Effect of Residual Oxygen Concentration on the Lattice Parameters of Aluminum Nitride Powder Prepared via Carbothermal Reduction Nitridation Reaction
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Synthesis
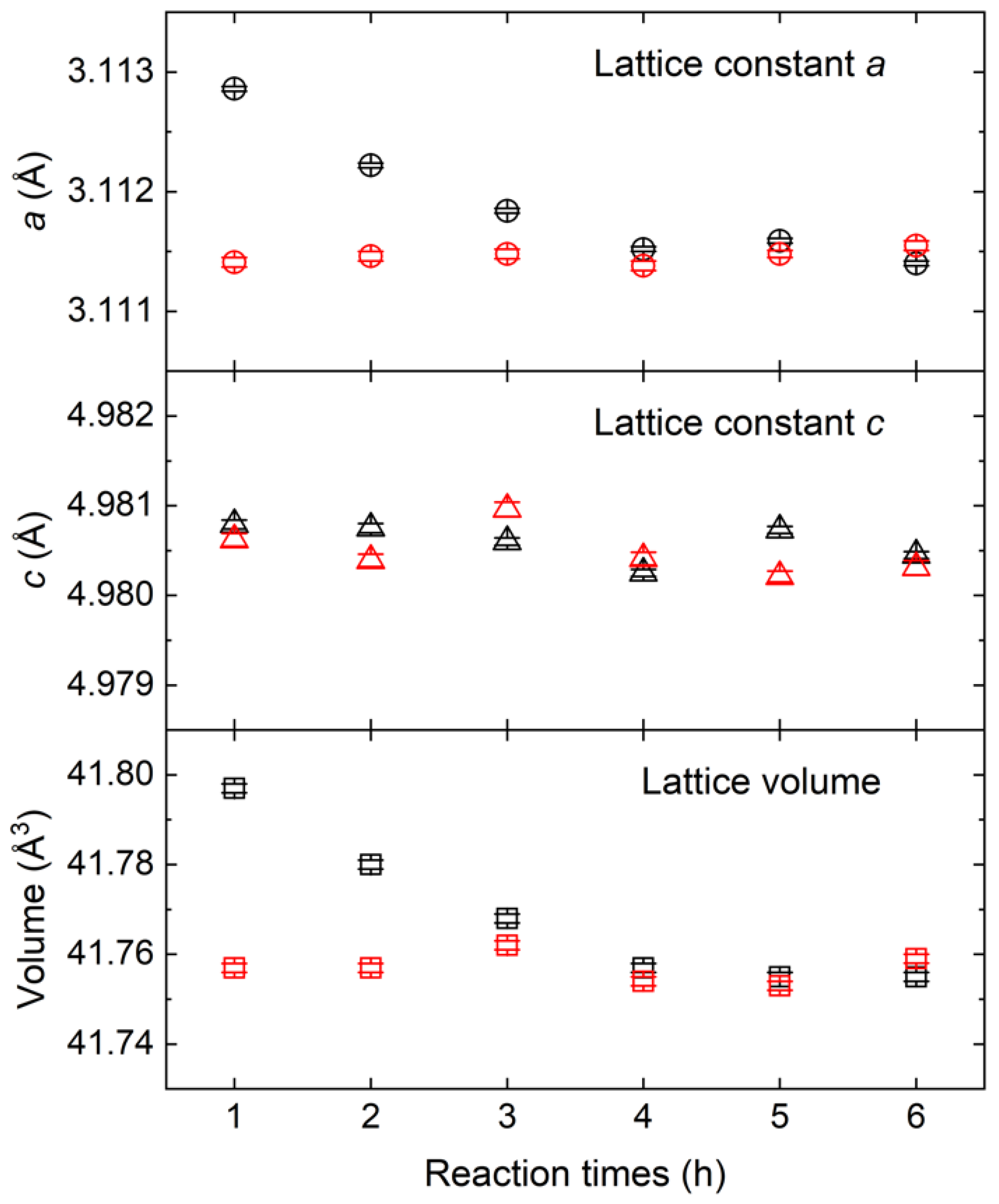
3.2. Oxygen Dissolution Effect on AlN Lattice
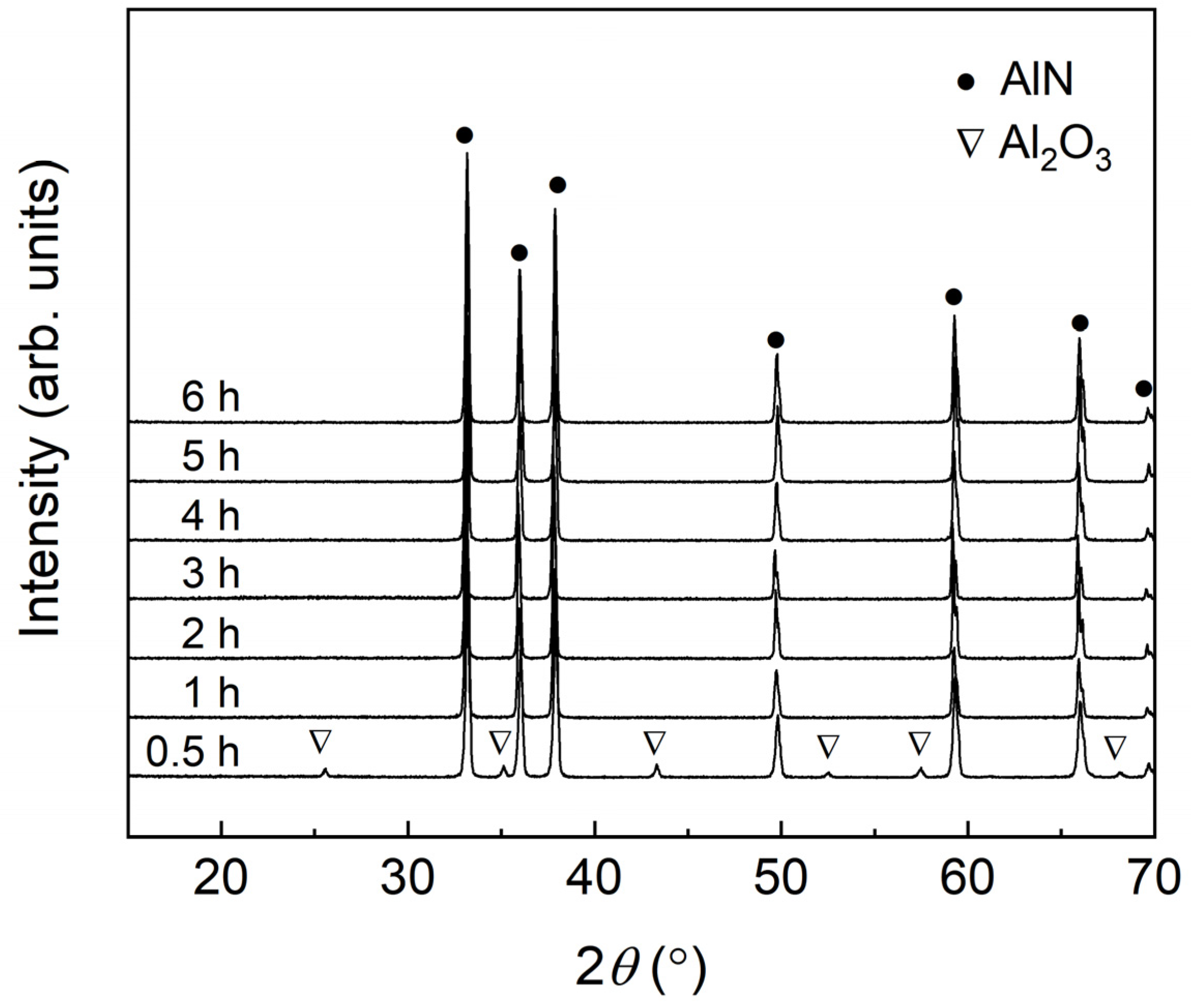
3.2.1. X-ray Diffraction
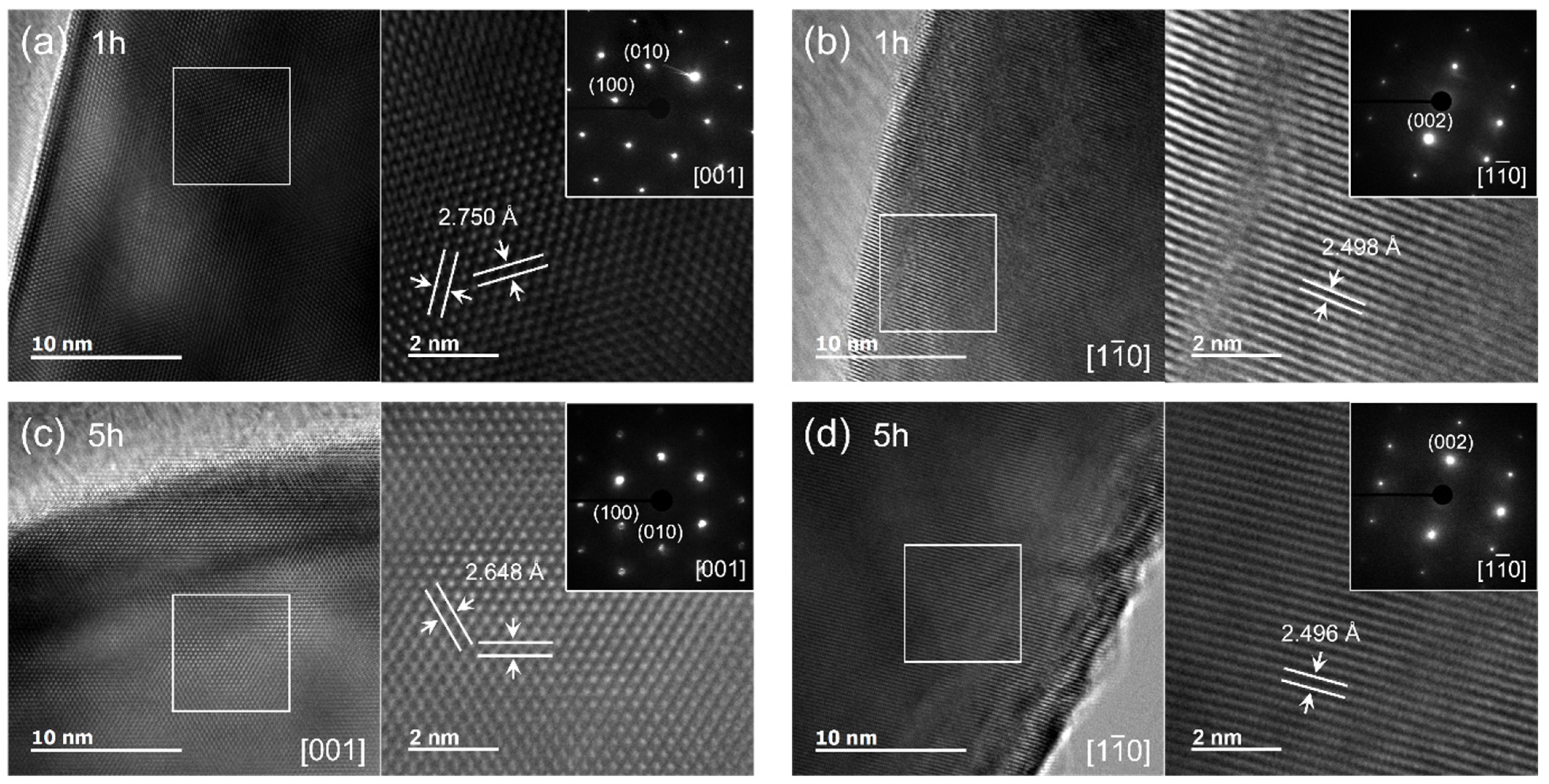
3.2.2. HRTEM Analysis
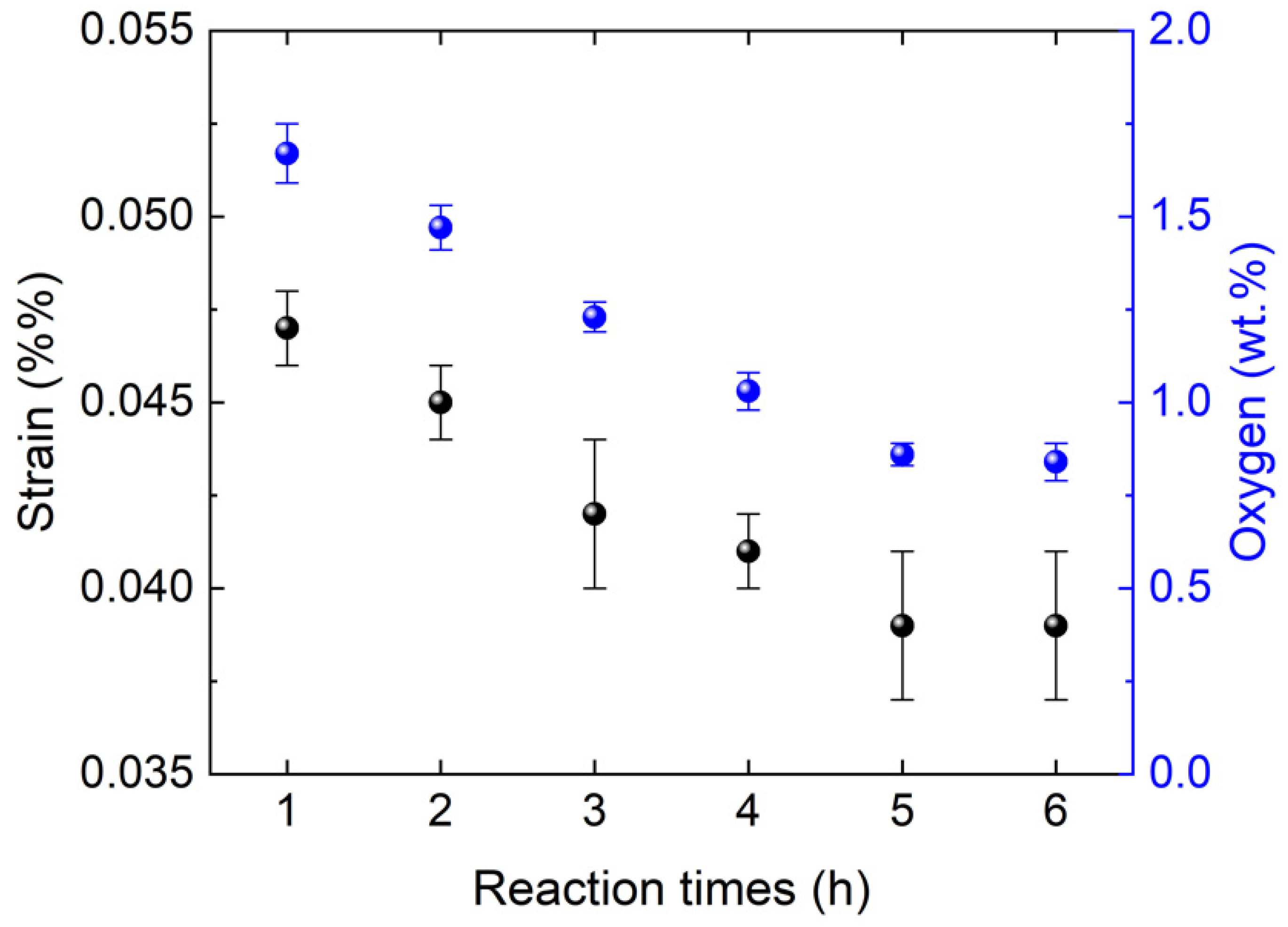
3.3. Micro-Strain Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Baik, Y.; Drew, R.A. Aluminum nitride: Processing and applications. Key Eng. Mater. 1996, 122, 553. [Google Scholar] [CrossRef]
- Kurokawa, Y.; Utsumi, K.; Takamizawa, H. Development and microstructural characterization of high-thermal-conductivity aluminum nitride ceramics. J. Am. Ceram. Soc. 1988, 71, 588–594. [Google Scholar] [CrossRef]
- Shang, Q.; Wang, Z.; Li, J.; Zhou, G.; Zhang, H.; Wang, S. Gel-tape-casting of aluminum nitride ceramics. J. Adv. Ceram. 2017, 6, 67–72. [Google Scholar] [CrossRef]
- Ide, T.; Komeya, K.; Meguro, T.; Tatami, J. Synthesis of AlN powder by carbothermal reduction-nitridation of various Al2O3 powders with CaF2. J. Am. Ceram. Soc. 1999, 82, 2993–2998. [Google Scholar] [CrossRef]
