Influence of Dy and Ho on the Phase Composition of the Ti-Al System Obtained by ‘Hydride Technology’
Abstract
1. Introduction
2. Materials and Methods
2.1. Obtaining Alloy
2.2. Research Methods
3. Results and Discussion
4. Conclusions
- The addition of 2 at.% Dy to Ti-Al system leads to the formation of the following phases: Ti (300), Ti (112), Ti3Al (402), Ti3Al (224), TiAl (301), DyAl2 (551), Dy (114), TiAl2 (Cmmm, orthorhombic), Dy3Al2 (P42nm, tetragonal), Dy3Al2 (3m, rhombohedral), Ti-Al (P4/mmm, tetragonal), Ti3Al (P63/mmc, hexagonal), Ti (Im-3m, cubic), Dy (P63/mmc, hexagonal), as well as Dy6Ti4Al43 (P63/mmc, hexagonal).
- The addition of 2 at % Ho to Ti-Al system leads to the formation of the following phases: TiAl2 (712), Ti3Al (004), Ho (114) Ho (203), HoAl3 (2215), HoAl2 (551), Ho3Al2 (P42nm, tetragonal), Ho6Ti4Al43 (P63/mmc, hexagonal), TiAl (P4/mmm, tetragonal) and Ti3Al (P63/mmc, hexagonal).
- A crystallographic database of stable and quasistable structures of the known elemental composition was created in the USPEX-SIESTA software by means of an evolutionary code. The calculations show that adding REM leads to a significant stabilizing effect in each Ti-Al-REM system without exception. It has been established that the lattice energies are equal to: EAl4Ti12Dy3 = −32,877.825 eV and EAl4Ti12Dy3 = −31,227.561 eV.
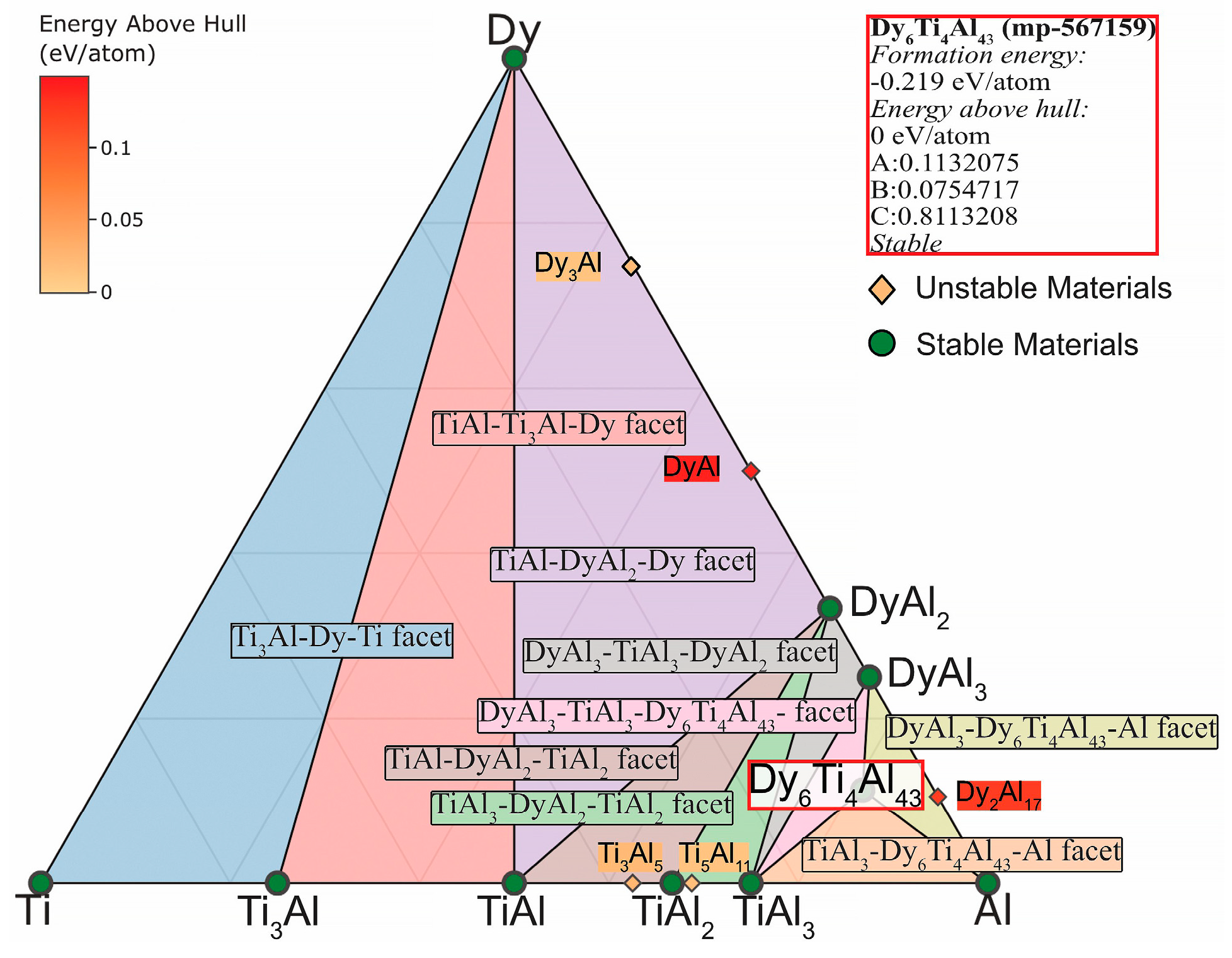
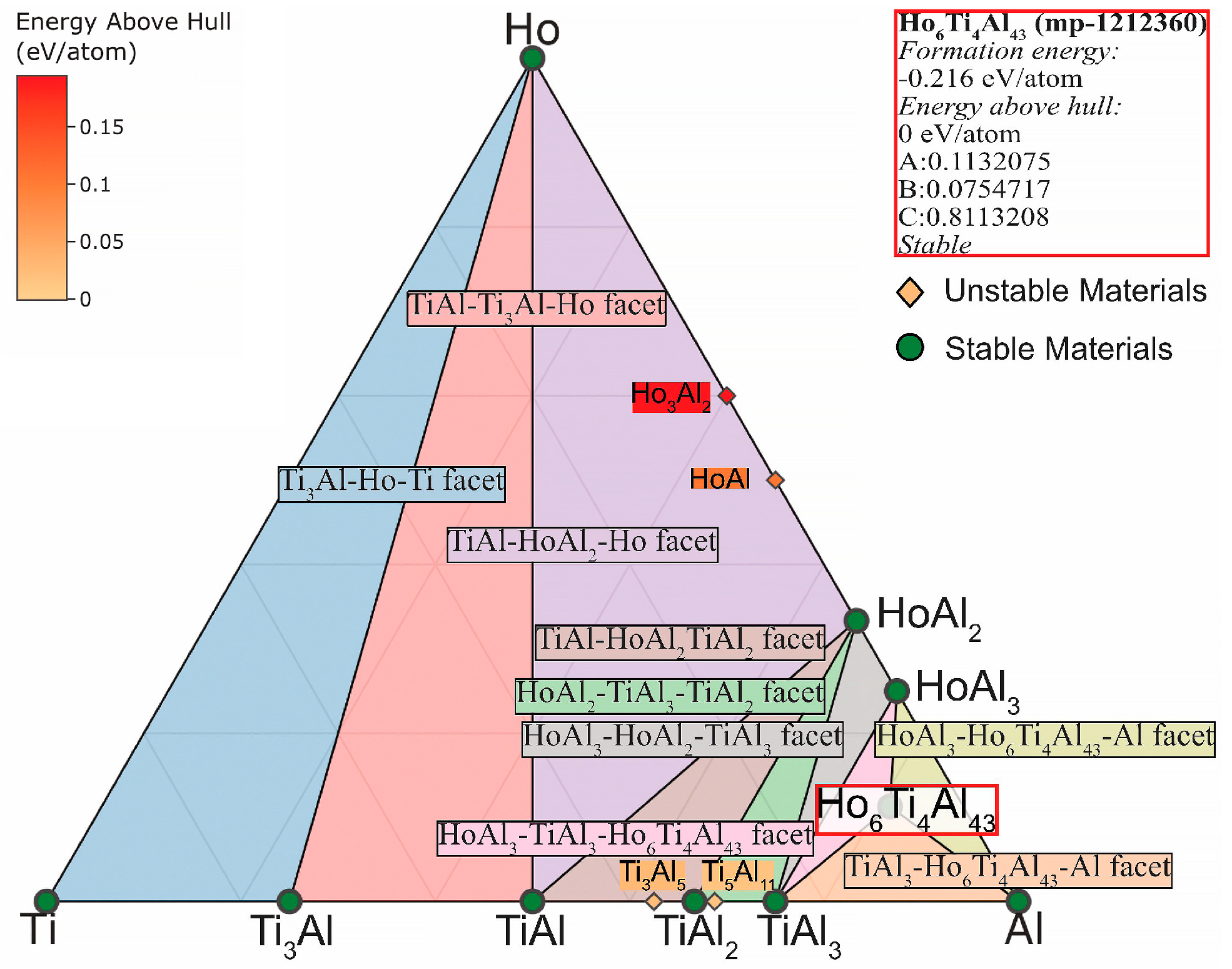
- The differences and commonality in the three-component TAD and TAH phase diagrams calculated using the Materials Project open database at a temperature of 1150 °C have been considered. The presence of ternary phases Dy₆Ti₄Al₄₃ and Ho₆Ti₄Al₄₃ with common faces MeAl3-Me₆Ti₄Al₄₃-Al, TiAl3-Me₆Ti₄Al₄₃-Al and MeAl3-TiAl3-Dy₆Ti₄Al₄₃ (Me = Dy, Ho) has been shown.
- The properties for ternary Dy₆Ti₄Al₄₃ and Ho₆Ti₄Al₄₃ phases have been theoretically calculated. The Ho₆Ti₄Al₄₃ phase has large values of predicted formation energy, Total magnetization, volume, bond length. However, the density of such phase is less.
- The addition of 2 at.% Dy increases the value of microhardness (1.61 ± 0.08 GPa) as compared with the case of adding 2 at.% Ho (1.47 ± 0.07 GPa).
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, G.; Lu, H.; Hu, X.; Lin, F.; Li, X.; Zhu, Q. Current progress in rheoforming of wrought aluminum alloys: A review. Metals 2020, 10, 238. [Google Scholar] [CrossRef]
- Bhatta, L.; Pesin, A.; Zhilyaev, A.P.; Tandon, P.; Kong, C.; Yu, H. Recent Development of Superplasticity in Aluminum Alloys: A Review. Metals 2020, 10, 77. [Google Scholar] [CrossRef]
- Berlanga-Labari, C.; Biezma-Moraleda, M.V.; Rivero, P.J. Corrosion of Cast Aluminum Alloys: A Review. Metals 2020, 10, 1384. [Google Scholar] [CrossRef]
- Wang, Q.D.; Lu, Y.Z.; Zeng, X.Q.; Ding, W.J.; Zhu, Y.P. Effects of RE microstructure and properties of AZ91 magnesium alloy. Trans. Nonferrous Met. Soc. China 2000, 10, 235–239. [Google Scholar]
- Jensen, J.; Mackintosh, A. Rare Earth Magnetism. In Structures and Excitations; Clarendon Press: Oxford, UK, 1991. [Google Scholar]
- Cheetham, A.K.; Fender, B.E.F. Neutron diffraction study of nonstoichiometric cerium hydride. J. Phys. Chem. 1972, 5, L35. [Google Scholar] [CrossRef]
- Lacaze, A.F.; Beranger, R.; Mardion, G.B.; Claudet, G.; Lacaze, A.A. Double acting reciprocating magnetic refrigerator: Recent improvements. In Advances in Cryogenic Engineering; Springer: Boston, MA, USA, 1984; pp. 573–579. [Google Scholar]
