Effects of Temperature and Pressure on Corrosion Behavior of HVOF-Sprayed Fe-Based Amorphous Coating on the Mg-RE Alloy for Dissolvable Plugging Tools
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
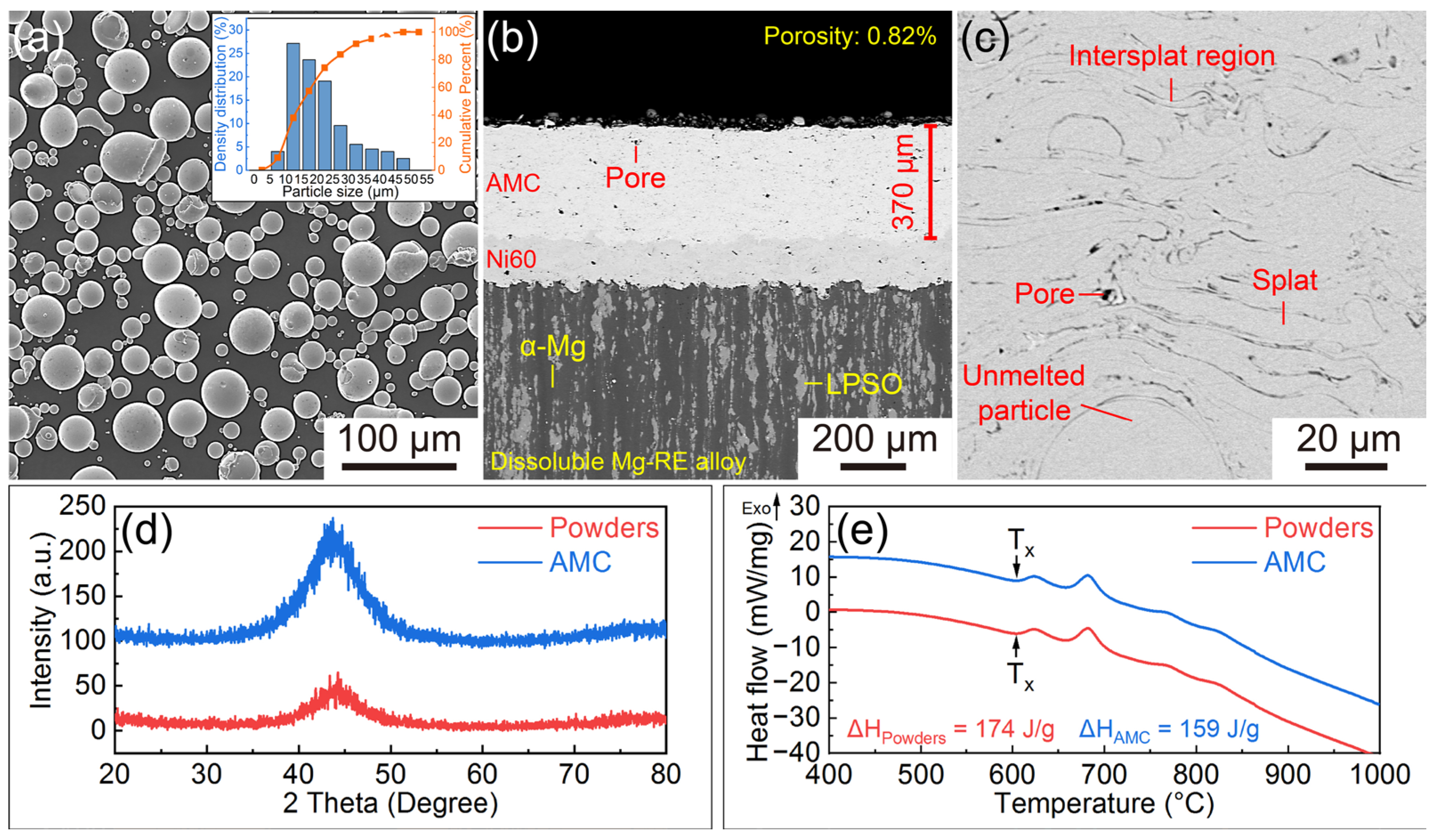
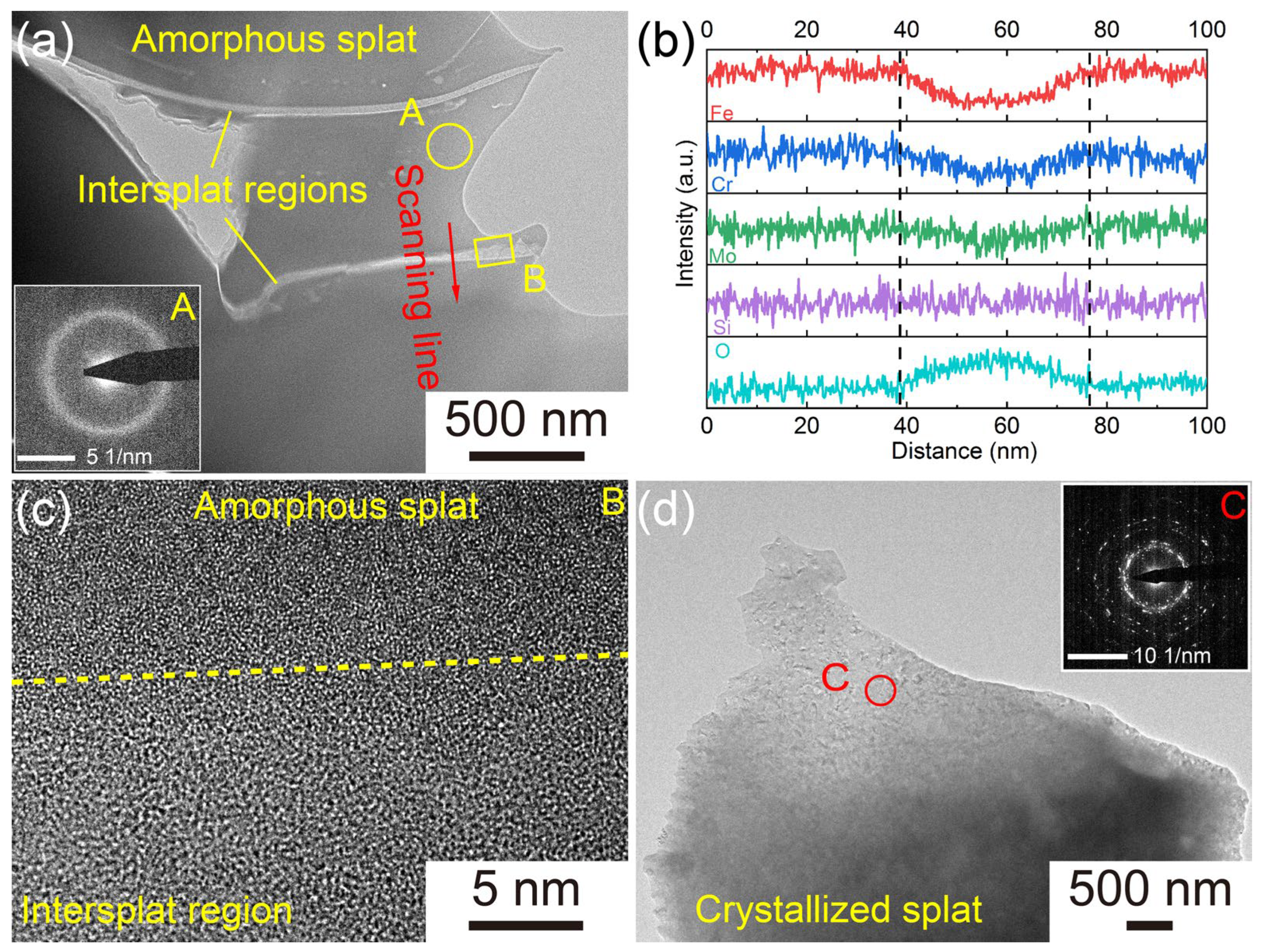
3.1. Characterization of the AMCs on Dissolvable Mg-RE Alloy
