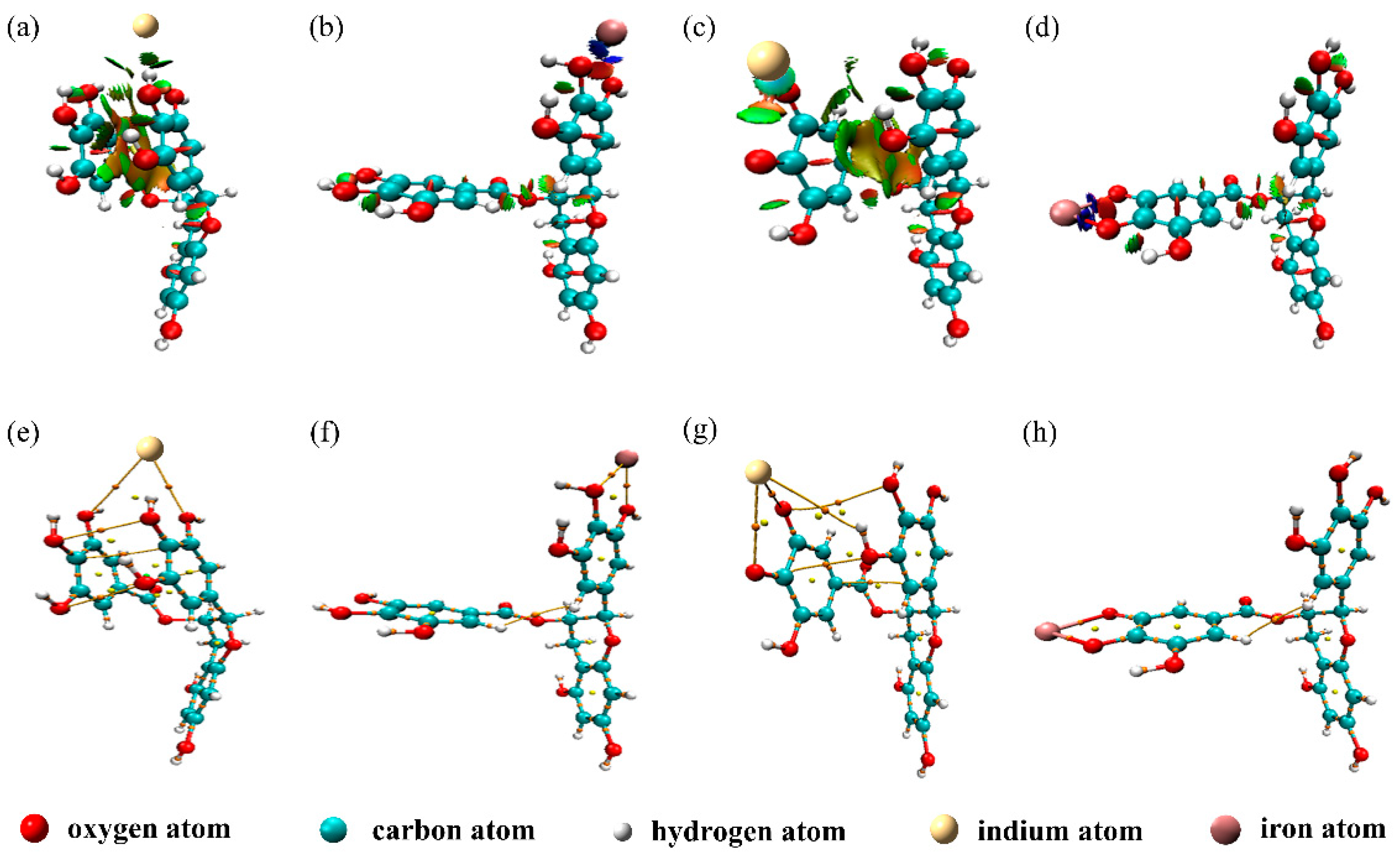
3.3. RDG Analysis
3.4. AIM Analysis
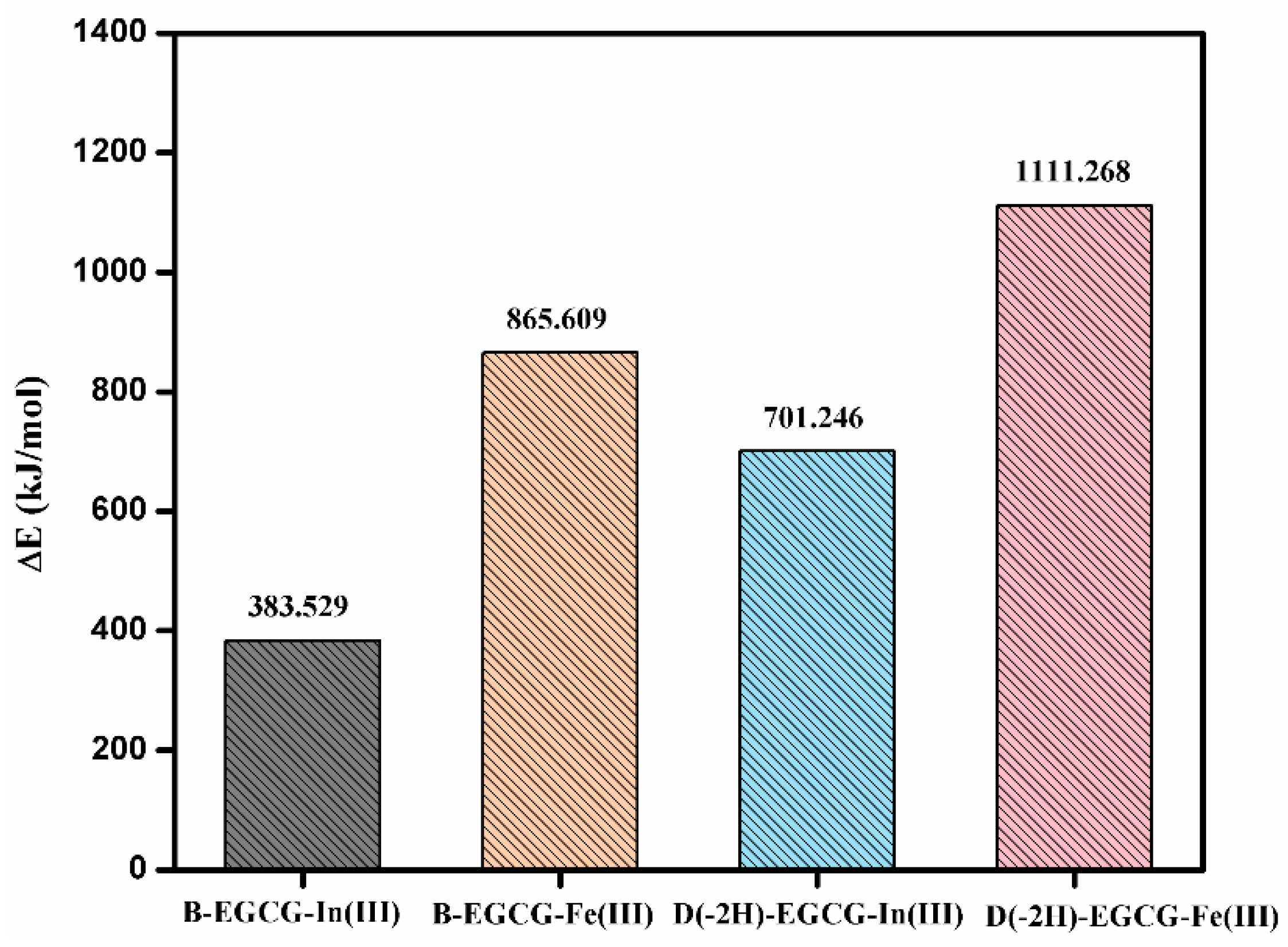
3.5. Binding Energy between EGCG and Indium/Iron Ions
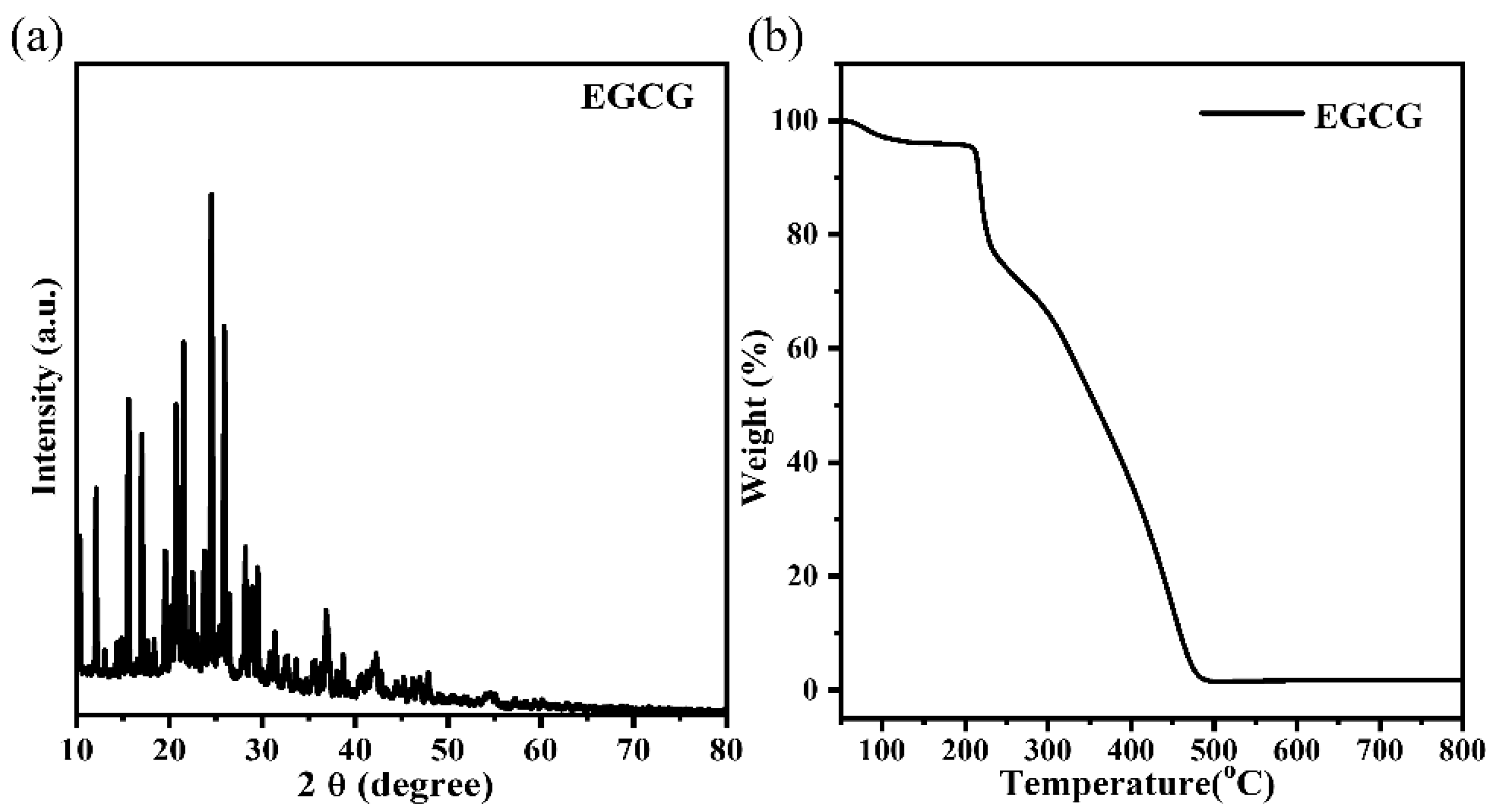
3.6. Characterization of the EGCG
3.7. Adsorption Kinetics Studies
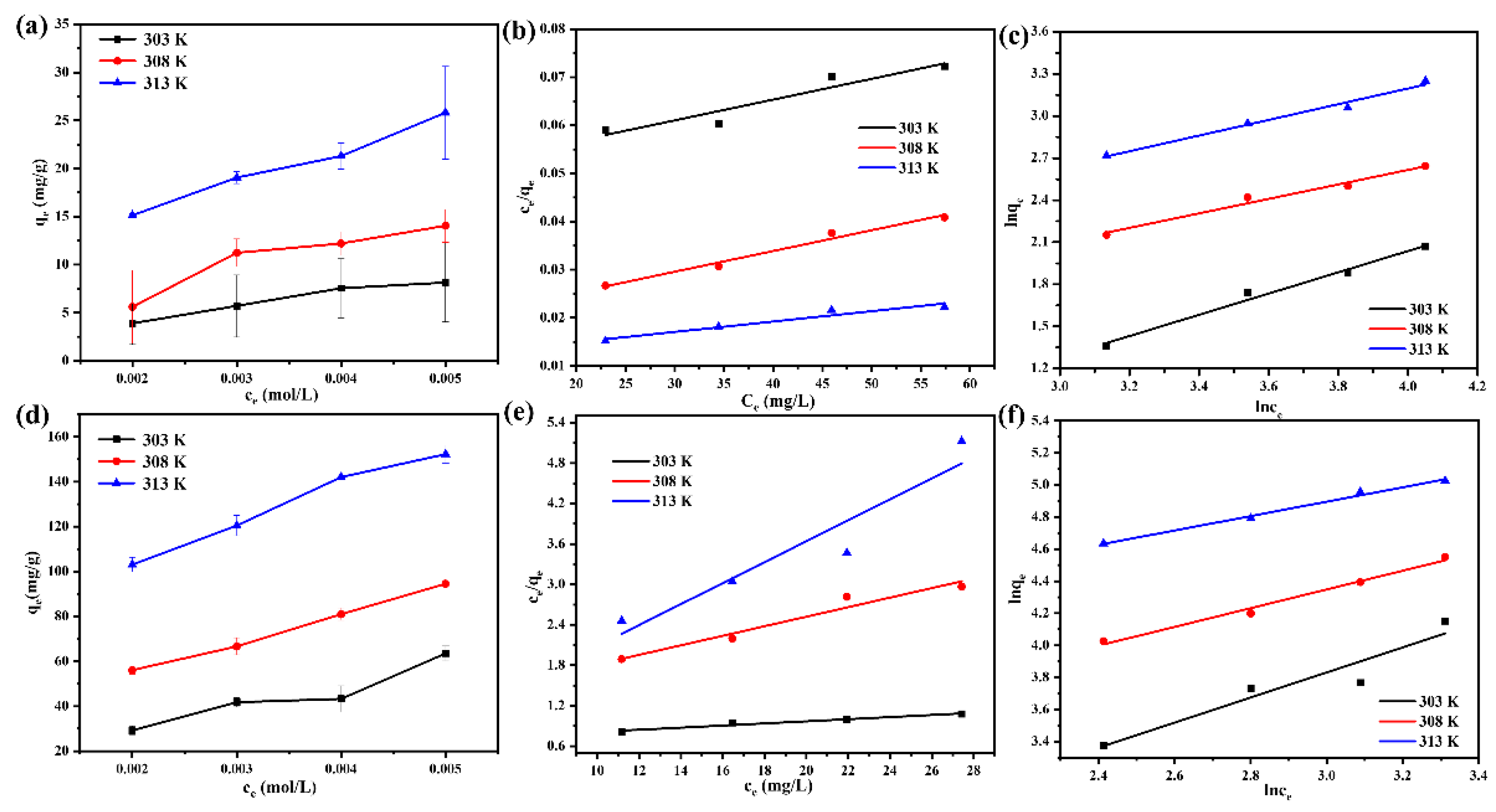
3.8. Adsorption Isotherm Studies and Thermodynamics Analysis
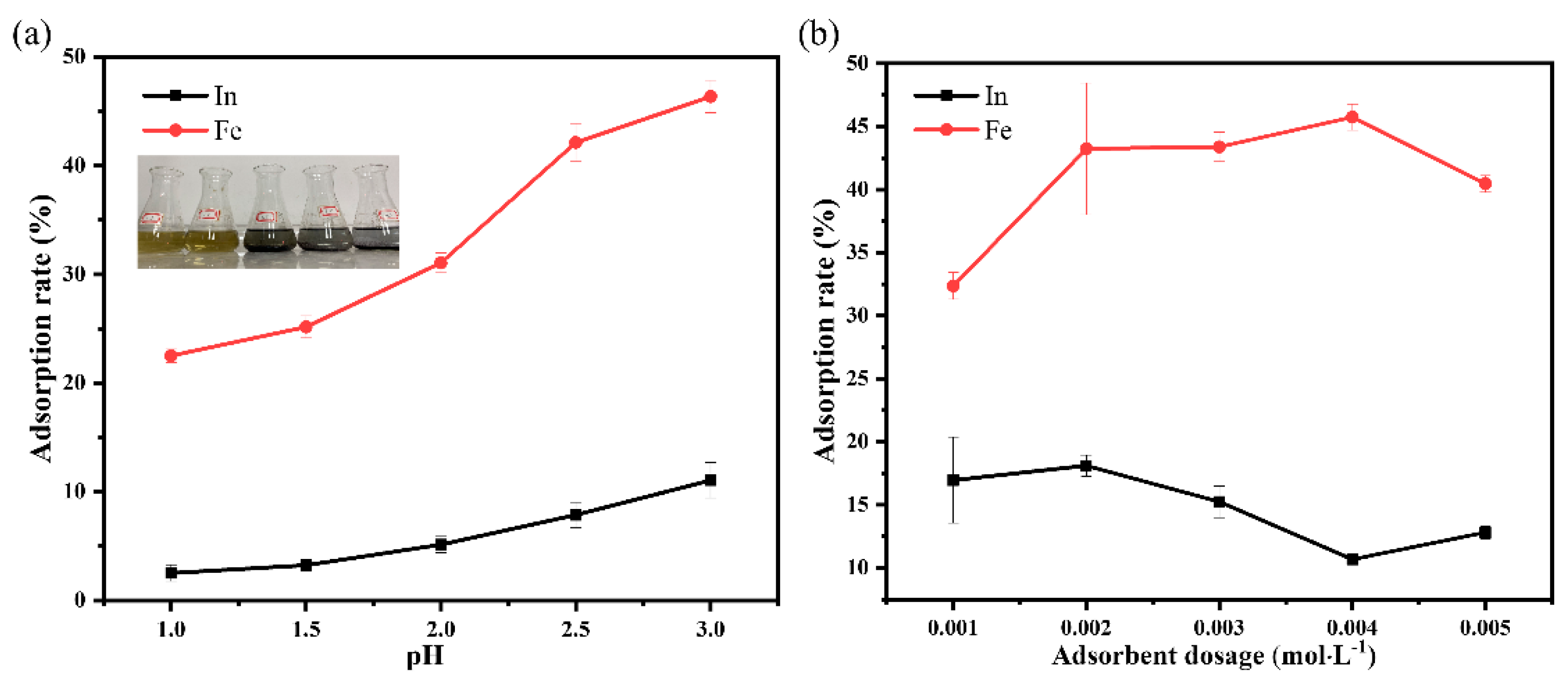
3.9. Selective Adsorption Experiments of Indium and Iron Ions
3.10. SEM Characterization of Indium/Iron Ion Adsorption by EGCG
3.11. XPS Analysis of the Indium/Iron Ion Adsorption by EGCG
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Swain, B.; Lee, C.G. Commercial indium recovery processes development from various e-(industry) waste through the insightful integration of valorization processes: A perspective. Waste Manag. 2019, 87, 597–611. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Lin, Y.-C.; Chien, S.-K.; Wu, T.-Y.; Chen, S.-C.; Cheng, P.-C.; Lai, C.-N. Efficient indium leaching and recovery from waste liquid crystal displays panels using microwave and ultrasound-assisted heating system. Sep. Purif. Technol. 2020, 250, 117154. [Google Scholar] [CrossRef]
- Deferm, C.; Onghena, B.; Nguyen, V.T.; Banerjee, D.; Fransaer, J.; Binnemans, K. Non-aqueous solvent extraction of indium from an ethylene glycol feed solution by the ionic liquid Cyphos IL 101: Speciation study and continuous counter-current process in mixer-settlers. RSC Adv. 2020, 10, 24595–24612. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Zheng, Y.; Li, X.; Wei, C.; Li, M.; Li, C.; Fan, G. Separation of copper and indium from zinc hydrometallurgy solution. Int. J. Chem. React. Eng. 2020, 18, 20200082. [Google Scholar] [CrossRef]
- Di Maria, A.; Van Acker, K. Turning Industrial Residues into Resources: An Environmental Impact Assessment of Goethite Valorization. Engineering 2018, 4, 421–429. [Google Scholar] [CrossRef]
- Virolainen, S.; Huhtanen, T.; Laitinen, A.; Sainio, T. Two alternative process routes for recovering pure indium from waste liquid crystal display panels. J. Clean. Prod. 2020, 243, 118599. [Google Scholar] [CrossRef]
- Fontana, D.; Forte, F.; Pietrantonio, M.; Pucciarmati, S. Recent developments on recycling end-of-life flat panel displays: A comprehensive review focused on indium. Crit. Rev. Environ. Sci. Technol. 2020, 51, 429–456. [Google Scholar] [CrossRef]
- Le, T.; Xiao, B.; Ju, S.; Peng, J.; Jiang, F. Separation of indium from impurities in T-type microreactor with D2EHPA. Hydrometallurgy 2019, 183, 79–86. [Google Scholar] [CrossRef]
- Fortin-Lecomte, C.; Tran, L.-H.; Rioux, G.; Coudert, L.; Blais, J.-F. Recovery of indium from acidic leach solutions of spent LCD panels using ion exchange. Hydrometallurgy 2022, 210, 105845. [Google Scholar] [CrossRef]
- Deferm, C.; Malaquias, J.C.; Onghena, B.; Banerjee, D.; Luyten, J.; Oosterhof, H.; Fransaer, J.; Binnemans, K. Electrodeposition of indium from the ionic liquid trihexyl(tetradecyl)phosphonium chloride. Green Chem. 2019, 21, 1517–1530. [Google Scholar] [CrossRef]