- Provenzano, V.; Shapiro, A.J.; Shull, R.D. Reduction of hysteresis losses in the magnetic refrigerant Gd5Ge2Si2 by the addition of iron. Nature 2004, 429, 853–857. [Google Scholar] [CrossRef]
- Takayama, S.; Tsutsui, N. Al-Sm and Al-Dy alloy thin films with low resistivity and high thermal stability for microelectronic conductor lines. Thin Solid Films 1996, 289, 289–294. [Google Scholar] [CrossRef]
- Ye, Y.; Li, P.; Novikov, L.S.; Avilkina, V.S.; He, L. Comparison of residual microstructures associated with impact craters in Al–Sc and Al–Ti alloys. Acta Mater. 2010, 58, 2520–2526. [Google Scholar] [CrossRef]
- Rovere, F.; Mayrhofer, P.H.; Reinholdt, A.; Mayer, J.; Schneider, J.M. The effect of yttrium incorporation on the oxidation resistance of Cr–Al–N coatings. Surf. Coat. Technol. 2008, 202, 5870–5875. [Google Scholar] [CrossRef]
- Mula, S.; Ghosh, S.; Pabi, S.K. Synthesis of an Al-based Al–Cr–Co–Ce alloy by mechanical alloying and its thermal stability. Mater. Sci. Eng. A 2008, 472, 208–213. [Google Scholar] [CrossRef]
- Pang, M.; Zhan, Y.; Du, Y. Solid state phase equilibria and intermetallic compounds of the Al–Cr–Ho system. J. Solid State Chem. 2013, 198, 344–356. [Google Scholar] [CrossRef]
- Gschneidner, K.A., Jr.; Pecharsky, V.K. Magnetocaloric materials. Annu. Rev. Mater. Res. 2000, 30, 387. [Google Scholar] [CrossRef]
- Zimm, C.; Jastrab, A.; Sternberg, A.; Pecharsky, V.; Gschneidner, K.; Osborne, M.; Anderson, I. Description and performance of a near-room temperature magnetic refrigerator. In Advances in Cryogenic Engineering; Springer: Boston, MA, USA, 1998; pp. 1759–1766. [Google Scholar] [CrossRef]
- Pecharsky, V.K.; Gschneidner, K.A., Jr. Effect of alloying on the giant magnetocaloric effect of Gd5 (Si2Ge2). J. Magn. Magn. Mater. 1997, 167, L179–L184. [Google Scholar] [CrossRef]
- Tan, Y.; Fang, H.; Chen, R.; Liu, Y.; Su, Y.; Guo, J.; Fu, H. Microalloying effects of Ho on microstructure evolution and high temperature properties of Ti46Al4Nb1Mo alloy. Intermetallics 2020, 126, 106883. [Google Scholar] [CrossRef]
- Su, Y.; Sui, Y.; Wang, X.; Cheng, J.; Wang, Y.; Liu, W.; Liu, X. Large magnetocaloric properties in single-crystal dysprosium titanate. Mater. Lett. 2012, 72, 15–17. [Google Scholar] [CrossRef]
- Sakakibara, T.; Tayama, T.; Matsuhira, K.; Takagi, S.; Hiroi, Z. Liquid–gas transition in the spin-ice dysprosium titanate. J. Magn. Magn. Mater. 2004, 272, 1312–1313. [Google Scholar] [CrossRef]
- Scharffe, S.; Kolland, G.; Valldor, M.; Cho, V.; Welter, J.F.; Lorenz, T. Heat transport of the spin-ice materials Ho2Ti2O7 and Dy2Ti2O7. J. Magn. Magn. Mater. 2015, 383, 83–87. [Google Scholar] [CrossRef]
- Lau, G.C.; Muegge, B.D.; McQueen, T.M.; Duncan, E.L.; Cava, R.J. Stuffed rare earth pyrochlore solid solutions. J. Solid State Chem. 2006, 179, 3126–3135. [Google Scholar] [CrossRef][Green Version]
- Pan, T.; Lin, C. Structural and Sensing Characteristics of Dy2O3 and Dy2TiO5 Electrolyte—Insulator—semiconductor pH Sensors. J. Phys. Chem. 2010, 114, 17914–17919. [Google Scholar] [CrossRef]
- Lin, Y.H.; Wang, S.H.; Wu, M.H.; Pan, T.M.; Lai, C.S.; Luo, J.D.; Chiou, C.C. Integrating solid-state sensor and microfluidic devices for glucose, urea and creatinine detection based on enzyme-carrying alginate microbeads. Biosens. Bioelectron. 2013, 43, 328–335. [Google Scholar] [CrossRef]
- Chen, F.; Pan, T. Physical and Electrical Properties of Dy2O3 and Dy2TiO5 Metal Oxide—High- k Oxide—Silicon-Type Nonvolatile Memory Devices. J. Electron. Mater. 2012, 41, 2197–2203. [Google Scholar] [CrossRef]
