The Preparation of a Novel Hyperbranched Antifouling Material and Application in the Protection of Marine Concrete
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
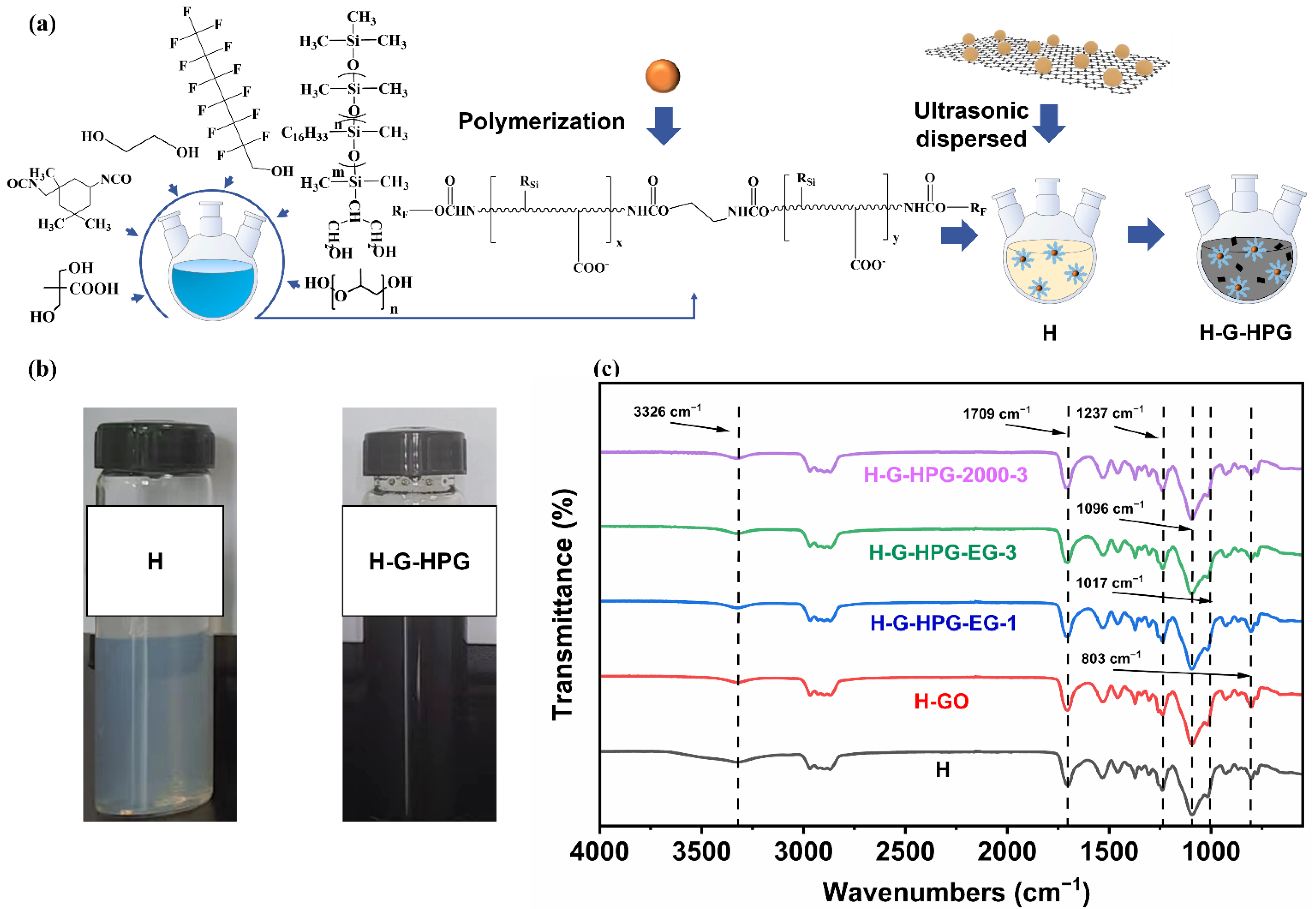
2.2. Methods of Synthesis and Preparation
2.2.1. Synthesis of Hyperbranched Polyglycerol (HPG)
2.2.2. Synthesis of Hyperbranched Waterborne Fluoro Silicone Polyurethane (H) Emulsion
2.2.3. Fabrication of HPG-Grafted GO (G-HPG)
2.2.4. Fabrication of the Hybrid Materials (H-G-HPG)
2.3. Test Methods
2.3.1. Chemical Structure
- d—spacing among GO and G-HPG sheets (nm);
- λ—0.15416 nm, the wavelength of X-ray radiation;
- θ—diffraction angle of GO and G-HPG sheets (°).