- Shen, Y.Y.; Wu, S.W.; Hou, C.H. Exploring the electrosorption selectivity and recovery of indium ions with capacitive deionization in acidic solution. J. Colloid Interface Sci. 2021, 586, 819–829. [Google Scholar] [CrossRef]
- Cui, J.; Zhu, N.; Mao, F.; Wu, P.; Dang, Z. Bioleaching of indium from waste LCD panels by Aspergillus niger: Method optimization and mechanism analysis. Sci. Total Environ. 2021, 790, 148151. [Google Scholar] [CrossRef] [PubMed]
- Pennesi, C.; Amato, A.; Occhialini, S.; Critchley, A.T.; Totti, C.; Giorgini, E.; Conti, C.; Beolchini, F. Adsorption of indium by waste biomass of brown alga Ascophyllum nodosum. Sci. Rep. 2019, 9, 16763. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Zhu, N.; Li, Y.; Cui, J.; Wu, P.; Wang, J. Simultaneous leaching and extraction of indium from waste LCDs with acidic ionic liquids. Hydrometallurgy 2019, 189, 105146. [Google Scholar] [CrossRef]
- Zürner, P.; Frisch, G. Leaching and Selective Extraction of Indium and Tin from Zinc Flue Dust Using an Oxalic Acid-Based Deep Eutectic Solvent. ACS Sustainable Chem. Eng. 2019, 7, 5300–5308. [Google Scholar] [CrossRef]
- Ampiaw, R.E.; Lee, W. Persimmon tannins as biosorbents for precious and heavy metal adsorption in wastewater: A review. Int. J. Environ. Sci. Technol. 2020, 17, 3835–3846. [Google Scholar] [CrossRef]