- Mrázek, J.; Potel, M.; Buršík, J.; Mráček, A.; Kallistová, A.; Jonášová, Š.; Kašík, I. Sol-gel synthesis and crystallization kinetics of dysprosium-titanate Dy2Ti2O7 for photonic applications. Mater. Chem. Phys. 2015, 168, 159–167. [Google Scholar] [CrossRef]
- Renuka, N.K.; Akhila, A.K. Preparation and photocatalytic activity of anatase titania modified with dysprosium oxide. J. Chem. Pharm. Sci. 2016, 1, 79–84. [Google Scholar]
- Gschneidner, K.A.; Pecharsky, V.K.; Tsokol, A.O. Recent developments in magnetocaloric materials. Rep. Prog. Phys. 2005, 68, 1479. [Google Scholar] [CrossRef]
- Trung, N.T.; Biharie, V.; Zhang, L.; Caron, L.; Buschow, K.H.J.; Brück, E. From single-to double-first-order magnetic phase transition in magnetocaloric Mn 1− x Cr x CoGe compounds. Appl. Phys. Lett. 2010, 96, 162507. [Google Scholar] [CrossRef]
- Lee, W.S.; Chen, T.H.; Lin, C.F.; Chen, M.S. Impact deformation behaviour and dislocation substructure of Al–Sc alloy. J. Alloy. Comp. 2010, 493, 580–589. [Google Scholar] [CrossRef]
- Karnesky, R.A.; Dunand, D.C.; Seidman, D.N. Seidman, Evolution of nanoscale precipitates in Al microalloyed with Sc and Er. Acta Mater. 2009, 57, 4022–4031. [Google Scholar] [CrossRef]
- Abzaev, Y.A.; Lider, A.M.; Klimenov, V.A.; Laptev, R.S.; Bordulev, Y.S.; Sadritdinova, G.D.; Mikhailov, A.A. Refinement of the structure of hydrogen–vacancy complexes in titanium by the Rietveld method. Phys. Solid State. 2016, 58, 1939–1944. [Google Scholar] [CrossRef]
- Crystallography Open Database. Available online: http://www.crystallography.net/ (accessed on 22 October 2022).
- Lyakhov, A.O.; Oganov, A.R.; Stokes, H.T.; Zhu, Q. New developments in evolutionary structure prediction algorithm USPEX. Comput. Phys. Commun. 2013, 184, 1172–1182. [Google Scholar] [CrossRef]
- Aarons, J. A New CASTEP and ONETEP Geometry Optimiser. Available online: http://www.hector.ac.uk/cse/distributedcse/reports/castep-geom/castep-geom/HTML/dCSE_project.html (accessed on 20 October 2022).
- Karakchieva, N.; Lepakova, O.; Abzaev, Y.; Sachkov, V.; Kurzina, I. The Influence of Scandium on the Composition and Structure of the Ti-Al Alloy Obtained by “Hydride Technology”. Nanomaterials 2021, 11, 918. [Google Scholar] [CrossRef]
- Karakchieva, N.; Artemenko, A.; Lepakova, O.; Sachkov, V.; Kurzina, I. Influence of Yttrium on the Phase Composition of the Ti-Al System Obtained by the ‘Hydride Technology’. Metals 2022, 12, 1481. [Google Scholar] [CrossRef]
- Zhou, H.; Liu, W.; Yuan, S.; Yan, J. The 500 °C isothermal section of the Al–Dy–Ti ternary system. J. Alloys Compd. 2002, 336, 218–221. [Google Scholar] [CrossRef]
- Raghavan, V. Al-Ho-Ti (Aluminum-Holmium-Titanium). J. Phase Equilibria Diffus. 2005, 26, 184. [Google Scholar] [CrossRef]
- Raghavan, V. Al-Dy-Ti (Aluminum-Dysprosium-Titanium). J. Phase Equilibria Diffus. 2005, 26, 178. [Google Scholar] [CrossRef]
- Bulanova, M.V.; Fartushna, Y.V.; Meleshevich, K.A.; Samelyuk, A.V. Phase equilibria during solidification in the Ti–TiAl–DyAl2–Dy region of the Ti–Dy–Al system. Powder Metall. Met. Ceram. 2014, 52, 686–708. [Google Scholar] [CrossRef]
- Nazarova, T.; Nazarov, K.; Sergeev, S.; Imayev, V.; Imayev, R. Microstructure and mechanical properties of intermetallic γ-TiAl alloy alloyed with dysprosium. Mater. Lett. 2017, 7, 307–311. [Google Scholar] [CrossRef][Green Version]
- The Materials Project. Materials Data on Ho6Ti4Al43 by Materials Project. 2020. Available online: https://materialsproject.org/materials/mp-1212360 (accessed on 20 October 2022).