- Molisani, A.L.; Yoshimura, H.L. Low-temperature synthesis of AlN powder with multicomponent additive systems by carbothermal reduction–nitridation method. Mater. Res. Bull. 2010, 45, 733–738. [Google Scholar] [CrossRef]
- Slack, G.A. Nonmetallic crystals with high thermal conductivity. J. Phys. Chem. Solids 1973, 34, 321–335. [Google Scholar] [CrossRef]
- Virkar, A.V.; Jackson, T.B.; Cutler, R.A. Thermodynamic and kinetic effects of oxygen removal on the thermal conductivity of aluminum nitride. J. Am. Ceram. Soc. 1989, 72, 2031–2042. [Google Scholar] [CrossRef]
- Buhr, H.; Müller, G.; Wiggers, H.; Aldinger, F.; Foley, P.; Roosen, A. Phase composition, oxygen content, and thermal conductivity of AIN(Y2O3) ceramics. J. Am. Ceram. Soc. 1991, 74, 718–723. [Google Scholar] [CrossRef]
- Harris, J.; Youngman, R.; Teller, R. On the nature of the oxygen-related defect in aluminum nitride. J. Mater. Res. 1990, 5, 1763–1773. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, H.; Zhang, S.; Wang, Y.; Zhang, Y.; Liu, C.; Zhang, D.; Jia, B.; Guo, D.; Chu, A.; et al. The Quantitative investigation of the lattice oxygen and grain edge oxygen on the thermal conductivity of aluminum nitride ceramics. J. Eur. Ceram. Soc. 2023, 43, 313–320. [Google Scholar] [CrossRef]
- De Baranda, P.S.; Knudsen, A.K.; Ruh, E. Effect of yttria on the thermal conductivity of aluminum nitride. J. Am. Ceram. Soc. 1994, 77, 1846–1850. [Google Scholar] [CrossRef]