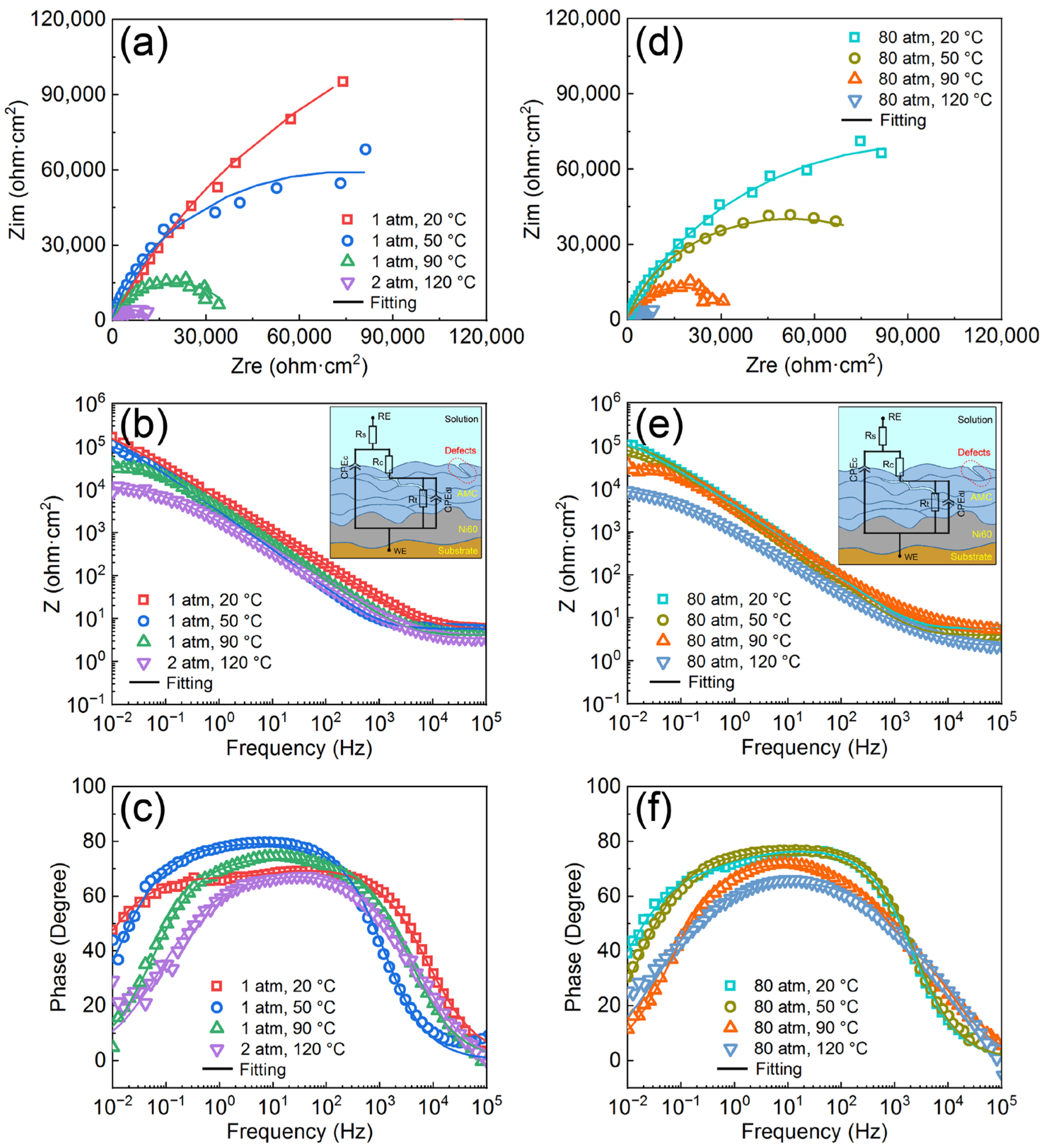
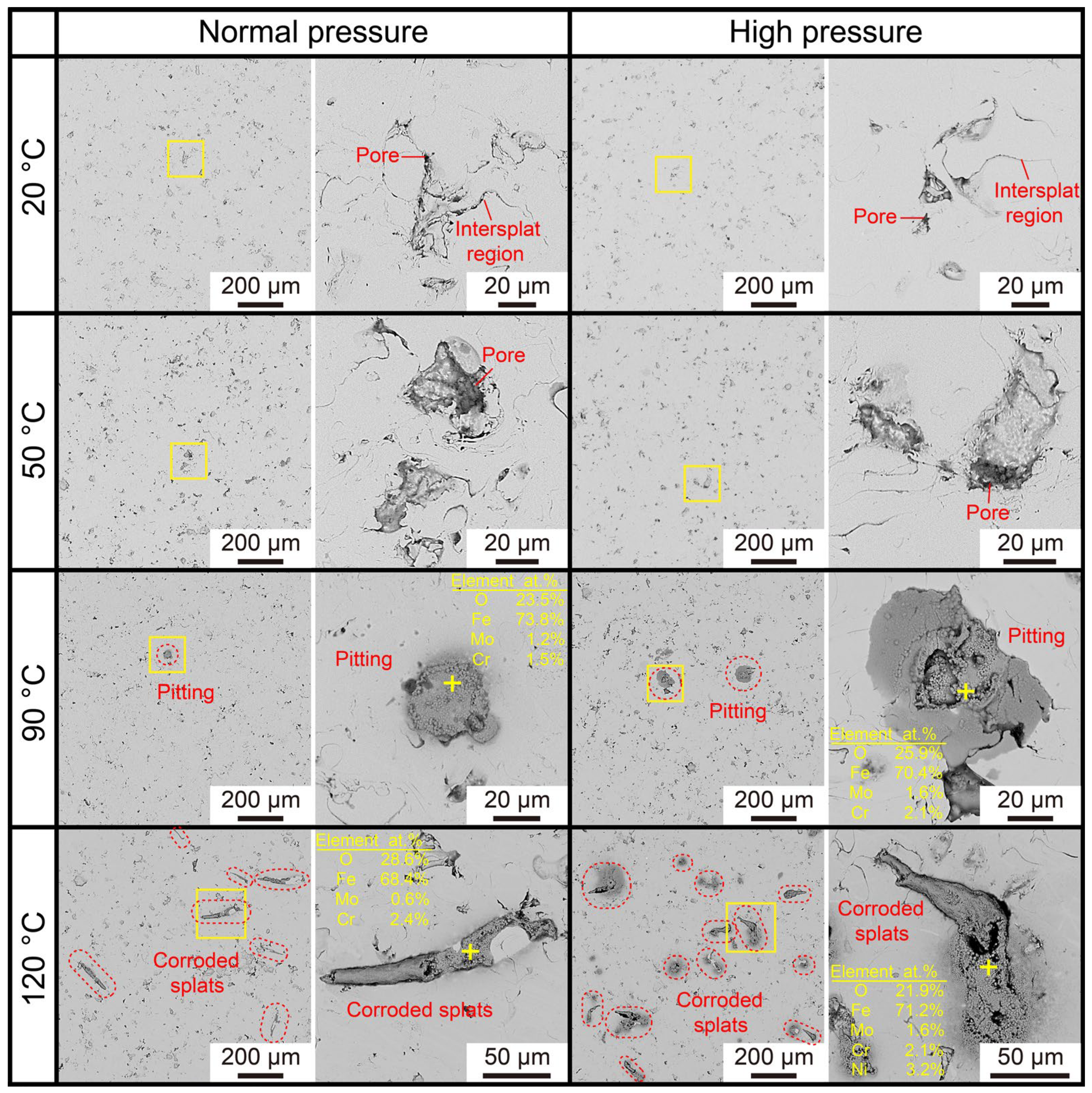
3.2. Corrosion Resistance of the AMCs on Dissolvable Mg-RE Alloy
3.3. Semiconductor Properties and Chemical Composition of Passive Films on the AMCs
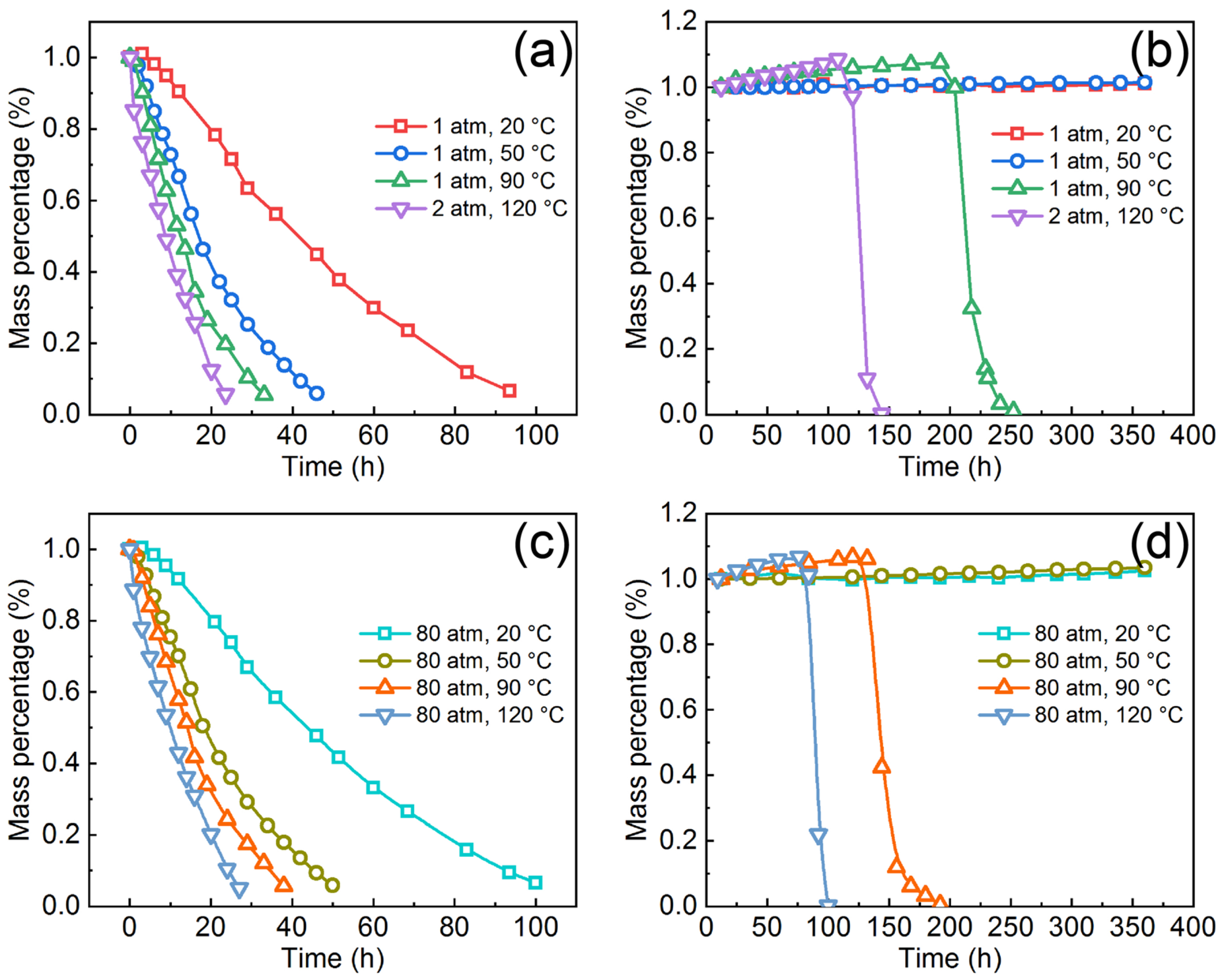
3.4. Long-Term Corrosion and Degradation of the Coated Dissolvable Mg-RE Alloy
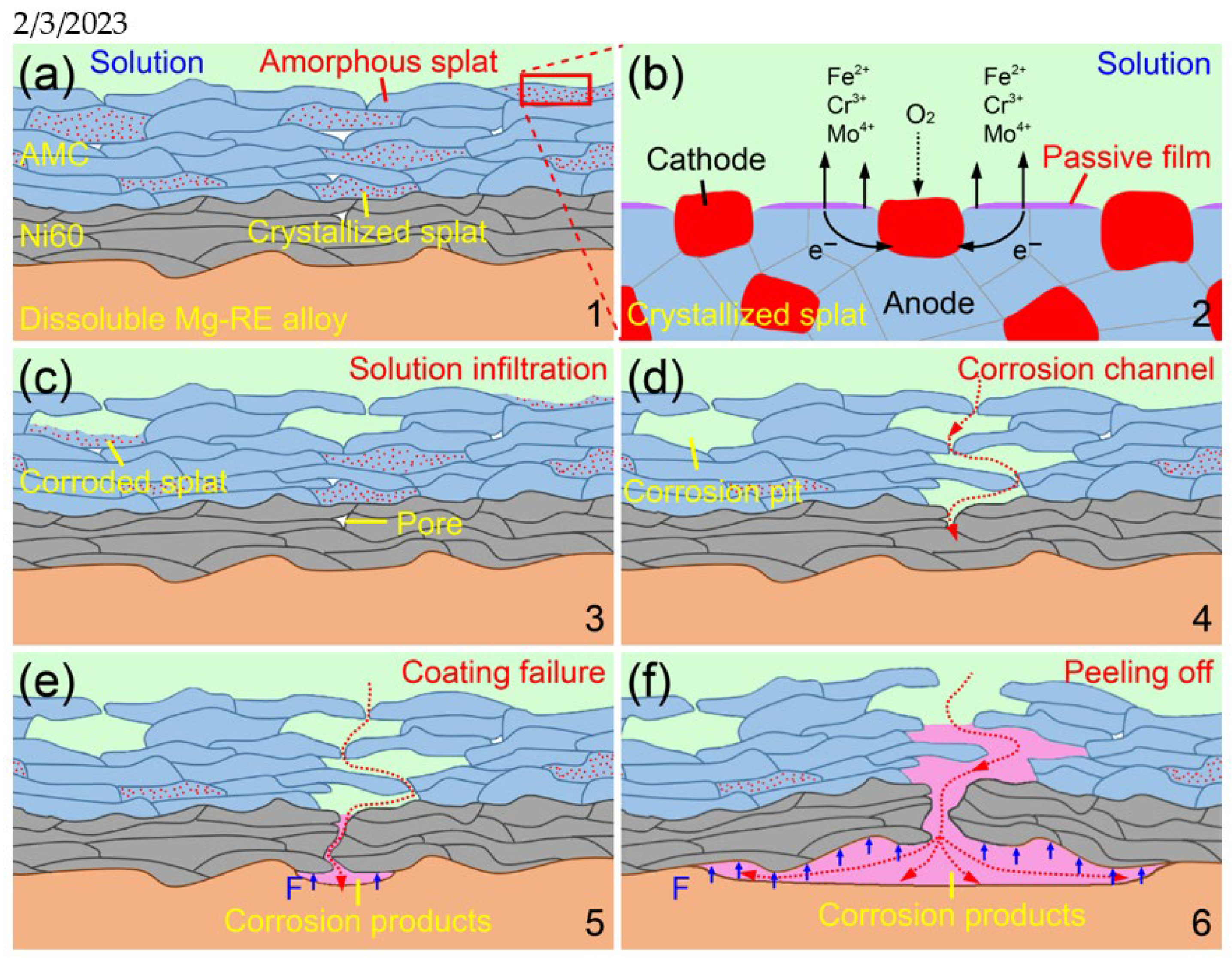
3.5. Corrosion Mechanism of the AMCs on Dissolvable Mg-RE Alloy
4. Conclusions
- (1)
- The AMCs on dissolvable Mg-RE alloy substrates possess low porosity (0.82%) and high amorphous contents (91.4%). In addition to the amorphous splats, inevitable pores and O-rich intersplat regions, the AMCs also contain a small number of crystallized splats.