- The Materials Project. Materials Data on Dy6Ti4Al43 by Materials Project. 2020. Available online: https://materialsproject.org/materials/mp-567159 (accessed on 20 October 2022).
- Wolff, M.W.; Niemann, S.; Ebel, T.; Jeitschko, W. Magnetic properties of rare-earth transition metal aluminides R6T4Al43 with Ho6Mo4Al43-type structure. J. Magn. Magn. Mater. 2001, 223, 1–15. [Google Scholar] [CrossRef]
- Niemann, S.; Jeitschko, W. Ternary Aluminides A6T4Al43 (A= Y, Nd, Sm, Gd-Lu, and U.; T= Ti, V, Nb, and Ta) with Ho6Mo4Al43 Type Structure. J. Solid State Chem. 1995, 116, 131–135. [Google Scholar] [CrossRef]
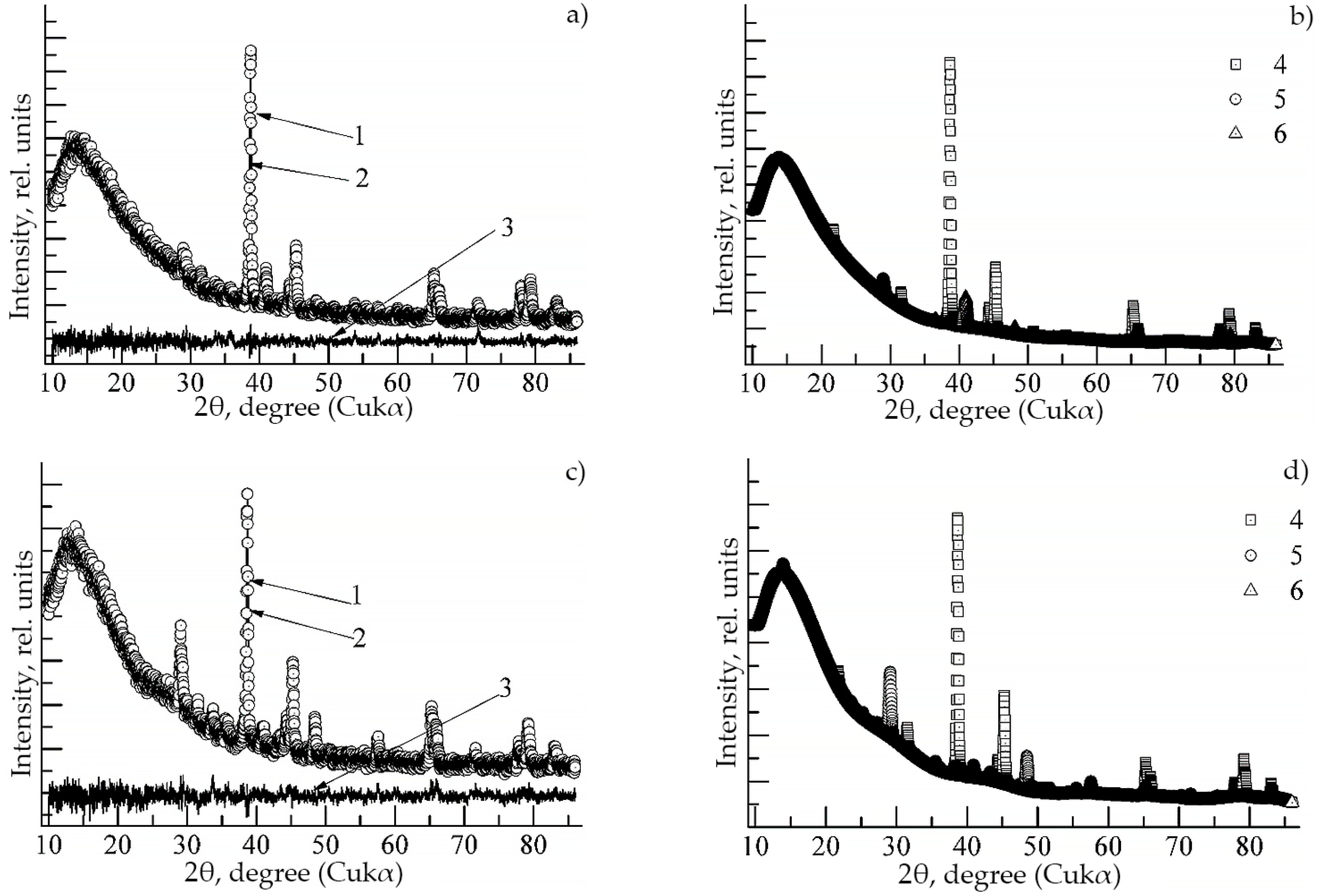

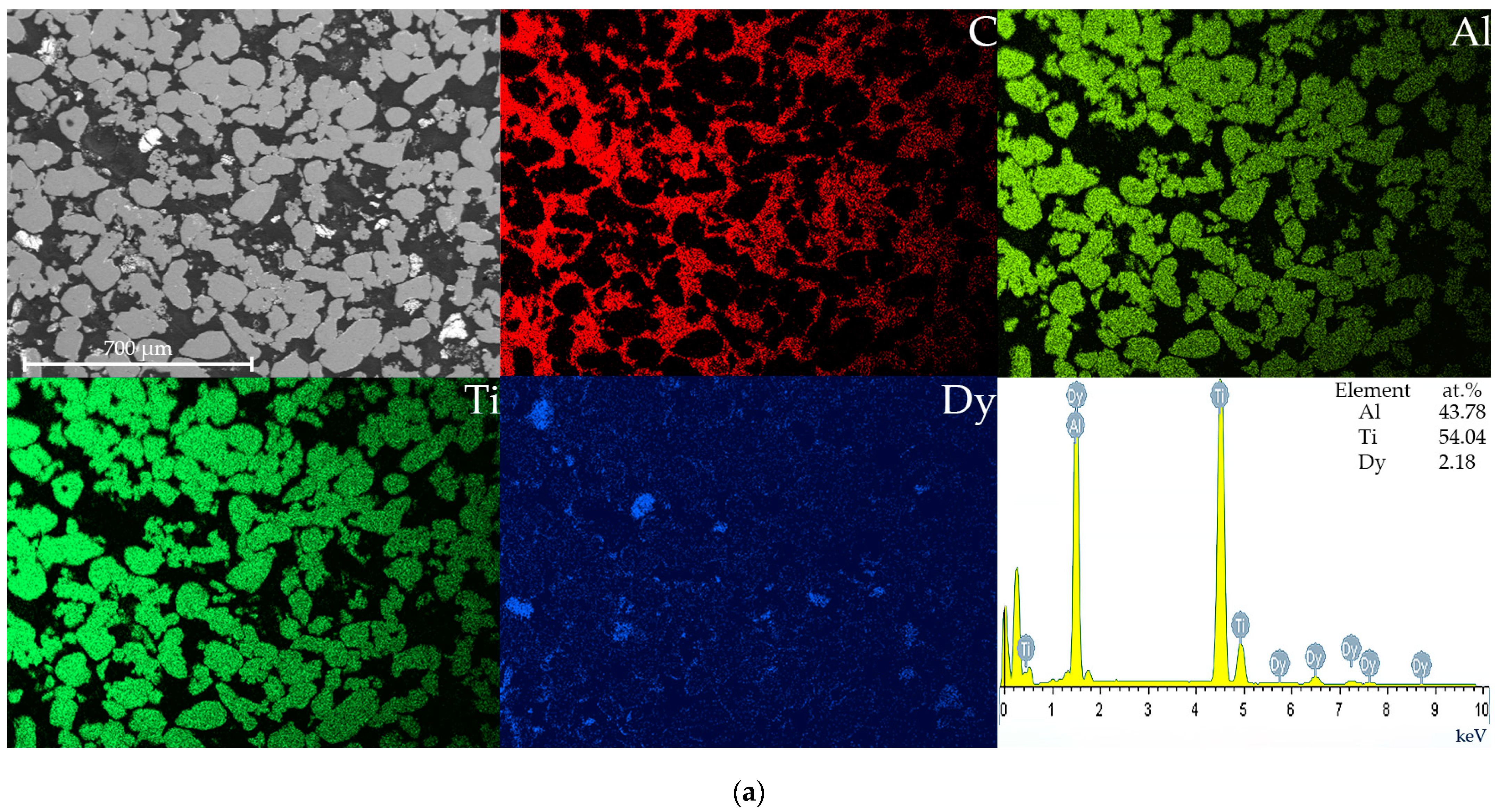

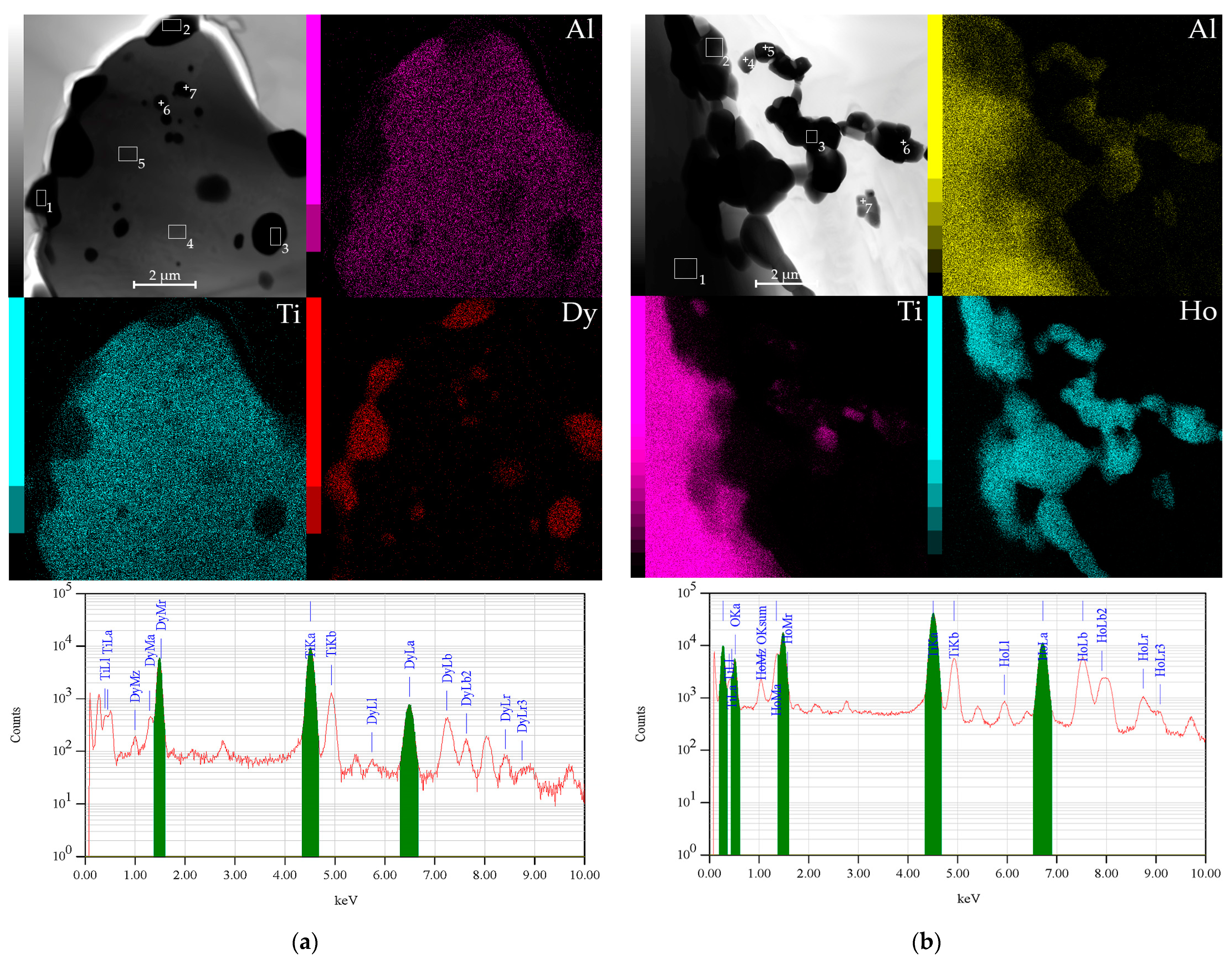
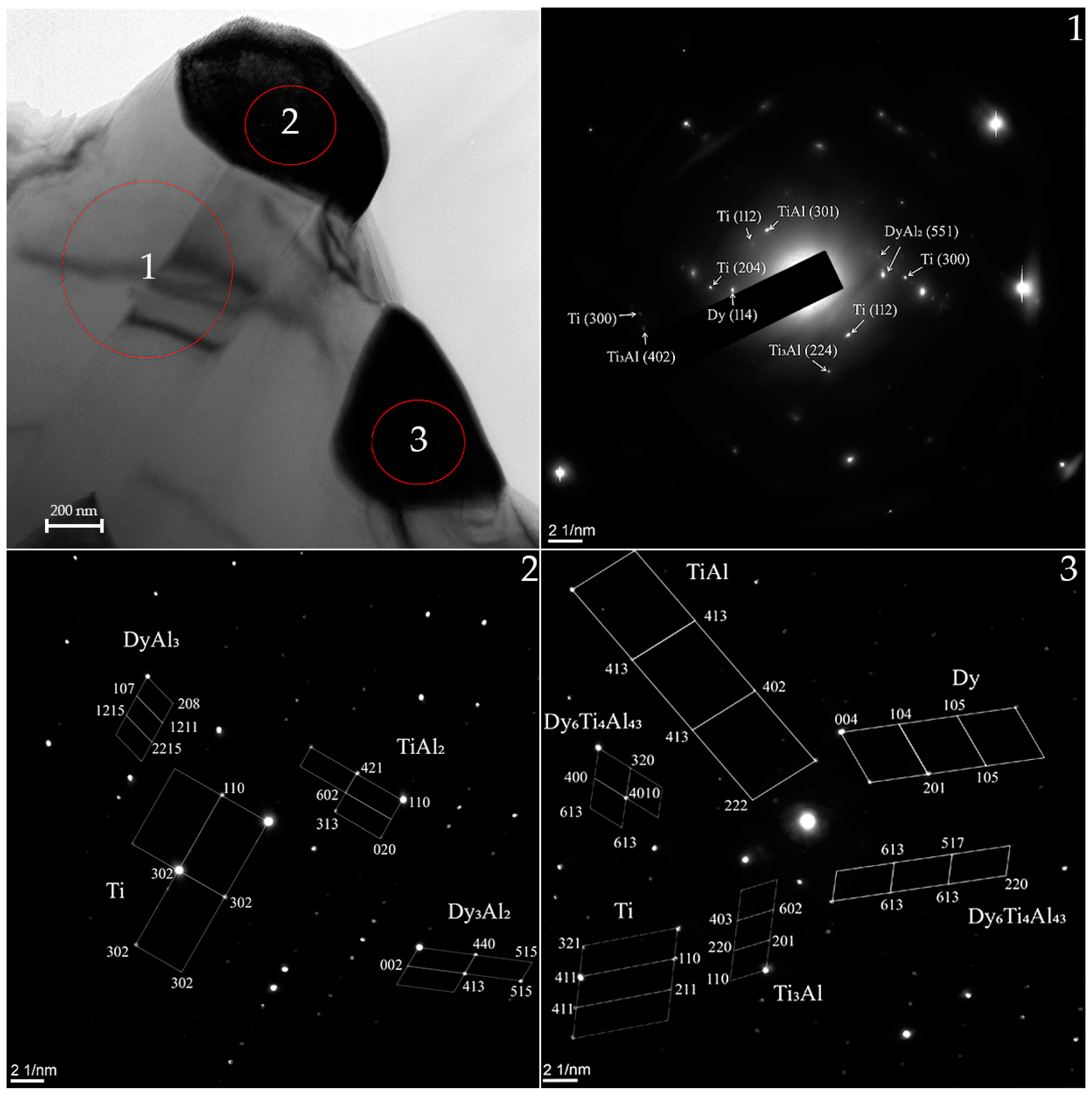
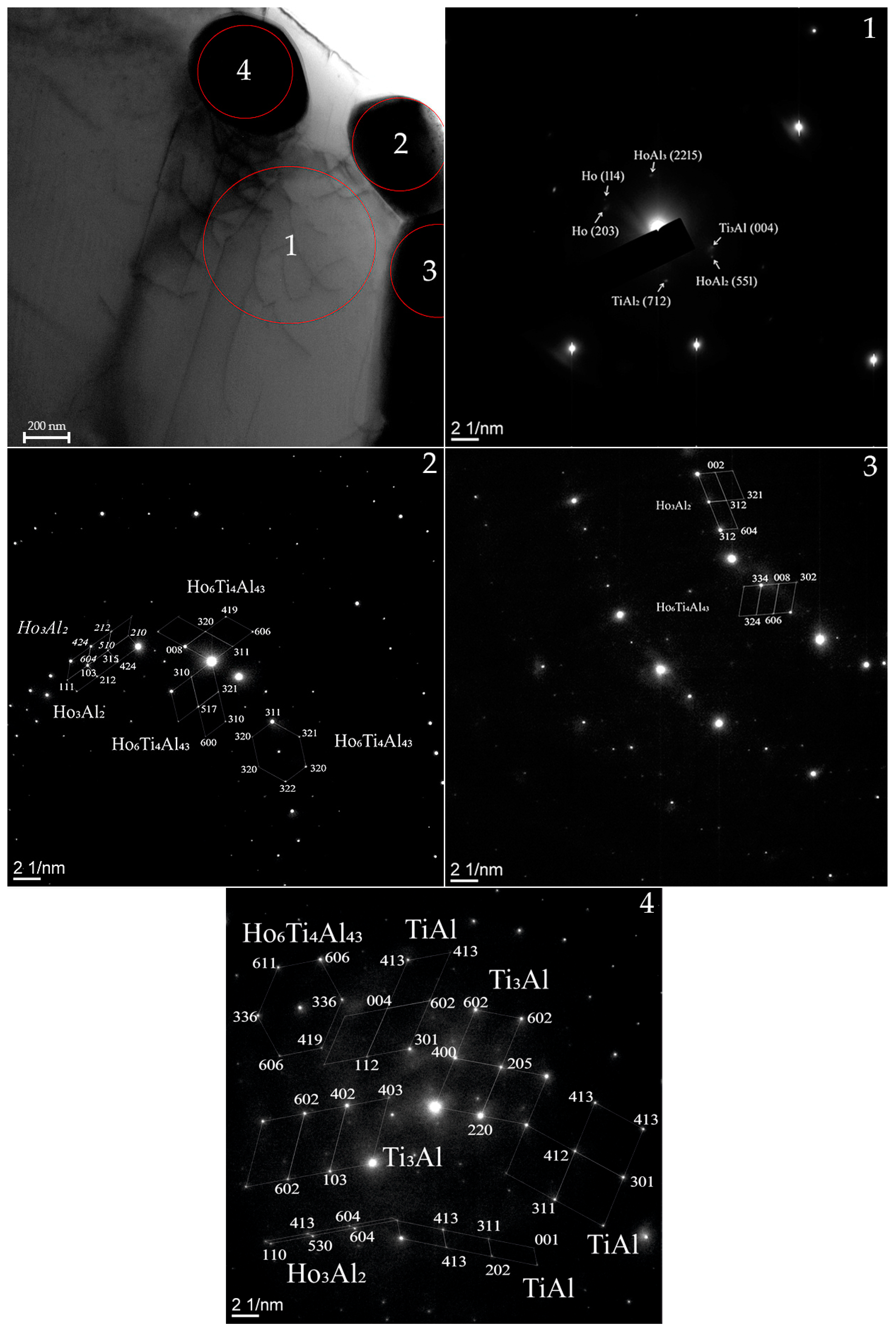
| Phase | State | a, Å | b, Å | c, Å | α | β | γ | V, Å3 | E, eV | Space Group | Fraction, % | Rwp, % |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Al-Ti | Reference | 2.837 | 2.837 | 4.059 | 90.00 | 90.00 | 90.00 | 32.677 | −1660.341 | P4/mmm, Tetragonal | 44.83 | 6.302 |