- Van de Walle, C.G. Effects of impurities on the lattice parameters of GaN. Phys. Rev. B 2003, 68, 165209. [Google Scholar] [CrossRef]
- Krysko, M.; Sarzynski, M.; Domagała, J.; Grzegory, I.; Łucznik, B.; Kamler, G.; Porowski, S.; Leszczyński, M. The influence of lattice parameter variation on microstructure of GaN single crystals. J. Alloys Compd. 2005, 401, 261–264. [Google Scholar] [CrossRef]
- Pandey, A.; Raman, R.; Dalal, S.; Kaur, D.; Kapoor, A.K. Structural and optical characteristics investigations in oxygen ion implanted GaN epitaxial layers. Mater. Sci. Semicond. Process. 2020, 107, 104833. [Google Scholar] [CrossRef]
- Lefort, P.; Billy, M. Mechanism of AlN formation through the carbothermal reduction of Al2O3 in a flowing N2 atmosphere. J. Am. Ceram. Soc. 1993, 76, 2295–2299. [Google Scholar] [CrossRef]
- Kobayashi, R.; Moriya, Y.; Imamura, M.; Oosawa, K.; Oh-Ishi, K. Relation between oxygen concentration in AlN lattice and thermal conductivity of AlN ceramics sintered with various sintering additives. J. Ceram. Soc. Jpn. 2011, 119, 291–294. [Google Scholar] [CrossRef]
- Potter, G.E.; Knudsen, A.F.; Tou, J.C.; Choudhury, A. Measurement of the Oxygen and Impurity Distribution in Polycrystalline Aluminum Nitride with Secondary Ion Mass Spectrometry. J. Am. Ceram. Soc. 1992, 75, 3215–3224. [Google Scholar] [CrossRef]