- (2)
- The corrosion resistance of AMCs decreases with the increase of temperature or pressure, but the AMCs still have excellent pitting resistance. High temperature and high pressure not only reduce the compactness of the passive films formed on the coating surfaces, but also increase the chemical activities of ions and metal elements at the interfaces of solution/AMC.
- (3)
- Regardless of temperature and pressure, the degradation time of the coated Mg-RE alloy is significantly longer than that of the bare Mg-RE alloy. When the temperature is lower than 50 °C, the coated Mg-RE alloy will not be degraded within 360 h. At 120 °C and 80 atm, the degradation time of the coated Mg-RE alloy is more than 87 h, longer than that of the bare Mg-RE alloy (27 h).
- (4)
- In long-term corrosion, the crystallized splats in AMCs are corroded preferentially because of the uneven passive films and potential differences. The corroded intersplat regions act as a bridge to connect the pores and the corroded crystallized splats, resulting in the formation of corrosion channels and the degradation of dissolvable Mg-RE alloy substrates.
- (5)
- This work provides an effective solution for the corrosion protection of the plugging tools made of dissolvable magnesium alloys. However, the hydrostatic pressure of 80 atm is insufficient for real downhole environments. Research on the corrosion behaviors of AMCs and magnesium alloys at higher hydrostatic pressure or under actual operating conditions still need to be performed in the future.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kalam, S.; Afagwu, C.; Jaberi, A.J.; Siddig, O.M.; Tariq, Z.; Mahmoud, M.; Abdulraheem, A. A review on non-aqueous fracturing techniques in unconventional reservoirs. J. Nat. Gas Sci. Eng. 2021, 95, 104223. [Google Scholar] [CrossRef]
- Monsef, R.; Salavati-Niasari, M. Architecturally robust tubular nano-clay grafted Li0.9Ni0.5Co0.5O2-x/LiFeO2 nanocomposites: New implications for electrochemical hydrogen storage. Fuel 2023, 332, 126015. [Google Scholar]
- Gholami, T.; Salavati-Niasari, M.; Varshoy, S. Electrochemical hydrogen storage capacity and optical properties of NiAl2O4/NiO nanocomposite synthesized by green method. Int. J. Hydrogen Energy 2017, 42, 5235–5245. [Google Scholar]
- Zheng, C.; Liu, Y.H.; Wang, H.X.; Chen, C.; Qin, J.; Liu, Z.K.; Shen, Y. Numerical simulation of the conveyance characteristics of fracturing ball in the horizontal section. J. Nat. Gas Sci. Eng. 2016, 34, 401–411. [Google Scholar] [CrossRef]
- Zheng, C.; Liu, Y.H.; Wang, H.X.; Qin, J.; Chen, C.; Liu, Z.K.; Shen, Y. Finite element analysis and experimental study on the deformation characteristics of an aluminum alloy fracturing ball. J. Nat. Gas Sci. Eng. 2016, 35, 203–210. [Google Scholar] [CrossRef]
- Sun, J.; Du, W.B.; Fu, J.J.; Liu, K.; Li, S.B.; Wang, Z.H.; Liang, H.X. A review on magnesium alloys for application of degradable fracturing tools. J. Magnes. Alloys 2022, 10, 2649–2672. [Google Scholar]
- Liu, Y.H.; Zhang, Z.R.; Wang, J.; Li, Y.; Li, H.X.; Jia, L.Y.; Wang, J.H.; Zhang, J.S. A novel Mg-Gd-Y-Zn-Cu-Ni alloy with excellent combination of strength and dissolution via peak-aging treatment. J. Magnes. Alloys 2022. [Google Scholar] [CrossRef]
