Computational Analysis on Antioxidant Activity of Four Characteristic Structural Units from Persimmon Tannin
Abstract
1. Introduction
2. Materials and Methods
2.1. Computational Methods
2.2. Computational Models
3. Results and Discussion
3.1. Structural Parameters of Molecules
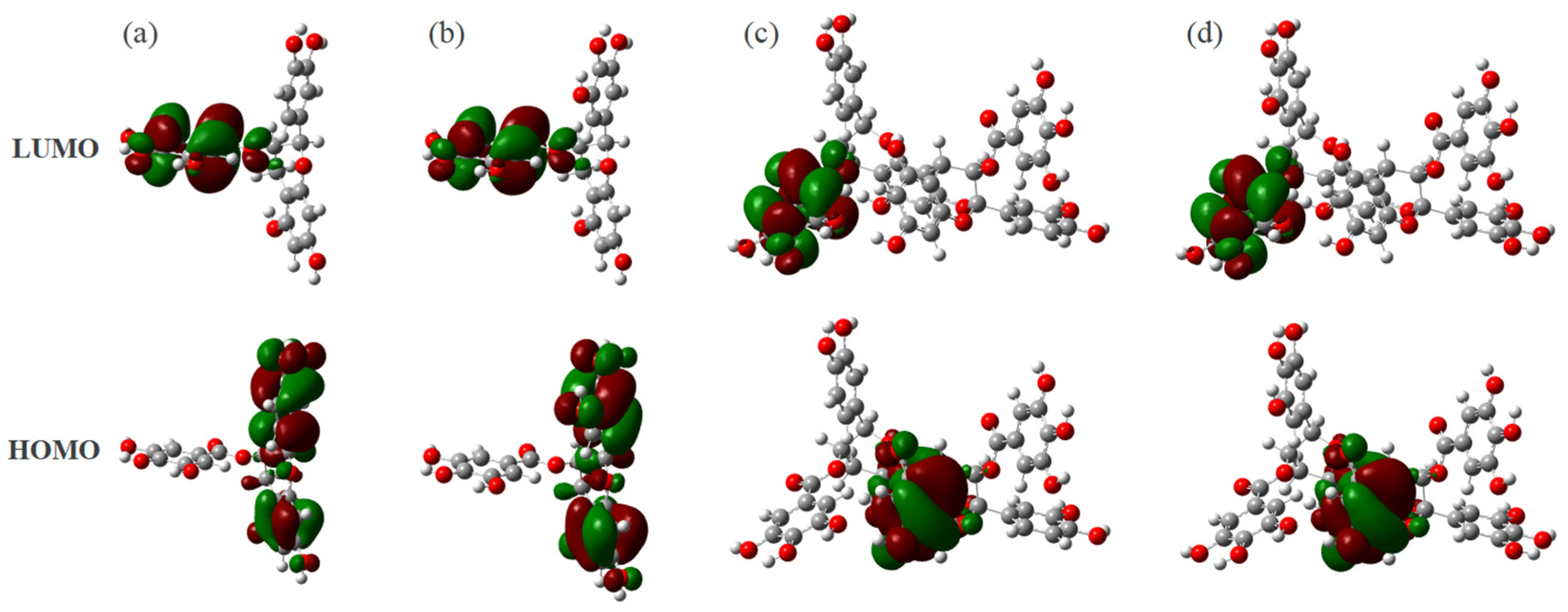
3.2. Frontier Molecular Orbitals
3.3. Molecular Electrostatic Potential (MEP)
3.4. Natural Bond Orbital (NBO) of Phenolic Hydroxyl Hydrogen Atoms
3.5. The Bond Dissociation Energy of Phenolic Hydroxyl Groups
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ruiz, L.M.; Salazar, C.; Jensen, E.; Ruiz, P.A.; Tiznado, W.; Quintanilla, R.A.; Barreto, M.; Elorza, A.A. Quercetin Affects Erythropoiesis and Heart Mitochondrial Function in Mice. Oxid. Med. Cell. Longev. 2015, 2015, 836301. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Birhman, K.; Raheja, I.; Sharma, S.K.; Kar, H.K. Quercetin: A wonder bioflavonoid with therapeutic potential in disease management. Asian Pac. J. Trop. Dis. 2016, 6, 248–252. [Google Scholar] [CrossRef]
- Liu, F.; Wang, S.; Chen, S. Adsorption behavior of Au(III) and Pd(II) on persimmon tannin functionalized viscose fiber and the mechanism. Int. J. Biol. Macromol. 2020, 152, 1242–1251. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.-G.; Qian, X.; Wang, Z.-M.; Ning, J.-L.; Qin, C.-K.; Huang, Z.-M.; Li, Y.-M.; He, N.; Lin, D.-H.; Zhou, Z.-D.; et al. Effects of Persimmon Tannin-Aloe vera Composite on Cytotoxic Activities, and Radioprotection Against X-rays Irradiated in Human Hepatoma and Hepatic Cells. J. Biomed. Nanotechnol. 2021, 17, 2043–2052. [Google Scholar] [CrossRef]
- Gao, L.; Wang, Z.; Qin, C.; Chen, Z.; Gao, M.; He, N.; Qian, X.; Zhou, Z.; Li, G. Preparation and application of iron oxide/persimmon tannin/ graphene oxide nanocomposites for efficient adsorption of erbium from aqueous solution. J. Rare Earths 2020, 38, 1344–1353. [Google Scholar] [CrossRef]
- Zhu, W.; Deng, X.; Peng, J.; Zou, B.; Li, C. A-type ECG and EGCG dimers inhibit 3T3-L1 differentiation by binding to cholesterol in lipid rafts. J. Nutr. Biochem. 2017, 48, 62–73. [Google Scholar] [CrossRef]
- Shin, Y.-J.; Shon, M.-S.; Kim, G.-N.; Lee, S.-C. Antioxidant and anti-adipogenic activities of persimmon tannins. Food Sci. Biotechnol. 2014, 23, 1689–1694. [Google Scholar] [CrossRef]
- Chang, Y.L.; Lin, J.T.; Lin, H.L.; Liao, P.L.; Wu, P.J.; Yang, D.J. Phenolic compositions and antioxidant properties of leaves of eight persimmon varieties harvested in different periods. Food Chem. 2019, 289, 74–83. [Google Scholar] [CrossRef]