- Liu, F.; Wang, S.; Chen, S. Adsorption behavior of Au(III) and Pd(II) on persimmon tannin functionalized viscose fiber and the mechanism. Int. J. Biol. Macromol. 2020, 152, 1242–1251. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Leverence, R.; Trombley, J.D.; Xu, S.; Yang, J.; Tian, Y.; Reed, J.D.; Hagerman, A.E. High molecular weight persimmon (Diospyros kaki L.) proanthocyanidin: A highly galloylated, A-linked tannin with an unusual flavonol terminal unit, myricetin. J. Agr. Food Chem. 2010, 58, 9033–9042. [Google Scholar] [CrossRef]
- Kavitha, V.U.; Kandasubramanian, B. Tannins for wastewater treatment. SN Appl. Sci. 2020, 2, 1081. [Google Scholar] [CrossRef]
- Zhang, S.; Dang, J.; Lin, J.; Liu, M.; Zhang, M.; Chen, S. Selective enrichment and separation of Ag(I) from electronic waste leachate by chemically modified persimmon tannin. J. Environ. Chem. Eng. 2021, 9, 104994. [Google Scholar] [CrossRef]
- Gao, L.; Wang, Z.; Qin, C.; Chen, Z.; Gao, M.; He, N.; Qian, X.; Zhou, Z.; Li, G. Preparation and application of iron oxide/persimmon tannin/graphene oxide nanocomposites for efficient adsorption of erbium from aqueous solution. J. Rare Earths 2020, 38, 1344–1353. [Google Scholar] [CrossRef]
- Qian, X.; Wang, Z.; Ning, J.; Qin, C.; Gao, L.; He, N.; Lin, D.; Zhou, Z.; Li, G. Protecting HaCaT cells from ionizing radiation using persimmon tannin-Aloe gel composite. Pharm. Biol. 2020, 58, 510–517. [Google Scholar] [CrossRef]