- Wang, J.; Li, T.; Li, H.X.; Ma, Y.Z.; Zhao, K.N.; Yang, C.L.; Zhang, J.S. Effect of trace Ni addition on microstructure, mechanical and corrosion properties of the extruded Mg-Gd-Y-Zr-Ni alloys for dissoluble fracturing tools. J. Magnes. Alloys 2021, 9, 1632–1643. [Google Scholar] [CrossRef]
- Saji, V.S. Review of rare-earth-based conversion coatings for magnesium and its alloys. J. Mater. Res. Technol. 2019, 8, 5012–5035. [Google Scholar]
- Saji, V.S. Organic conversion coatings for magnesium and its alloys. J. Ind. Eng. Chem. 2019, 75, 20–37. [Google Scholar] [CrossRef]
- Darband, G.B.; Aliofkhazraei, M.; Hamghalam, P.; Valizade, N. Plasma electrolytic oxidation of magnesium and its alloys: Mechanism, properties and applications. J. Magnes. Alloys 2017, 5, 4–132. [Google Scholar] [CrossRef]
- Liu, L.; Yu, S.R.; Zhu, G.; Li, Q.; Liu, E.Y.; Xiong, W.; Wang, B.Y.; Yang, X.Z. Corrosion and wear resistance of micro-arc oxidation coating on glass microsphere reinforced Mg alloy composite. J. Mater. Sci. 2021, 56, 15379–15396. [Google Scholar]
- Gray, J.E.; Luan, B. Protective coatings on magnesium and its alloys-a critical review. J. Alloys Compd. 2002, 336, 88–113. [Google Scholar] [CrossRef]
- Lu, X.Y.; Feng, X.G.; Zuo, Y.; Zhang, P.; Zheng, C.B. Improvement of protection performance of Mg-rich epoxy coating on AZ91D magnesium alloy by DC anodic oxidation. Prog. Org. Coat. 2017, 104, 188–198. [Google Scholar]
- Lu, X.Y.; Feng, X.G.; Zuo, Y.; Zheng, C.B.; Lu, S.; Xu, L. Evaluation of the micro-arc oxidation treatment effect on the protective performance of a Mg-rich epoxy coating on AZ91D magnesium alloy. Surf. Coat. Technol. 2015, 270, 227–235. [Google Scholar] [CrossRef]
- Thakur, A.; Gharde, S.; Kandasubramanian, B. Electroless nickel fabrication on surface modified magnesium substrates. Def. Technol. 2019, 15, 636–644. [Google Scholar]
- Yang, H.Y.; Guo, X.W.; Chen, X.B.; Birbilis, N. A homogenisation pre-treatment for adherent and corrosion-resistant Ni electroplated coatings on Mg-alloy AZ91D. Corros. Sci. 2014, 79, 41–49. [Google Scholar]
- Istrate, B.; Mareci, D.; Munteanu, C.; Stanciu, S.; Luca, D.; Crimu, C.I.; Kamel, E. In vitro electrochemical properties of biodegradable ZrO2-CaO coated MgCa alloy using atmospheric plasma spraying. J. Optoelectron. Adv. Mater. 2015, 17, 1186–1192. [Google Scholar]
- Wang, X.J.; Zhang, L.Y.; Zhou, X.L.; Wu, W.; Jie, X.H. Corrosion behavior of Al2O3-reinforced graphene encapsulated Al composite coating fabricated by low pressure cold spraying. Surf. Coat. Technol. 2020, 386, 125486. [Google Scholar] [CrossRef]
- Daroonparvar, M.; Bakhsheshi-Rad, H.R.; Saberi, A.; Razzaghi, M.; Kasar, A.K.; Ramakrishna, S.; Menezes, P.L.; Misra, M.; Ismail, A.F.; Sharif, S.; et al. Surface modification of magnesium alloys using thermal and solid-state cold spray processes: Challenges and latest progresses. J. Magnes. Alloys 2022, 10, 2025–2061. [Google Scholar]
- Zhang, S.D.; Zhang, W.L.; Wang, S.G.; Gu, X.J.; Wang, J.Q. Characterisation of three-dimensional porosity in an Fe-based amorphous coating and its correlation with corrosion behaviour. Corros. Sci. 2015, 93, 211–221. [Google Scholar] [CrossRef]