2.3.2. Hydrophobicity
2.3.3. Mechanical Properties
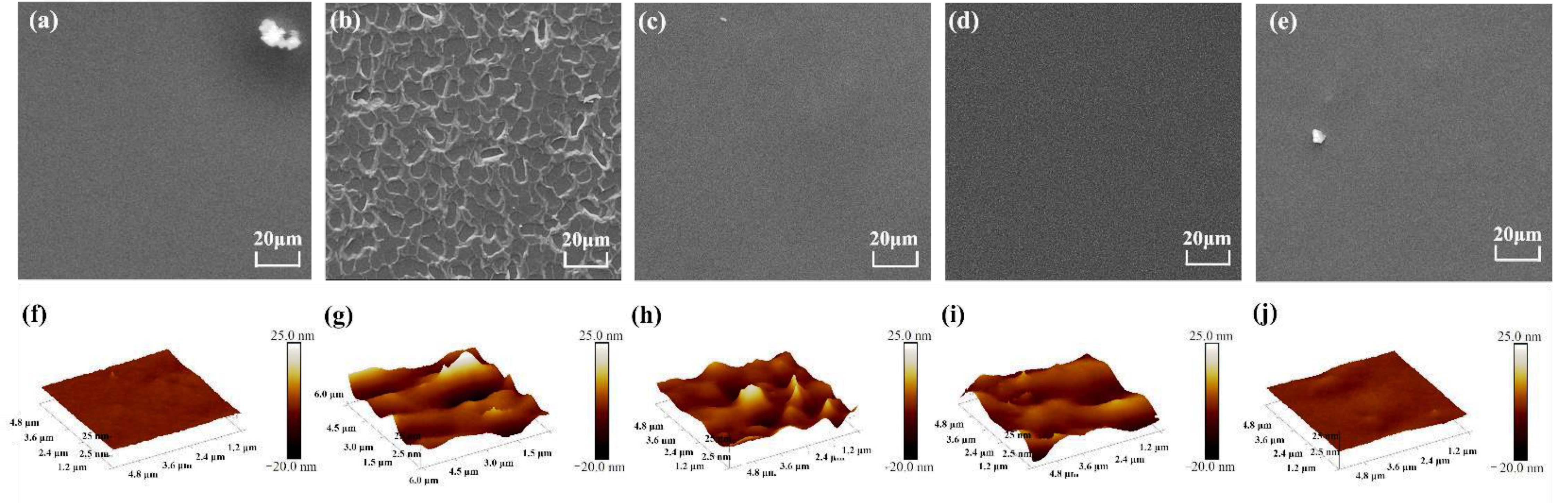
2.3.4. Surface Morphology
2.3.5. Antifouling Properties
- A
- —Absorbance at λ = 280 nm;
- A
- C—Concentration of BSA (mg/mL).
- Q—BSA absorption per square centimeter (μg/cm2);
- Ci—Concentration of BSA before the test (mg/mL);
- Ce—Concentration of BSA after the test (mg/mL);
- V—the volume of PBS solution of BSA (mL);
- S—Area of each sample piece (cm2).
- A
- —Absorbance at λ = 600 nm;
- A
- C—Concentration of P. tricornutum (CFU/mL).