- Kumar, N.; Gusain, A.; Kumar, J.; Singh, R.; Hota, P.K. Anti-oxidation properties of 2-substituted furan derivatives: A mechanistic study. J. Lumin. 2021, 230, 117725. [Google Scholar] [CrossRef]
- Wei, J.; Liang, Q.; Guo, Y.; Zhang, W.; Wu, L. A Deep Insight in the Antioxidant Property of Carnosic Acid: From Computational Study to Experimental Analysis. Foods 2021, 10, 2279. [Google Scholar] [CrossRef]
- Jensen, J.H.; Swain, C.J.; Olsen, L. Prediction of pKa Values for Druglike Molecules Using Semiempirical Quantum Chemical Methods. J. Phys. Chem. A 2017, 121, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Leverence, R.; Trombley, J.D.; Xu, S.; Yang, J.; Tian, Y.; Reed, J.D.; Hagerman, A.E. High molecular weight persimmon (Diospyros kaki L.) proanthocyanidin: A highly galloylated, A-linked tannin with an unusual flavonol terminal unit, myricetin. J. Agric. Food Chem. 2010, 58, 9033–9042. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tang, H.; Hou, B.; Zhang, P.; Wang, Q.; Zhang, B.-L.; Huang, Y.-W.; Wang, Y.; Xiang, Z.-M.; Zi, C.-T.; et al. Synthesis, antioxidant activity, and density functional theory study of catechin derivatives. RSC Adv. 2017, 7, 54136–54141. [Google Scholar] [CrossRef]
- Boulmokh, Y.; Belguidoum, K.; Meddour, F.; Amira-Guebailia, H. Investigation of antioxidant activity of epigallocatechin gallate and epicatechin as compared to resveratrol and ascorbic acid: Experimental and theoretical insights. Struct. Chem. 2021, 32, 1907–1923. [Google Scholar] [CrossRef]
- Manini, P.; Bietti, M.; Galeotti, M.; Salamone, M.; Lanzalunga, O.; Cecchini, M.M.; Reale, S.; Crescenzi, O.; Napolitano, A.; De Angelis, F.; et al. Characterization and Fate of Hydrogen-Bonded Free-Radical Intermediates and Their Coupling Products from the Hydrogen Atom Transfer Agent 1,8-Naphthalenediol. ACS Omega 2018, 3, 3918–3927. [Google Scholar] [CrossRef]
- Rammohan, A.; Bhaskar, B.V.; Camilo, A.; Gunasekar, D.; Gu, W.; Zyryanov, G.V. In silico, in vitro antioxidant and density functional theory based structure activity relationship studies of plant polyphenolics as prominent natural antioxidants. Arabian J. Chem. 2020, 13, 3690–3701. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, A.; Shi, P.; Zhang, H. A Theoretical Study on the Antioxidant Activity of Piceatannol and Isorhapontigenin Scavenging Nitric Oxide and Nitrogen Dioxide Radicals. PLoS ONE 2017, 12, e0169773. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar]