| Refined | 2.826 | 2.826 | 4.072 | 90.00 | 90.00 | 90.00 | 32.525 | |||||
| Al3Ti3 | Reference | 6.294 | 4.109 | 4.292 | 113.31 | 92.71 | 92.50 | 101.596 | −4954.206 | P1, Triclinic | 44.34 | |
| Refined | 6.267 | 4.093 | 4.288 | 113.70 | 91.93 | 92.44 | 100.44 | |||||
| Al4Ti12Ho3 | Reference | 5.764 | 5.764 | 4.664 | 90.00 | 90.00 | 120.00 | 134.20 | −32,877.825 | P6/mmm, Hexagonal | 5.55 | |
| Refined | 5.731 | 5.731 | 4.773 | 90.00 | 90.00 | 120.00 | 135.77 |
| Phase | State | a, Å | b, Å | c, Å | α | β | γ | V, Å3 | E, eV | Space Group | Fraction, % | Rwp, % |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Al-Ti | Reference | 2.837 | 2.837 | 4.059 | 90.00 | 90.00 | 90.00 | 32.677 | −1660.341 | P4/mmm, Tetragonal | 65.04 | 6.504 |
| Refined | 2.826 | 2.826 | 4.074 | 90.00 | 90.00 | 90.00 | 32.537 | |||||
| Al3Ti3 | Reference | 6.339 | 4.150 | 4.234 | 113.36 | 93.36 | 92.52 | 101.79 | −4978.606 | P1, Triclinic | 16.88 | |
| Al4Ti12Dy3 | Reference | 5.764 | 5.764 | 4.664 | 90.00 | 90.00 | 120.00 | 132.56 | −31,227.561 | P6/mmm, Hexagonal | 11.20 | |
| Refined | 5.771 | 5.771 | 4.657 | 90.00 | 90.00 | 120.00 | 134.34 |