- Q—P. tricornutum attachment per square centimeter (CFU/cm2);
- C0—Concentration of P. tricornutum before the test (mg/mL);
- Ci—Concentration of P. tricornutum after the test (mg/mL);
- V—the volume of P. tricornutum culture solution (ml);
- R—Radium of each sample piece (cm).
3. Results and Discussion
3.1. Characterizations of Hyperbranched Polyglycerol (HPG), HPG-Grafted GO (G-HPG), and Hybrid Materials (H-G-HPG)
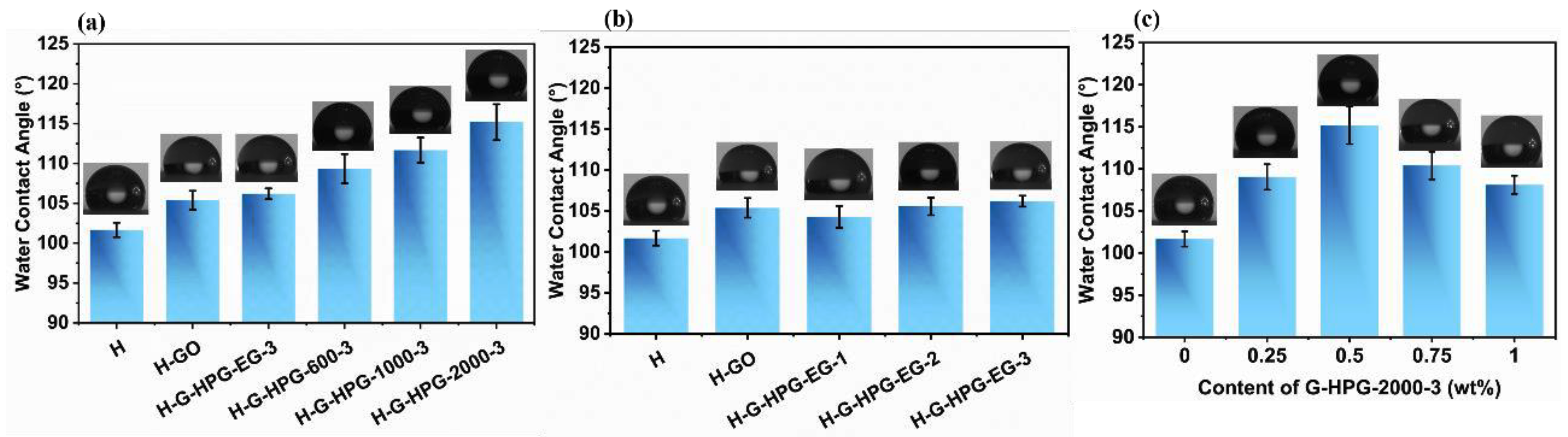
3.2. Hydrophobicity of H-G-HPG
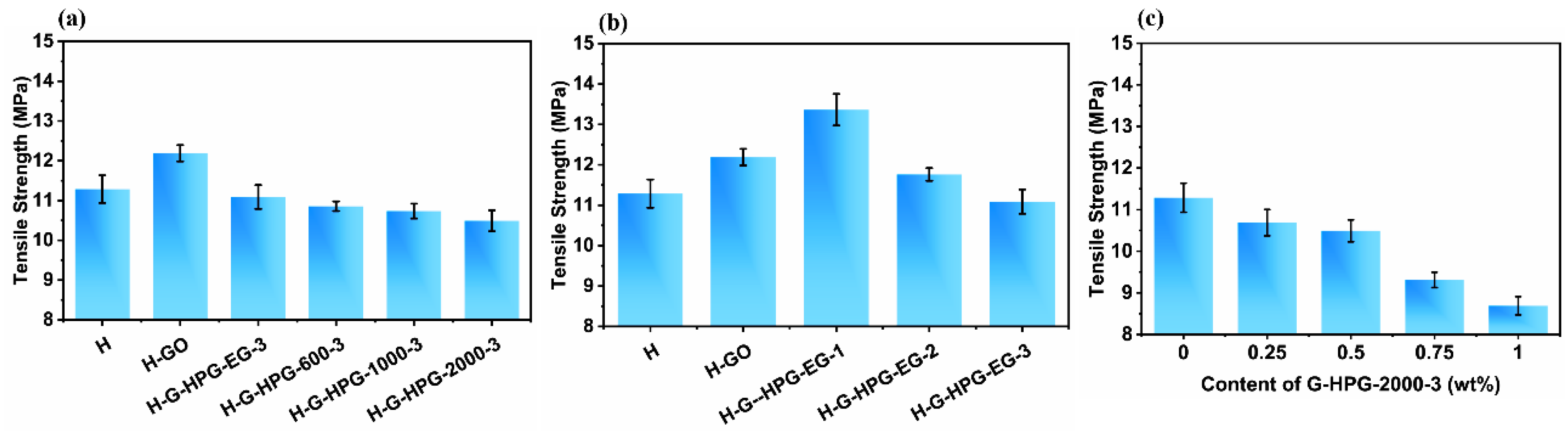
3.3. Mechanical Properties of H-G-HPG
3.4. Antifouling Properties of H-G-HPG
4. Conclusions
- (1)
- The hydrophobicity of hybrid materials can be raised by introducing 0.5 wt% G-HPG graftings hyperbranched polyglycerol with higher generation numbers and backbone molecular weights for forming ester groups, exposure of the hydrophobic center of graphene oxide sheets, and increasing agglomeration among the ether groups. The highest water contact angle of 115.19° could be reached by introducing 0.5 wt% G-HPG-2000-3 in H material.
- (2)
- The mechanical properties of hybrid materials slightly decreased by introducing 0.5 wt% G-HPG graftings hyperbranched polyglycerol with higher generation numbers and backbone molecular weights for the increasing amount of soft hyperbranched polyglycerol segments and decreasing amount of strengthening graphene oxide. The tensile strength of H-G-HPG-2000-3 decreased from 11.29 MPa to 10.49 MPa, and the pencil hardness and adhesion grade of H-G-HPG-2000-3 was same as H of 2H and grade 2
- (3)
- The antifouling properties of hybrid materials can be significantly raised by introducing 0.5 wt% G-HPG graftings hyperbranched polyglycerol with higher generation numbers and backbone molecular weights for the increase in hydrophobicity and the more significant steric hindrance among hybrid materials and biofoulings. The lowest BSA absorption of 2.33 μg/cm2 and P. tricornutum attachment of 1.289 × 104 CFU/cm2 could be reached by introducing 0.5 wt% G-HPG-2000-3 in H materials.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qiu, H.; Feng, K.; Gapeeva, A.; Meurisch, K.; Kaps, S.; Li, X.; Yu, L.; Mishra, Y.K.; Adelung, R.; Baum, M. Functional polymer materials for modern marine biofouling control. Prog. Polym. Sci. 2022, 127, 101516. [Google Scholar] [CrossRef]