- Pardo, A.; Casajús, P.; Mohedano, M.; Coy, A.E.; Viejo, F.; Torres, B.; Matykina, E. Corrosion protection of Mg/Al alloys by thermal sprayed aluminium coatings. Appl. Surf. Sci. 2009, 255, 6968–6977. [Google Scholar]
- García-Rodríguez, S.; López, A.J.; Torres, B.; Rams, J. 316L stainless steel coatings on ZE41 magnesium alloy using HVOF thermal spray for corrosion protection. Surf. Coat. Technol. 2016, 287, 9–19. [Google Scholar] [CrossRef]
- Palanisamy, K.; Gangolu, S.; Antony, J.M. Effects of HVOF spray parameters on porosity and hardness of 316L SS coated Mg AZ80 alloy. Surf. Coat. Technol. 2022, 448, 128898. [Google Scholar]
- Carboneras, M.; López, M.D.; Rodrigo, P.; Campo, M.; Torres, B.; Otero, E.; Rams, J. Corrosion behaviour of thermally sprayed Al and Al/SiCp composite coatings on ZE41 magnesium alloy in chloride medium. Corros. Sci. 2010, 52, 761–768. [Google Scholar] [CrossRef]
- López, A.J.; Torres, B.; Taltavull, C.; Rams, J. Influence of high velocity oxygen-fuel spraying parameters on the wear resistance of Al-SiC composite coatings deposited on ZE41A magnesium alloy. Mater. Des. 2013, 43, 144–152. [Google Scholar] [CrossRef]
- Jonda, E.; Szala, M.; Sroka, M.; Łatka, L.; Walczak, M. Investigations of cavitation erosion and wear resistance of cermet coatings manufactured by HVOF spraying. Appl. Surf. Sci. 2023, 608, 155071. [Google Scholar] [CrossRef]
- Clavijo-Mejía, G.A.; Hermann-Muñoz, J.A.; Rincón-López, J.A.; Ageorges, H.; Muñoz-Saldaña, J. Bovine-derived hydroxyapatite coatings deposited by high-velocity oxygen-fuel and atmospheric plasma spray processes: A comparative study. Surf. Coat. Technol. 2020, 381, 125193. [Google Scholar]
- Mardali, M.; Salimijazi, H.; Karimzadeh, F.; Luthringer-Feyerabend, B. The effect of an MgO intermediate layer on a nanostructured HA coating fabricated by HVOF on an Mg alloy. Surf. Coat. Technol. 2019, 374, 1071–1077. [Google Scholar]
- Sun, Y.J.; Yang, R.; Xie, L.; Wang, W.R.; Li, Y.B.; Wang, S.L.; Li, H.X.; Zhang, J.M.; Zhang, J.S. Interfacial bonding mechanism and properties of HVOF-sprayed Fe-based amorphous coatings on LA141 magnesium alloy substrate. Surf. Coat. Technol. 2021, 426, 127801. [Google Scholar] [CrossRef]
- Zhang, H.R.; Wang, S.L.; Yang, X.L.; Hao, S.L.; Chen, Y.H.; Li, H.X.; Pan, D. Interfacial characteristic and microstructure of Fe-based amorphous coating on magnesium alloy. Surf. Coat. Technol. 2021, 425, 127659. [Google Scholar]
- Sun, Y.J.; Wang, W.C.; Li, H.X.; Xie, L.; Li, Y.B.; Wang, S.L.; Wang, W.R.; Zhang, J.M.; Zhang, J.S. High-Performance HVOF-Sprayed Fe-Based Amorphous Coating on LA141 Magnesium Alloy via Optimizing Oxygen Flow and Kerosene Flow. Materials 2021, 14, 4786. [Google Scholar]
- Guo, S.F.; Pan, F.S.; Zhang, H.J.; Zhang, D.F.; Wang, J.F.; Miao, J.; Su, C.; Zhang, C. Fe-based amorphous coating for corrosion protection of magnesium alloy. Mater. Des. 2016, 108, 624–631. [Google Scholar]
- Nayak, S.K.; Kumar, A.; Laha, T. Fe-based metallic glass coatings by thermal spraying: A focused review on corrosion properties and related degradation mechanisms. Int. Mater. Rev. 2022, 67, 1–82. [Google Scholar] [CrossRef]