- Kasori, M.; Ueno, F. Thermal Conductivity Improvement of YAG Added AlN Ceramics in the Grain Boundary Elimination Process. J. Eur. Ceram. Soc. 1995, 15, 435–443. [Google Scholar] [CrossRef]
- Schulz, H.; Tiemann, K.H. Crystal structure refinement of AlN and GaN. Solid State Commun. 1977, 23, 815–819. [Google Scholar] [CrossRef]
- Kumagai, Y.; Kubota, Y.; Nagashima, T.; Kinoshita, T.; Dalmau, R.; Schlesser, R.; Moody, B.; Xie, J.; Murakami, H.; Koukitu, A. Preparation of a Freestanding AlN Substrate from a Thick AlN Layer Grown by Hydride Vapor Phase Epitaxy on a Bulk AlN Substrate Prepared by Physical Vapor Transport. Appl. Phys. Exp. 2012, 5, 055504. [Google Scholar] [CrossRef]
- Wagner, J.-M.; Bechstedt, F. Phonon deformation potentials of α-GaN and -AlN: An ab initio calculation. Appl. Phys. Lett. 2000, 77, 346–348. [Google Scholar] [CrossRef]
- Rodríguez-Carvajal, J. Recent advances in magnetic structure determination by neutron powder diffraction. Phys. B Condens. Matter 1993, 192, 55–69. [Google Scholar] [CrossRef]
- Rodriguez-Carvajal, J. Commission on powder diffraction (IUCr). Newsletter 2001, 26, 12. [Google Scholar]
- Bagherian, S.; Zak, A.K. X-ray peak broadening and optical properties analysis of SnO2 nanosheets prepared by sol-gel method. Mater. Sci. Semicond. Process. 2016, 56, 52–58. [Google Scholar] [CrossRef]