- Chen, L.; Duan, Y.; Cui, M.; Huang, R.; Su, R.; Qi, W.; He, Z. Biomimetic surface coatings for marine antifouling: Natural antifoulants, synthetic polymers and surface microtopography. Sci. Total. Environ. 2021, 766, 144469. [Google Scholar] [CrossRef]
- Tan, J.; Liang, X.; Yang, J.; Zhou, S. Sol–gel-derived hard coatings from tetraethoxysilane and organoalkoxysilanes bearing zwitterionic and isothiazolinone groups and their antifouling behaviors. J. Mater. Chem. B 2021, 10, 406–417. [Google Scholar] [CrossRef] [PubMed]
- Abbina, S.; Vappala, S.; Kumar, P.; Siren, E.M.J.; La, C.C.; Abbasi, U.; Brooks, D.E.; Kizhakkedathu, J.N. Hyperbranched polyglycerols: Recent advances in synthesis, biocompatibility and biomedical applications. J. Mater. Chem. B 2017, 5, 9249–9277. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Xie, R.; Xie, Q.; Ma, C.; Zhang, G. Simultaneous realization of antifouling, self-healing, and strong substrate adhesion via a bioinspired self-stratification strategy. Chem. Eng. J. 2022, 449, 137875. [Google Scholar] [CrossRef]
- Tian, Y.; Liu, Y.; Su, Z.; Wang, S.; Zhang, B.; Zhang, H.; Zhang, Q. Biomimetic Brushlike Slippery Coatings with Mechanically Robust, Self-Cleaning, and Icephobic Properties. ACS Appl. Mater. Interfaces 2020, 12, 54041–54052. [Google Scholar] [CrossRef]
- Zhao, Y.; Xu, T.; Hu, J.-M. A robust, room-temperature curable and molecular-level superhydrophobic coating with excellent antibacterial and antifouling properties. Chem. Eng. J. 2022, 450, 136557. [Google Scholar] [CrossRef]
- Yang, J.; Li, J.; Jia, X.; Li, Y.; Song, H. Fabrication of Robust and Transparent Slippery Coating with Hot Water Repellency, Antifouling Property, and Corrosion Resistance. ACS Appl. Mater. Interfaces 2020, 12, 28645–28654. [Google Scholar] [CrossRef]
- Leonardi, A.K.; Medhi, R.; Zhang, A.; Düzen, N.; Finlay, J.A.; Clarke, J.L.; Clare, A.S.; Ober, C.K. Investigation of N-Substituted Morpholine Structures in an Amphiphilic PDMS-Based Antifouling and Fouling-Release Coating. Biomacromolecules 2022, 23, 2697–2712. [Google Scholar] [CrossRef]
- Sheng, T.; Kong, L.; Wang, Y. Crosslinking of polyimide atomic-layer-deposited on polyethersulfone membranes for synergistically enhanced performances. J. Membr. Sci. 2015, 486, 161–168. [Google Scholar] [CrossRef]
- Joshi, M.; Adak, B.; Butola, B. Polyurethane nanocomposite based gas barrier films, membranes and coatings: A review on synthesis, characterization and potential applications. Prog. Mater. Sci. 2018, 97, 230–282. [Google Scholar] [CrossRef]