- Souza, C.A.C.; Ribeiro, D.V.; Kiminami, C.S. Corrosion resistance of Fe-Cr-based amorphous alloys: An overview. J. Non-Cryst. Solids 2016, 442, 56–66. [Google Scholar]
- Zhang, C.; Chan, K.C.; Wu, Y.; Liu, L. Pitting initiation in Fe-based amorphous coatings. Acta Mater. 2012, 60, 4152–4159. [Google Scholar] [CrossRef]
- Zhang, S.D.; Wu, J.; Qi, W.B.; Wang, J.Q. Effect of porosity defects on the long-term corrosion behaviour of Fe-based amorphous alloy coated mild steel. Corros. Sci. 2016, 110, 57–70. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, Z.W.; Chen, Q.; Liu, L. Effect of hydrostatic pressure on the corrosion behavior of HVOF-sprayed Fe-based amorphous coating. J. Alloys Compd. 2018, 758, 108–115. [Google Scholar]
- Sun, H.; Wu, X.; Han, E.H. Effects of temperature on the oxide film properties of 304 stainless steel in high temperature lithium borate buffer solution. Corros. Sci. 2009, 51, 2840–2847. [Google Scholar] [CrossRef]
- Huang, J.B.; Wu, X.Q.; Han, E.H. Electrochemical properties and growth mechanism of passive films on Alloy 690 in high-temperature alkaline environments. Corros. Sci. 2010, 52, 3444–4352. [Google Scholar]
- Zhang, C.; Zhang, Z.W.; Liu, L. Degradation in pitting resistance of 316L stainless steel under hydrostatic pressure. Electrochim. Acta 2016, 210, 401–406. [Google Scholar] [CrossRef]
- Kan, B.; Wu, W.J.; Yang, Z.X.; Zhang, X.W.; Li, J.X. Effects of hydrostatic pressure and pH on the corrosion behavior of 2205 duplex stainless steel. J. Electroanal. Chem. 2021, 886, 115134. [Google Scholar] [CrossRef]
- Zhang, T.; Yang, Y.G.; Shao, Y.W.; Meng, G.Z.; Wang, F.H. A stochastic analysis of the effect of hydrostatic pressure on the pit corrosion of Fe-20Cr alloy. Electrochim. Acta 2009, 54, 3915–3922. [Google Scholar] [CrossRef]
- Ma, R.Y.; Shen, Y.F.; Wang, C.G.; Dong, J.H.; Ke, W. Effect of hydrostatic pressure on the thermodynamic and kinetic behavior of metal electrode reactions. Electrochim. Acta 2022, 424, 140617. [Google Scholar]
- Jiang, R.J.; Chen, C.F.; Zheng, S.Q. The non-linear fitting method to analyze the measured M-S plots of bipolar passive films. Electrochim. Acta 2010, 55, 2498–2504. [Google Scholar] [CrossRef]
- Wang, L.W.; Tian, H.Y.; Gao, H.; Xie, F.Z.; Zhao, K.; Cui, Z.Y. Electrochemical and XPS analytical investigation of the accelerative effect of bicarbonate/carbonate ions on AISI 304 in alkaline environment. Appl. Surf. Sci. 2019, 492, 792–807. [Google Scholar] [CrossRef]
- Yang, Z.X.; Kan, B.; Li, J.X.; Su, Y.J.; Qiao, L.J. Hydrostatic pressure effects on corrosion behavior of X70 pipeline steel in a simulated deep-sea environment. J. Electroanal. Chem. 2018, 822, 123–133. [Google Scholar]
- Wang, X.H.; Fan, L.; Ding, K.K.; Xu, L.K.; Guo, W.M.; Hou, J.; Duan, T.G. Pitting corrosion of 2Cr13 stainless steel in deep-sea environment. J. Mater. Sci. Technol. 2021, 64, 187–194. [Google Scholar] [CrossRef]
- Sun, Y.J.; Yang, R.; Xie, L.; Li, Y.B.; Wang, S.L.; Li, H.X.; Wang, W.R.; Zhang, J.S. Interfacial bonding and corrosion behaviors of HVOF-sprayed Fe-based amorphous coating on 8090 Al-Li alloy. Surf. Coat. Technol. 2022, 436, 128316. [Google Scholar] [CrossRef]