| TAD | TAH | |||||||
|---|---|---|---|---|---|---|---|---|
| Spectrum | Element, at % | Spectrum | Element, at % | |||||
| Ti | Al | Dy | Ti | Al | Ho | O | ||
| 1 | 13.11 | 1.87 | 85.02 | 1 | 72.63 | 26.63 | 0.2 | 0.53 |
| 2 | 12.66 | 3.3 | 84.04 | 2 | 10.39 | 4.56 | 47.85 | 37.20 |
| 3 | 13.28 | 4.02 | 82.71 | 3 | 12.77 | 4.45 | 60.45 | 22.33 |
| 4 | 59.12 | 40.82 | 0.06 | 4 | 2.38 | 5.37 | 31.52 | 60.73 |
| 5 | 62.22 | 37.69 | 0.09 | 5 | 7.59 | 3.39 | 48.11 | 40.92 |
| 6 | 56.87 | 37.05 | 6.08 | 6 | 20.41 | 5.36 | 47.99 | 26.24 |
| 7 | 51.9 | 28.81 | 19.28 | 7 | 8.15 | 2.81 | 35.21 | 53.82 |
| Properties | Ho₆Ti₄Al₄₃ [42] | Dy₆Ti₄Al₄₃ [43] | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Space Group | P63/mcm (No. 193) | P63/mcm (No. 193) | ||||||||
| Predicted Formation Energy | −0.338 eV/atom | −0.340 eV/atom | ||||||||
| Magnetic Ordering | Non-magnetic | Non-magnetic | ||||||||
| Total Magnetization | 0.33 µB/f.u. | 0.41 µB/f.u | ||||||||
| Thermodynamic Stability | Yes | Yes | ||||||||
| Density | 4.12 g·cm⁻3 | 4.08 g·cm⁻3 | ||||||||
| Volume | 1887.56 Å3 | 1895.57 Å3 | ||||||||