- Feng, K.; Ni, C.; Yu, L.; Zhou, W.; Li, X. Synthesis and evaluation of acrylate resins suspending indole derivative structure in the side chain for marine antifouling. Colloids Surfaces B Biointerfaces 2019, 184, 110518. [Google Scholar] [CrossRef] [PubMed]
- Dafforn, K.A.; Lewis, J.A.; Johnston, E.L. Antifouling strategies: History and regulation, ecological impacts and mitigation. Mar. Pollut. Bull. 2011, 62, 453–465. [Google Scholar] [CrossRef]
- Xu, W.; Wang, W.; Hao, L.; Zhao, W.; Liu, H.; Wang, X. Effect of generation number on properties of fluoroalkyl-terminated hyperbranched polyurethane latexs and its films. J. Appl. Polym. Sci. 2020, 137, 49215. [Google Scholar] [CrossRef]
- Gogoi, S.; Karak, N. Biobased Biodegradable Waterborne Hyperbranched Polyurethane as an Ecofriendly Sustainable Material. ACS Sustain. Chem. Eng. 2014, 2, 2730–2738. [Google Scholar] [CrossRef]
- Ghosh, S.; Chatterjee, K. Poly(Ethylene Glycol) Functionalized Graphene Oxide in Tissue Engineering: A Review on Recent Advances. Int. J. Nanomed. 2020, 15, 5991–6006. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.K.; Narayan, R.; Raju, K.; Aminabhavi, T.M. Hyperbranched polyurethane (HBPU)-urea and HBPU-imide coatings: Effect of chain extender and NCO/OH ratio on their properties. Prog. Org. Coat. 2012, 74, 134–141. [Google Scholar] [CrossRef]
- Džunuzović, J.V.; Stefanović, I.S.; Džunuzović, E.S.; Dapčević, A.; Šešlija, S.I.; Balanč, B.D.; Lama, G.C. Polyurethane networks based on polycaprolactone and hyperbranched polyester: Structural, thermal and mechanical investigation. Prog. Org. Coat. 2019, 137. [Google Scholar] [CrossRef]
- Wei, Q.; Becherer, T.; Noeske, P.-L.M.; Grunwald, I.; Haag, R. A Universal Approach to Cross linked Hierarchical Polymer Multi layers as Stable and Highly Effective Antifouling Coatings. Adv. Mater. 2014, 26, 2688–2693. [Google Scholar] [CrossRef]
- Bencherif, H.; Meddour, F.; Elshorbagy, M.H.; Khalid Hossain, M.; Cuadrado, A.; Abdi, M.A.; Bendib, T.; Kouda, S.; Alda, J. Performance enhancement of (FAPbI3)1-x(MAPbBr3)x perovskite solar cell with an optimized design. Micro Nanostructures 2022, 171, 207403. [Google Scholar] [CrossRef]
- Selim, M.S.; Azzam, A.M.; Higazy, S.A.; El-Safty, S.A.; Shenashen, M.A. Novel graphene-based ternary nanocomposite coatings as ecofriendly antifouling brush surfaces. Prog. Org. Coat. 2022, 167, 106803. [Google Scholar] [CrossRef]