- Williamson, G.; Hall, W. X-ray line broadening from filed aluminium and wolfram. Acta Metall. 1953, 1, 22–31. [Google Scholar] [CrossRef]
- Van de Walle, C.G. DX-center formation in wurtzite and zinc-blende AlxGa1−xN. Phys. Rev. B 1998, 57, R2033–R2036. [Google Scholar] [CrossRef]
- Hirata, K.; Mori, Y.; Yamada, H.; Uehara, M.; Anggraini, S.A.; Akiyama, M. Significant Enhancement of Piezoelectric Response in AlN by Yb Addition. Materials 2021, 14, 309. [Google Scholar] [CrossRef]
- Winiarski, M.J. Electronic Structure of Ternary Alloys of Group III and Rare Earth Nitrides. Materials 2021, 14, 4115. [Google Scholar] [CrossRef]
- Fitzgerald, J.J.; Kohl, S.D.; Piedra, G.P. Observation of Four-Coordinate Aluminum Oxynitride (AlO4−xNx) Environments in AlON Solids by MAS 27Al NMR at 14 T. Chem. Mater. 1994, 6, 1915–1917. [Google Scholar] [CrossRef]
- Jung, W.; Chae, S. 27Al MAS NMR spectroscopic identification of reaction intermediates in the carbothermal reduction and nitridation of alumina. Mater. Chem. Phys. 2010, 123, 610–613. [Google Scholar] [CrossRef]
| Reaction Time (h) | O (wt%/at%) * | C (ppm) * | AlN3O NMR Peak Area (%) ** |
|---|---|---|---|
| 1 | 1.67(8)/4.28(21) | - | - |
| 2 | 1.47(6)/3.77(15) | 1691(16) | 11.37 |
| 3 | 1.23(4)/3.15(10) | 1527(14) | 8.63 |