- Liu, F.; Zhou, Z.; Li, G. Persimmon tannin functionalized polyacrylonitrile fiber for highly efficient and selective recovery of Au(III) from aqueous solution. Chemosphere 2021, 264, 128469. [Google Scholar] [CrossRef] [PubMed]
- Mananghaya, M.R.; Santos, G.N.; Yu, D. Small transition metal cluster adsorbed on graphene and graphene nanoribbons: A density functional based tight binding molecular dynamics study. Org. Electron. 2018, 63, 355–361. [Google Scholar] [CrossRef]
- Jiang, T.; Wan-Fu, M.A.; Xie, N.; Zhou, P. Study on the Interaction of EGCG with Zn(II) Based on Experiments and Density Functional Theory. Acta Phys. Chim. Sin. 2011, 27, 2291–2296. [Google Scholar]
- Liu, Z.G.; Chen, T.; Hu, C.H.; Wang, D.H.; Li, G.Y. Calculation and analysis of interaction between characteristic functional group of persimmon tannin and metal ions. Acta Phys. Sin. 2021, 70, 123101. [Google Scholar] [CrossRef]
- Goerigk, L.; Grimme, S. A thorough benchmark of density functional methods for general main group thermochemistry, kinetics, and noncovalent interactions. Phys. Chem. Chem. Phys. 2011, 13, 6670–6688. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Zimeri, J.; Tong, C.H. Degradation Kinetics of (−)-Epigallocatechin Gallate as a Function of pH and Dissolved Oxygen in a Liquid Model System. J. Food Sci. 1999, 64, 753–758. [Google Scholar] [CrossRef]
- Chen, K.; Chen, C.; Ren, X.; Alsaedi, A.; Hayat, T. Interaction mechanism between different facet TiO2 and U(VI): Experimental and density-functional theory investigation. Chem. Eng. J. 2019, 359, 944–954. [Google Scholar] [CrossRef]