- Hossain, M.K.; Rubel, M.; Akbar, A.; Ahmed, M.H.; Haque, N.; Rahman, F.; Hossain, J.; Hossain, K.M. A review on recent applications and future prospects of rare earth oxides in corrosion and thermal barrier coatings, catalysts, tribological, and environmental sectors. Ceram. Int. 2022, 48, 32588–32612. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, R.; Song, D.; Yu, J.; Sun, G.; Liu, Q.; Han, S.; Liu, J.; Zhang, H.; Wang, J. Guanidine-functionalized graphene to improve the antifouling performance of boron acrylate polymer. Prog. Org. Coat. 2021, 159, 106396. [Google Scholar] [CrossRef]
- Islami, M.; Zarrabi, A.; Tada, S.; Kawamoto, M.; Isoshima, T.; Ito, Y. Controlled quercetin release from high-capacity-loading hyperbranched polyglycerol-functionalized graphene oxide. Int. J. Nanomed. 2018, 13, 6059–6071. [Google Scholar] [CrossRef] [PubMed]
- Mu, S.; Li, G.; Liang, Y.; Wu, T.; Ma, D. Hyperbranched polyglycerol-modified graphene oxide as an efficient drug carrier with good biocompatibility. Mater. Sci. Eng. C 2017, 78, 639–646. [Google Scholar] [CrossRef]
- Rafiee, Z.; Omidi, S. Modification of carbon-based nanomaterials by polyglycerol: Recent advances and applications. RSC Adv. 2021, 12, 181–192. [Google Scholar] [CrossRef]
- Wen, J.; Sun, Z.; Xiang, J.; Fan, H.; Chen, Y.; Yan, J. Preparation and characteristics of waterborne polyurethane with various lengths of fluorinated side chains. Appl. Surf. Sci. 2019, 494, 610–618. [Google Scholar] [CrossRef]
- Thomas, A.; Müller, S.S.; Frey, H. Beyond Poly(ethylene glycol): Linear Polyglycerol as a Multifunctional Polyether for Biomedical and Pharmaceutical Applications. Biomacromolecules 2014, 15, 1935–1954. [Google Scholar] [CrossRef]
- Li, P.; Sun, S.; Dong, A.; Hao, Y.; Shi, S.; Sun, Z.; Gao, G.; Chen, Y. Developing of a novel antibacterial agent by functionalization of graphene oxide with guanidine polymer with enhanced antibacterial activity. Appl. Surf. Sci. 2015, 355, 446–452. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, R.; Shi, Y.; Li, H.; Liu, Y.; Zhou, C. Synthesis and surface migration of polydimethylsiloxane and perfluorinated polyether in modified waterborne polyurethane. Polym. Bull. 2019, 76, 5517–5535. [Google Scholar] [CrossRef]
- Zhang, H.; Liang, T.; Liu, Y.; Misra, R.; Zhao, Y. Low-surface-free-energy GO/FSiAC coating with self-healing function for anticorrosion and antifouling applications. Surf. Coat. Technol. 2021, 425, 127690. [Google Scholar] [CrossRef]