- Frish, M.; Trucks, G.; Schlegel, H.; Scuseria, G.; Robb, M.; Cheeseman, J.; Scalmani, G.; Barone, V.; Mennucci, B.; Paterson, G. Gaussian 09, Revision A. 02; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Zhang, Y.; Zhong, L.; Zhou, B.; Chen, J.-Y.; Li, C.-M. Interaction of characteristic structural elements of persimmon tannin with Chinese cobra PLA2. Toxicon 2013, 74, 34–43. [Google Scholar] [CrossRef]
- Wang, G.; Xue, Y.; An, L.; Zheng, Y.; Dou, Y.; Zhang, L.; Liu, Y. Theoretical study on the structural and antioxidant properties of some recently synthesised 2,4,5-trimethoxy chalcones. Food Chem. 2015, 171, 89–97. [Google Scholar] [CrossRef]
- Xue, Y.; Liu, Y.; Luo, Q.; Wang, H.; Chen, R.; Liu, Y.; Li, Y. Antiradical Activity and Mechanism of Coumarin-Chalcone Hybrids: Theoretical Insights. J. Phys. Chem. A 2018, 122, 8520–8529. [Google Scholar] [CrossRef] [PubMed]
- Kongpichitchoke, T.; Hsu, J.L.; Huang, T.C. Number of Hydroxyl Groups on the B-Ring of Flavonoids Affects Their Antioxidant Activity and Interaction with Phorbol Ester Binding Site of PKCdelta C1B Domain: In Vitro and in Silico Studies. J. Agric. Food Chem. 2015, 63, 4580–4586. [Google Scholar] [CrossRef] [PubMed]
- de Souza Farias, S.A.; da Costa, K.S.; Martins, J.B.L. Analysis of Conformational, Structural, Magnetic, and Electronic Properties Related to Antioxidant Activity: Revisiting Flavan, Anthocyanidin, Flavanone, Flavonol, Isoflavone, Flavone, and Flavan-3-ol. ACS Omega 2021, 6, 8908–8918. [Google Scholar] [CrossRef] [PubMed]
- Mu, W.; Ma, Y.; Fang, D.; Wang, R.; Zhang, H. Computational Insights into the Diels-Alder-alike Reactions of 1-Iodo-2-Lithio-o-Carborane with Fulvenes. Acta Chim. Sin. 2018, 76, 55–61. [Google Scholar] [CrossRef]



| ECG | EGCG | A−ECG Dimer | A−ECG Dimer | |
|---|---|---|---|---|
| RC5−OH | 0.96022 | 0.96021 | 0.97281 | 0.97256 |
| RC7−OH | 0.96048 | 0.96046 | 0.97196 | 0.97205 |
| RC11−OH | 0.95988 | 0.95996 | 0.96676 | 0.96682 |
| RC12−OH | 0.96433 | 0.96349 | 0.97068 | 0.97030 |
| RC13−OH | − | 0.96423 | − | 0.97080 |
| RC19−OH | 0.96411 | 0.96412 | 0.97085 | 0.97074 |
| RC20−OH | 0.96427 | 0.96421 | 0.97089 | 0.97083 |
| RC21−OH | 0.96040 | 0.96036 | 0.96704 | 0.96699 |
| RC19′−OH | − | − | 0.97324 | 0.97314 |
| RC20′−OH | − | − | 0.97128 | 0.97121 |
| RC21′−OH | − | − | 0.96732 | 0.96726 |
| D4-3-2-9 | 177.05255 | 176.23593 | −176.13482 | −176.25620 |
| D4-3-15-16 | −81.30450 | −80.94205 | −78.78486 | −78.95503 |
| D1-2-7′-5′ | − | − | −86.92627 | −86.80530 |
| ECG | EGCG | A−ECG Dimer | A−EGCG Dimer | |
|---|---|---|---|---|
| ΔE(LUMO) | −0.00967 | −0.00834 | −0.01853 | −0.01882 |