- Navarro, R.E.; Santacruz, H.; Inoue, M. Complexation of epigallocatechin gallate (a green tea extract, egcg) with Mn2+: Nuclear spin relaxation by the paramagnetic ion. J. Inorg. Biochem. 2005, 99, 584–588. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Chen, F. Bond order analysis based on the Laplacian of electron density in fuzzy overlap space. J Phys. Chem. A 2013, 117, 3100–3108. [Google Scholar] [CrossRef]
- Johnson, E.R.; Keinan, S.; Mori-Sánchez, P.; Contreras-García, J.; Cohen, A.J.; Yang, W. Revealing Noncovalent Interactions. J. Am. Chem. Soc. 2010, 132, 6498–6506. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Bader, R.F.W. A quantum theory of molecular structure and its applications. Chem Rev. 1991, 91, 893–928. [Google Scholar] [CrossRef]
- Xie, H.; Liu, C.; Gao, J.; Shi, J.; Ni, F.; Luo, X.; He, Y.; Ren, G.; Luo, Z. Fabrication of Zein-Lecithin-EGCG complex nanoparticles: Characterization, controlled release in simulated gastrointestinal digestion. Food Chem. 2021, 365, 130542. [Google Scholar] [CrossRef]
- Pan, J.; Li, M.; Zhang, S.; Jiang, Y.; Lv, Y.; Liu, J.; Liu, Q.; Zhu, Y.; Zhang, H. Effect of epigallocatechin gallate on the gelatinisation and retrogradation of wheat starch. Food Chem. 2019, 294, 209–215. [Google Scholar] [CrossRef]