| Bond length | Ho–Ho—3.49 Å | Dy–Dy—3.51 Å | ||||||||
| Ho–Ti—3.50 Å | Dy–Ti—3.52 Å | |||||||||
| Ho–Al—from 3.08 to 3.47 Å | Dy–Al—from 3.08 to 3.49 Å | |||||||||
| Atomic Positions | Wyckoff | Element | X | Y | Z | Wyckoff | Element | X | Y | Z |
| 2b | Ti | 0 | 0 | 0 | 2b | Ti | 0 | 0 | 1/2 | |
| 6g | Ti | 0 | 0.27 | 1/4 | 6g | Ti | 0.73 | 0.73 | 3/4 | |
| 6g | Al | 0 | 0.85 | 1/4 | 6g | Al | 0 | 0.15 | 1/4 | |
| 8h | Al | 2/3 | 1/3 | 0.13 | 8h | Al | 1/3 | 2/3 | 0.63 | |
| 12i | Al | 0.49 | 0.25 | 0 | 12i | Al | 0.75 | 0.5 | 0 | |
| 12j | Al | 0.6 | 0.45 | 1/4 | 12j | Al | 0.4 | 0.85 | 3/4 | |
| 12k | Ho | 0 | 0.47 | 0.1 | 12k | Dy | 0.53 | 0 | 0.1 | |
| 12k | Al | 0 | 0.84 | 0.62 | 12k | Al | 0 | 0.75 | 0.97 | |
| 12k | Al | 0 | 0.75 | 0.03 | 12k | Al | 0.16 | 0 | 0.62 | |
| 24l | Al | 0.39 | 0.24 | 0.16 | 24l | Al | 0.84 | 0.61 | 0.84 | |
| Vizualization | 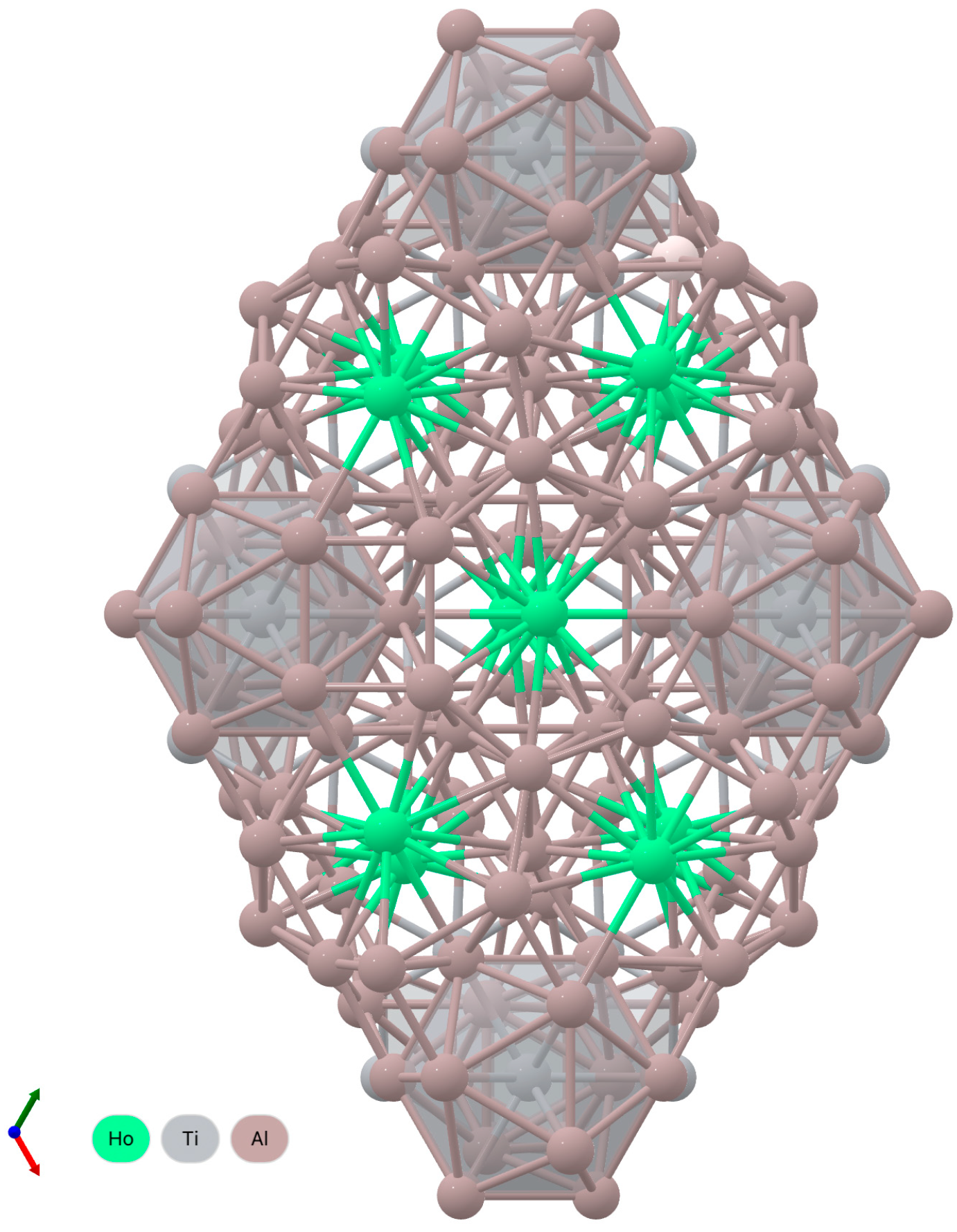 | 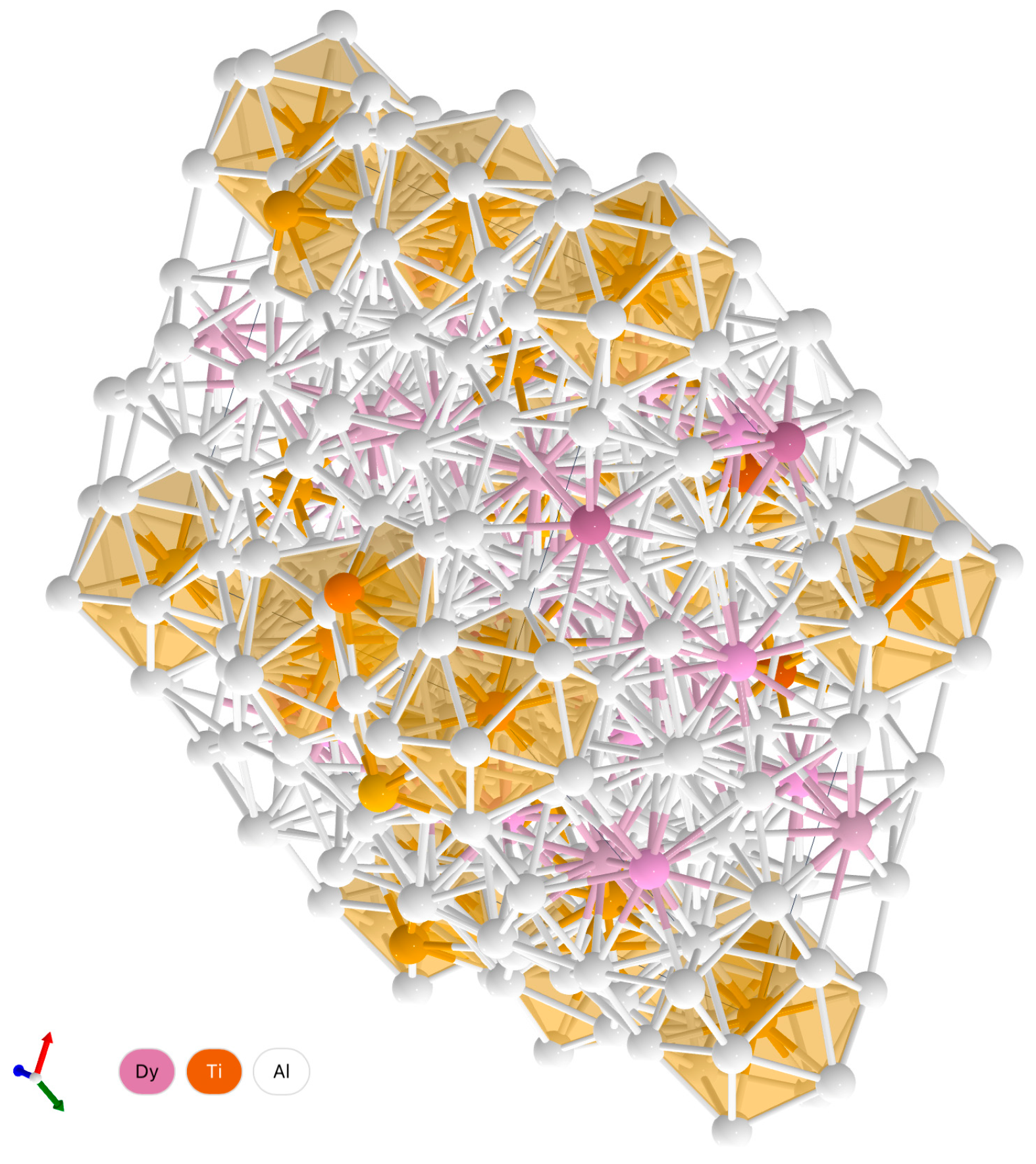 | ||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karakchieva, N.; Artemenko, A.; Sokolov, S.; Amelichkin, I.; Knyazev, A.; Vorozhtsov, A.; Abzaev, Y.; Sachkov, V.; Kurzina, I. Influence