- Gan, Z.; Zhang, C.; Zhang, Z.R.; Chen, Z.J.; Liu, L. Crystallization-dependent transition of corrosion resistance of an Fe-based bulk metallic glass under hydrostatic pressures. Corros. Sci. 2021, 179, 109098. [Google Scholar]
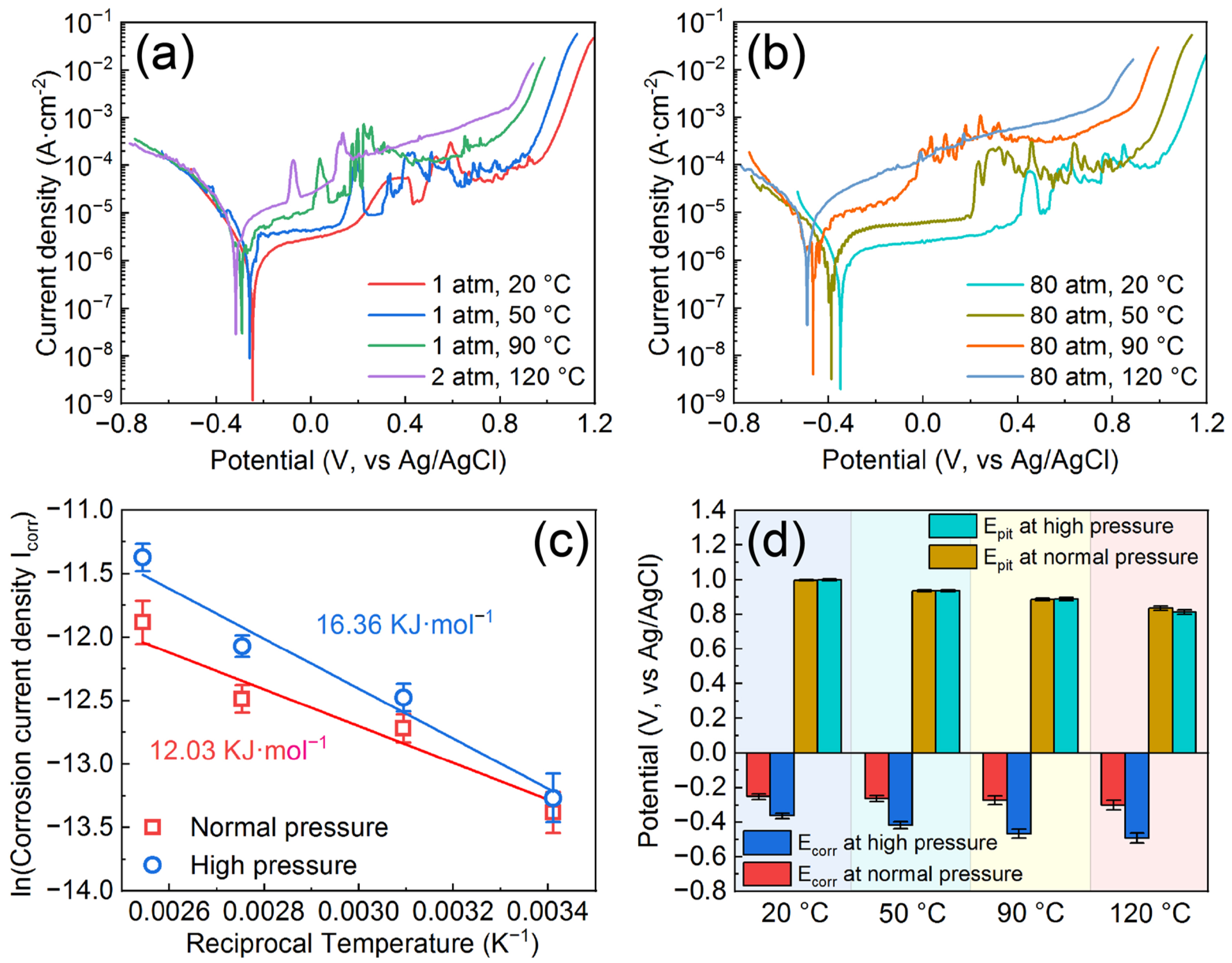
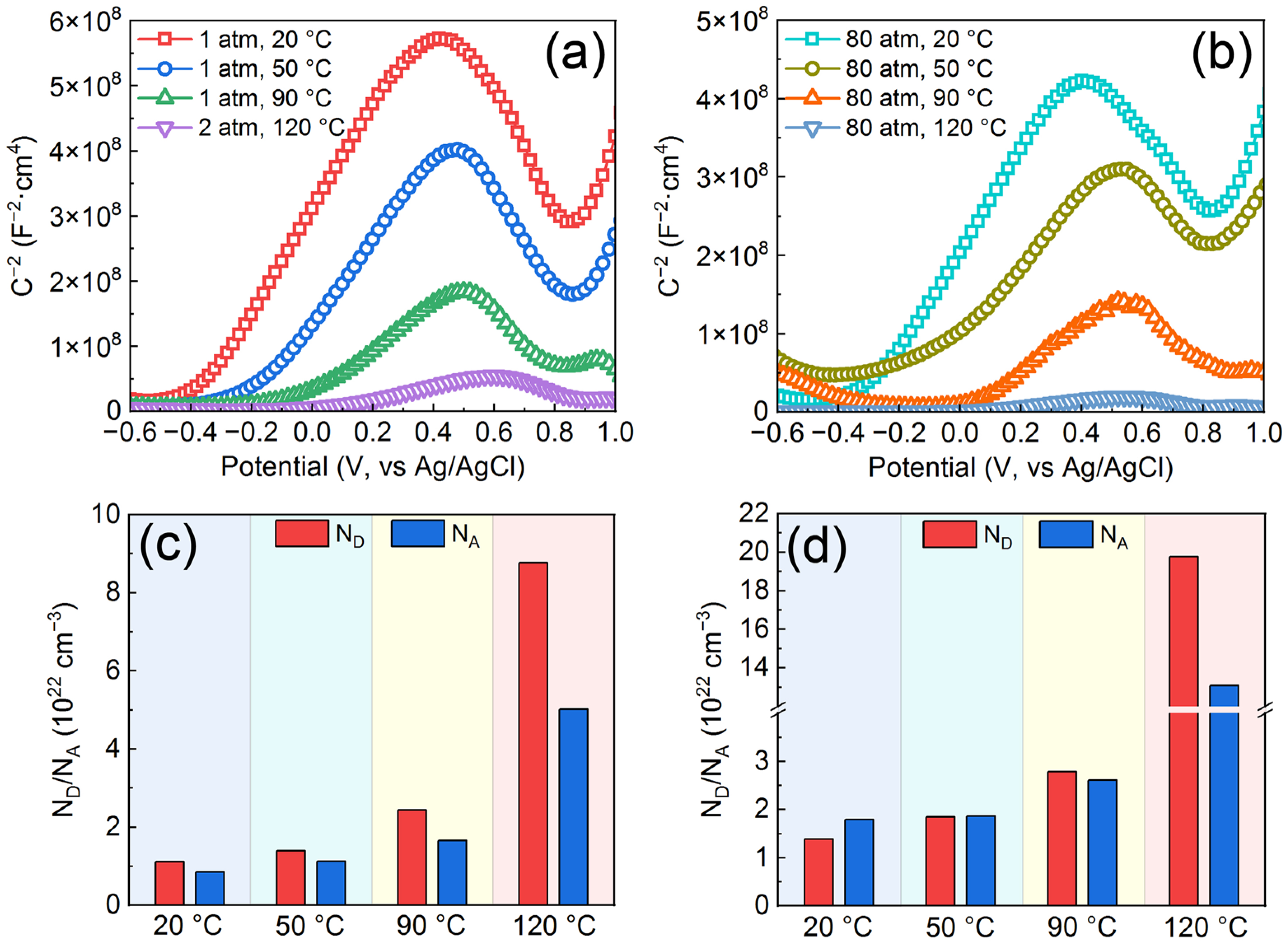
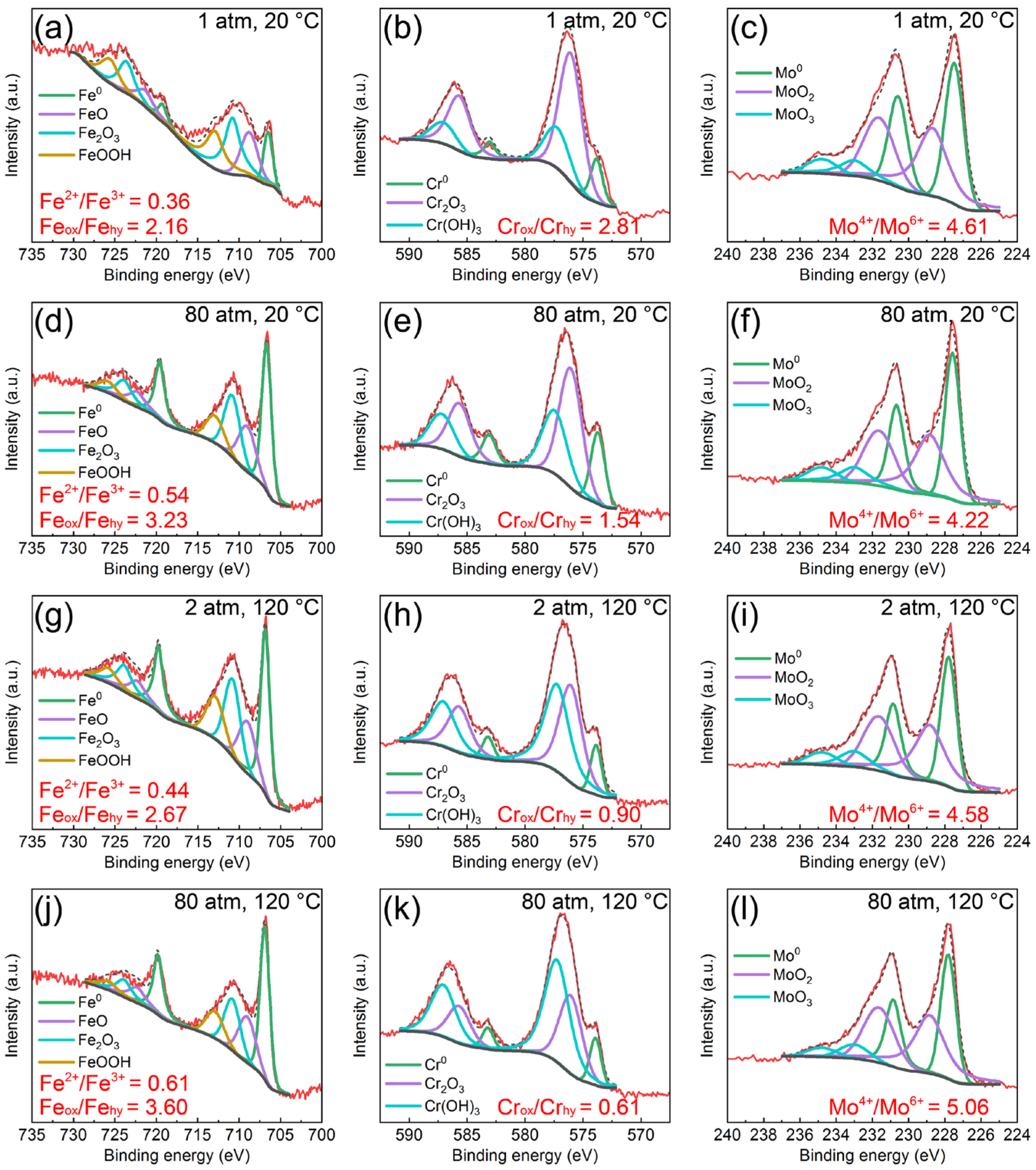
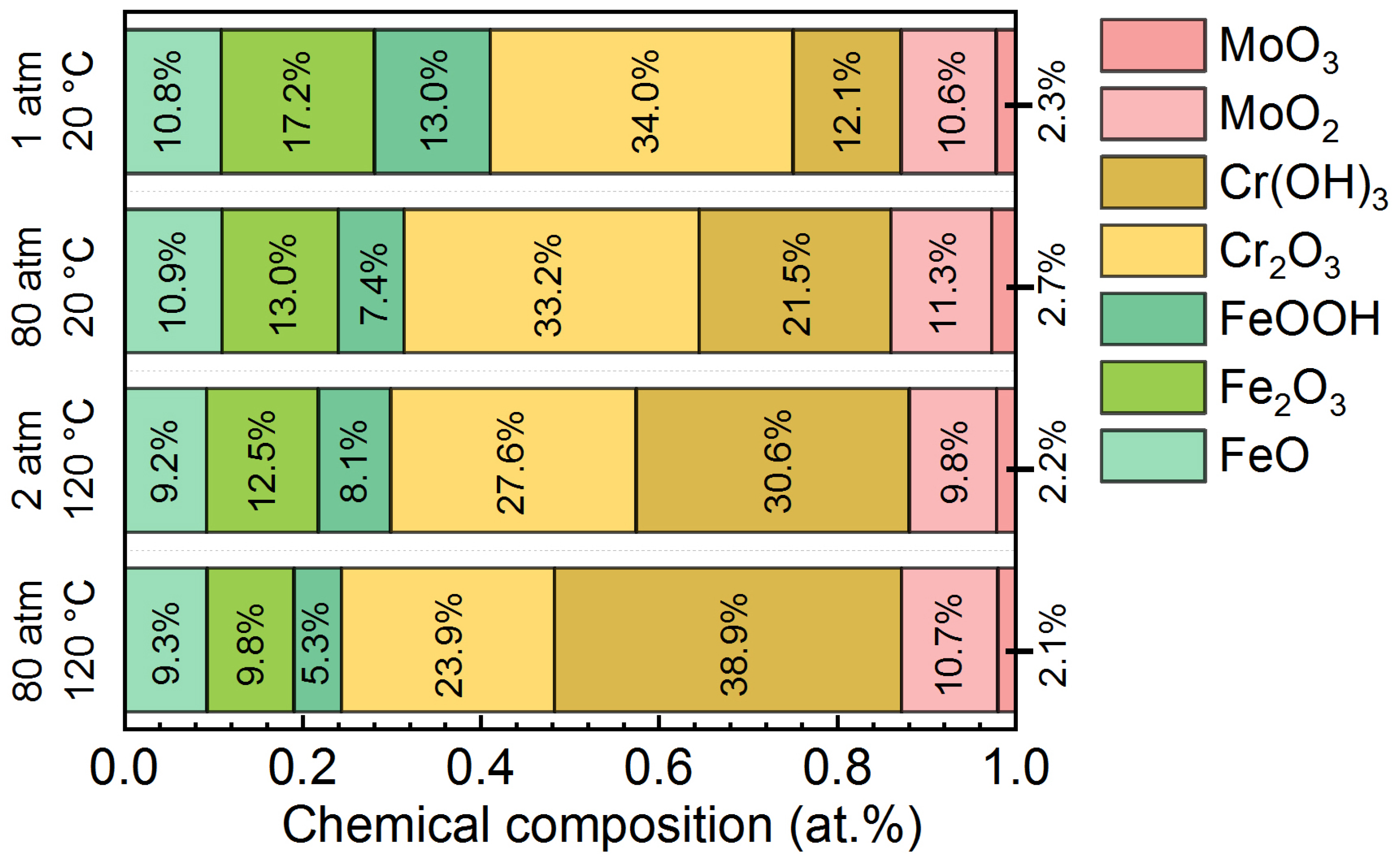
| Samples | Icorr (μA/cm2) | Ecorr (VAg/AgCl) | Ipass (μA/cm2) | Epit (VAg/AgCl) |
|---|---|---|---|---|
| 1 atm, 20 °C | 1.54 ± 0.29 | −0.252 ± 0.018 | 13.45 ± 3.54 | 0.997 ± 0.005 |
| 1 atm, 50 °C | 2.99 ± 0.37 | −0.264 ± 0.022 | 27.92 ± 3.82 | 0.936 ± 0.006 |
| 1 atm, 90 °C | 3.77 ± 0.48 | −0.274 ± 0.030 | 52.37 ± 10.33 | 0.885 ± 0.008 |
| 2 atm, 120 °C | 6.90 ± 0.99 | −0.301 ± 0.033 | 120.65 ± 24.21 | 0.834 ± 0.015 |
| 80 atm, 20 °C | 1.73 ± 0.41 | −0.364 ± 0.021 | 14.11 ± 3.12 | 0.999 ± 0.005 |
| 80 atm, 50 °C | 3.81 ± 0.45 | −0.418 ± 0.026 | 43.26 ± 6.78 | 0.937 ± 0.005 |
| 80 atm, 90 °C | 5.71 ± 0.59 | −0.467 ± 0.031 | 86.72 ± 13.94 | 0.886 ± 0.012 |
| 80 atm, 120 °C | 11.52 ± 1.63 | −0.492 ± 0.034 | 235.55 ± 41.31 | 0.812 ± 0.016 |
| Sample | Rs (Ω·cm2) | Rc (Ω·cm2) | CPEc (μF·cm−2) | CPEc-n | Rt (Ω·cm2) | CPEdl (μF·cm−2) | CPEdl-n |
|---|---|---|---|---|---|---|---|
| 1 atm, 20 °C | 5.36 ± 0.07 | 29,506 ± 1887 | 36.2 ± 1.73 | 0.78 ± 0.01 | 362,170 ± 18,027 | 7.76 ± 0.52 | 0.69 ± 0.01 |
| 1 atm, 50 °C | 5.73 ± 0.06 | 19,397 ± 640 | 55.3 ± 1.21 | 0.89 ± 0.01 | 144,720 ± 9696 | 9.5 ± 0.57 | 0.61 ± 0.01 |
| 1 atm, 90 °C | 3.95 ± 0.05 | 29.12 ± 1.13 | 28.9 ± 0.86 | 0.88 ± 0.01 | 39,389 ± 472 | 59.6 ± 0.56 | 0.83 ± 0.01 |
| 2 atm, 120 °C | 3.40 ± 0.07 | 14.29 ± 0.64 | 14.4 ± 0.96 | 0.74 ± 0.01 | 11,357 ± 227 | 12.3 ± 0.37 | 0.85 ± 0.01 |
| 80 atm, 20 °C | 5.54 ± 0.03 | 21,662 ± 1732 | 45.8 ± 0.76 | 0.86 ± 0.01 | 179,990 ± 13,679 | 17.3 ± 0.85 | 0.63 ± 0.01 |
| 80 atm, 50 °C | 4.94 ± 0.02 | 16,397 ± 836 | 56.0 ± 0.59 | 0.87 ± 0.01 | 89,779 ± 3129 | 12.1 ± 0.34 | 0.65 ± 0.01 |
| 80 atm, 90 °C | 3.25 ± 0.04 | 22.94 ± 1.08 | 32.2 ± 0.89 | 0.81 ± 0.01 | 34,556 ± 449 | 28.4 ± 0.63 | 0.81 ± 0.01 |
| 80 atm, 120 °C | 2.30 ± 0.04 | 4.12 ± 0.21 | 15.7 ± 0.54 | 0.84 ± 0.01 | 10,758 ± 215 | 15.7 ± 0.66 | 0.94 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Li, H.; Yang, J.; Zhang, J. Effects of Temperature and Pressure on Corrosion Behavior of HVOF-Sprayed Fe-Based Amorphous Coating on the Mg-RE Alloy for Dissolvable Plugging Tools. Materials 2023, 16, 1313. https://doi.org/10.3390/ma16031313
Sun Y, Li H, Yang J, Zhang J. Effects of Temperature and Pressure on Corrosion Behavior of HVOF-Sprayed Fe-Based Amorphous Coating on the Mg-RE Alloy for Dissolvable Plugging Tools. Materials. 2023; 16(3):1313. https://doi.org/10.3390/ma16031313
Chicago/Turabian StyleSun, Yijiao, Hongxiang Li, Jun Yang, and Jishan Zhang. 2023. "Effects of Temperature and Pressure on Corrosion Behavior of HVOF-Sprayed Fe-Based Amorphous Coating on the Mg-RE Alloy for Dissolvable Plugging Tools" Materials 16, no. 3: 1313. https://doi.org/10.3390/ma16031313
APA StyleSun, Y., Li, H., Yang, J., & Zhang, J. (2023). Effects of Temperature and Pressure on Corrosion Behavior of HVOF-Sprayed Fe-Based Amorphous Coating on the Mg-RE Alloy for Dissolvable Plugging Tools. Materials, 16(3), 1313. https://doi.org/10.3390/ma16031313