- Li, B.; Li, M.; Zhang, P.; Pan, Y.; Huang, Z.; Xiao, H. Remediation of Cd (II) ions in aqueous and soil phases using novel porous cellulose/chitosan composite spheres loaded with zero-valent iron nanoparticles. React. Funct. Polym. 2022, 173, 105210. [Google Scholar] [CrossRef]
- Van Roosendael, S.; Regadío, M.; Roosen, J.; Binnemans, K. Selective recovery of indium from iron-rich solutions using an Aliquat 336 iodide supported ionic liquid phase (SILP). Sep. Purif. Technol. 2019, 212, 843–853. [Google Scholar] [CrossRef]
- Li, M.; Tang, S.; Liu, R.; Meng, X.; Feng, J.; Zhou, L.; Chen, Y. Experimental and DFT studies on highly selective separation of indium ions using silica gel/graphene oxide based ion-imprinted composites as a sorbent. Chem. Eng. Res. Des. 2021, 168, 135–145. [Google Scholar] [CrossRef]
- Gao, X.; Cao, Z.; Li, C.; Liu, J.; Liu, X.; Guo, L. Activated carbon fiber modified with hyperbranched polyethylenimine and phytic acid for the effective adsorption and separation of In(III). New J. Chem. 2022, 46, 18952–18960. [Google Scholar] [CrossRef]


| EGCG–Metal Complex | Compound Bond | Bond Length (Å) | Mayer Bond Order |
|---|---|---|---|
| B-EGCG–In(III) | 3′O-In | 3.1418 | 0.0425 |
| 4′O-In | 4.0454 | 0.0188 | |
| B-EGCG–Fe(III) | 3′O-Fe | 2.1682 | 0.2211 |
| 4′O-Fe | 2.2139 | 0.2193 | |
| D(-2H)-EGCG–In(III) | 4″O-In | 2.9289 | 0.0742 |
| 5″O-In | 2.6831 | 0.1183 | |
| D(-2H)-EGCG–Fe(III) | 4″O-Fe | 2.0819 | 0.3321 |
| 5″O-Fe | 2.0721 | 0.3623 |
| EGCG–Metal Complex | Compound Bond | ρ(BCP) (a.u.) |
|---|---|---|
| B-EGCG–In(III) | 3′O-In | 0.00917 |
| 4′O-In | 0 | |