of Dy and Ho on the Phase Composition of the Ti-Al System Obtained by ‘Hydride Technology’. Materials 2022, 15, 8584. https://doi.org/10.3390/ma15238584
Karakchieva N, Artemenko A, Sokolov S, Amelichkin I, Knyazev A, Vorozhtsov A, Abzaev Y, Sachkov V, Kurzina I. Influence of Dy and Ho on the Phase Composition of the Ti-Al System Obtained by ‘Hydride Technology’. Materials. 2022; 15(23):8584. https://doi.org/10.3390/ma15238584
Chicago/Turabian StyleKarakchieva, Natalia, Alina Artemenko, Sergei Sokolov, Ivan Amelichkin, Alexey Knyazev, Alexander Vorozhtsov, Yuri Abzaev, Victor Sachkov, and Irina Kurzina. 2022. "Influence of Dy and Ho on the Phase Composition of the Ti-Al System Obtained by ‘Hydride Technology’" Materials 15, no. 23: 8584. https://doi.org/10.3390/ma15238584
APA StyleKarakchieva, N., Artemenko, A., Sokolov, S., Amelichkin, I., Knyazev, A., Vorozhtsov, A., Abzaev, Y., Sachkov, V., & Kurzina, I. (2022). Influence of Dy and Ho on the Phase Composition of the Ti-Al System Obtained by ‘Hydride Technology’. Materials, 15(23), 8584. https://doi.org/10.3390/ma15238584