- Soleyman, R.; Adeli, M. Impact of dendritic polymers on nanomaterials. Polym. Chem. 2014, 6, 10–24. [Google Scholar] [CrossRef]
- Caron, G.; Kihlberg, J.; Ermondi, G. Intramolecular hydrogen bonding: An opportunity for improved design in medicinal chemistry. Med. Res. Rev. 2019, 39, 1707–1729. [Google Scholar] [CrossRef]
- Baier, R.E. Surface behaviour of biomaterials: The theta surface for biocompatibility. J. Mater. Sci. Mater. Med. 2006, 17, 1057–1062. [Google Scholar] [CrossRef] [PubMed]




| Sample | EG (mol) | PEG600 (mol) | PEG1000 (mol) | PEG2000 (mol) | Glycerol (mol) |
|---|---|---|---|---|---|
| HPG-EG-1 | 0.1 | - | - | - | 0.2 |
| HPG-EG-2 | 0.1 | - | - | - | 0.6 |
| HPG-EG-3 | 0.1 | - | - | - | 1.4 |
| HPG-600-3 | - | 0.1 | - | - | 1.4 |
| HPG-1000-3 | - | - | 0.1 | - | 1.4 |
| HPG-2000-3 | - | - | - | 0.1 | 1.4 |
| Samples | Mn a | MW a | PDI b | DB c |
|---|---|---|---|---|
| HPG-EG-1 | 415 | 532 | 1.28 | / |
| HPG-EG-2 | 578 | 931 | 1.61 | / |
| HPG-EG-3 | 794 | 1263 | 1.59 | 0.58 |
| HPG-600-3 | 1113 | 1759 | 1.58 | 0.54 |
| HPG-1000-3 | 1621 | 2594 | 1.60 | 0.53 |
| HPG-2000-3 | 3376 | 3680 | 1.09 | 0.50 |
| Sample | Pencil Hardness | Adhesion |
|---|---|---|
| H | 2H | 2 |
| H-GO | 3H | 0 |
| H-G-HPG-EG-1 | 2H | 0 |
| H-G-HPG-EG-2 | 2H | 1 |
| H-G-HPG-EG-3 | 2H | 1 |
| H-G-HPG-600-3 | 2H | 1 |
| H-G-HPG-1000-3 | 2H | 2 |
| H-G-HPG-2000-3 | 2H | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, J.; Qi, S.; Ran, Q.; Dong, L. The Preparation of a Novel Hyperbranched Antifouling Material and Application in the Protection of Marine Concrete. Materials 2022, 15, 8402. https://doi.org/10.3390/ma15238402
Xie J, Qi S, Ran Q, Dong L. The Preparation of a Novel Hyperbranched Antifouling Material and Application in the Protection of Marine Concrete. Materials. 2022; 15(23):8402. https://doi.org/10.3390/ma15238402
Chicago/Turabian StyleXie, Junhao, Shuai Qi, Qianping Ran, and Lei Dong. 2022. "The Preparation of a Novel Hyperbranched Antifouling Material and Application in the Protection of Marine Concrete" Materials 15, no. 23: 8402. https://doi.org/10.3390/ma15238402
APA StyleXie, J., Qi, S., Ran, Q., & Dong, L. (2022). The Preparation of a Novel Hyperbranched Antifouling Material and Application in the Protection of Marine Concrete. Materials, 15(23), 8402. https://doi.org/10.3390/ma15238402