| B-EGCG–Fe(III) | 3′O-Fe | 0.04504 |
| 4′O-Fe | 0.04102 | |
| D(-2H)-EGCG–In(III) | 4″O-In | 0.01464 |
| 5″O-In | 0.02335 | |
| D(-2H)-EGCG–Fe(III) | 4″O-Fe | 0.06035 |
| 5″O-Fe | 0.06289 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Wang, Z.; Gan, W.; Liu, S.; Zhang, J.; Ran, Z.; Wu, C.; Hu, C.; Wang, D.; Chen, T.; et al. Computational and Experimental Investigation of the Selective Adsorption of Indium/Iron Ions by the Epigallocatechin Gallate Monomer. Materials 2022, 15, 8251. https://doi.org/10.3390/ma15228251
Liu Z, Wang Z, Gan W, Liu S, Zhang J, Ran Z, Wu C, Hu C, Wang D, Chen T, et al. Computational and Experimental Investigation of the Selective Adsorption of Indium/Iron Ions by the Epigallocatechin Gallate Monomer. Materials. 2022; 15(22):8251. https://doi.org/10.3390/ma15228251
Chicago/Turabian StyleLiu, Zhigao, Zhongmin Wang, Weijiang Gan, Songlin Liu, Jianglin Zhang, Zhaojin Ran, Chenxi Wu, Chaohao Hu, Dianhui Wang, Tao Chen, and et al. 2022. "Computational and Experimental Investigation of the Selective Adsorption of Indium/Iron Ions by the Epigallocatechin Gallate Monomer" Materials 15, no. 22: 8251. https://doi.org/10.3390/ma15228251
APA StyleLiu, Z., Wang, Z., Gan, W., Liu, S., Zhang, J., Ran, Z., Wu, C., Hu, C., Wang, D., Chen, T., & Li, G. (2022). Computational and Experimental Investigation of the Selective Adsorption of Indium/Iron Ions by the Epigallocatechin Gallate Monomer. Materials, 15(22), 8251. https://doi.org/10.3390/ma15228251