| ΔE(HOMO) | −0.26608 | −0.26621 | −0.24692 | −0.24728 |
| ΔE(LUMO−HOMO) | 0.25641 | 0.25787 | 0.22839 | 0.22846 |
| ECG | EGCG | A−ECG Dimer | A−EGCG Dimer | |
|---|---|---|---|---|
| H atom of C5−OH | 0.47085 | 0.47075 | 0.50132 | 0.50142 |
| H atom of C7−OH | 0.46627 | 0.47075 | 0.48776 | 0.48819 |
| H atom of C11−OH | 0.47974 | 0.47945 | 0.48034 | 0.48096 |
| H atom of C12−OH | 0.48122 | 0.49287 | 0.48326 | 0.49387 |
| H atom of C13−OH | − | 0.48211 | − | 0.48249 |
| H atom of C19−OH | 0.48326 | 0.48274 | 0.48361 | 0.48279 |
| H atom of C20−OH | 0.49639 | 0.49591 | 0.49704 | 0.49448 |
| H atom of C21−OH | 0.48357 | 0.48314 | 0.48400 | 0.48346 |
| H atom of C19′−OH | − | − | 0.49534 | 0.49505 |
| H atom of C20′−OH | − | − | 0.50472 | 0.50315 |
| H atom of C21′−OH | − | − | 0.48661 | 0.48616 |
| ECG | EGCG | A−ECG Dimer | A−EGCG Dimer | |
|---|---|---|---|---|
| BDE(C5−OH) | 0.147299 | 0.147108 | 0.154702 | 0.154792 |
| BDE(C7−OH) | 0.154142 | 0.154006 | 0.154463 | 0.154576 |
| BDE(C11−OH) | 0.134529 | 0.137308 | − | − |
| BDE(C12−OH) | 0.150097 | 0.137218 | − | − |
| BDE(C13−OH) | − | 0.15258 | − | − |
| BDE(C19−OH) | 0.153795 | 0.154378 | − | − |
| BDE(C20−OH) | 0.140509 | 0.140191 | − | − |
| BDE(C21−OH) | 0.142142 | 0.141735 | − | − |
| BDE(C19′−OH) | − | − | 0.149704 | 0.149647 |
| BDE(C20′−OH) | − | − | 0.142017 | 0.142053 |
| BDE(C21′−OH) | − | − | 0.143425 | 0.143471 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Liu, Z.; Wu, C.; Liu, S.; Wang, D.; Hu, C.; Chen, T.; Ran, Z.; Gan, W.; Li, G. Computational Analysis on Antioxidant Activity of Four Characteristic Structural Units from Persimmon Tannin. Materials 2023, 16, 320. https://doi.org/10.3390/ma16010320
Wang Z, Liu Z, Wu C, Liu S, Wang D, Hu C, Chen T, Ran Z, Gan W, Li G. Computational Analysis on Antioxidant Activity of Four Characteristic Structural Units from Persimmon Tannin. Materials. 2023; 16(1):320. https://doi.org/10.3390/ma16010320
Chicago/Turabian StyleWang, Zhongmin, Zhigao Liu, Chenxi Wu, Songlin Liu, Dianhui Wang, Chaohao Hu, Tao Chen, Zhaojin Ran, Weijiang Gan, and Guiyin Li. 2023. "Computational Analysis on Antioxidant Activity of Four Characteristic Structural Units from Persimmon Tannin" Materials 16, no. 1: 320. https://doi.org/10.3390/ma16010320
APA StyleWang, Z., Liu, Z., Wu, C., Liu, S., Wang, D., Hu, C., Chen, T., Ran, Z., Gan, W., & Li, G. (2023). Computational Analysis on Antioxidant Activity of Four Characteristic Structural Units from Persimmon Tannin. Materials, 16(1), 320. https://doi.org/10.3390/ma16010320