| 4 | 1.03(5)/2.64(13) | 1412(13) | 7.17 |
| 5 | 0.86(3)/2.20(8) | 1087(10) | 4.72 |
| 6 | 0.84(5)/2.15(13) | 1132(10) | 4.75 |
| Commercial | 0.85(5)/2.17(13) | - | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Ahn, H.; Kim, S.-J.; Kim, J.-Y.; Pee, J.-H. Effect of Residual Oxygen Concentration on the Lattice Parameters of Aluminum Nitride Powder Prepared via Carbothermal Reduction Nitridation Reaction. Materials 2022, 15, 8926. https://doi.org/10.3390/ma15248926
Kim J, Ahn H, Kim S-J, Kim J-Y, Pee J-H. Effect of Residual Oxygen Concentration on the Lattice Parameters of Aluminum Nitride Powder Prepared via Carbothermal Reduction Nitridation Reaction. Materials. 2022; 15(24):8926. https://doi.org/10.3390/ma15248926
Chicago/Turabian StyleKim, Jaegyeom, Heewon Ahn, Seung-Joo Kim, Jong-Young Kim, and Jae-Hwan Pee. 2022. "Effect of Residual Oxygen Concentration on the Lattice Parameters of Aluminum Nitride Powder Prepared via Carbothermal Reduction Nitridation Reaction" Materials 15, no. 24: 8926. https://doi.org/10.3390/ma15248926
APA StyleKim, J., Ahn, H., Kim, S.-J., Kim, J.-Y., & Pee, J.-H. (2022). Effect of Residual Oxygen Concentration on the Lattice Parameters of Aluminum Nitride Powder Prepared via Carbothermal Reduction Nitridation Reaction. Materials, 15(24), 8926. https://doi.org/10.3390/ma15248926







