Effect of Residual Oxygen Concentration on the Lattice Parameters of Aluminum Nitride Powder Prepared via Carbothermal Reduction Nitridation Reaction
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Synthesis
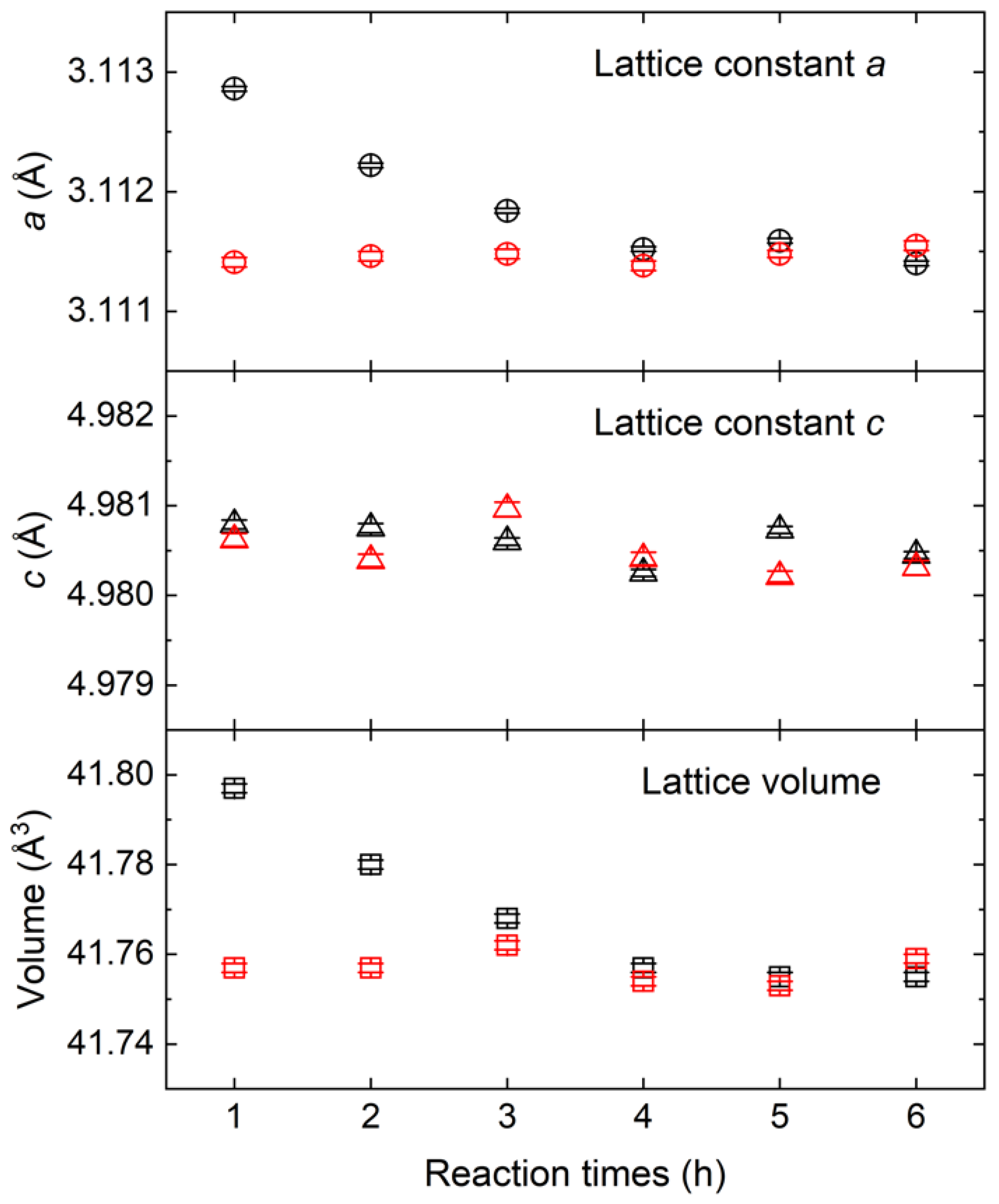
3.2. Oxygen Dissolution Effect on AlN Lattice
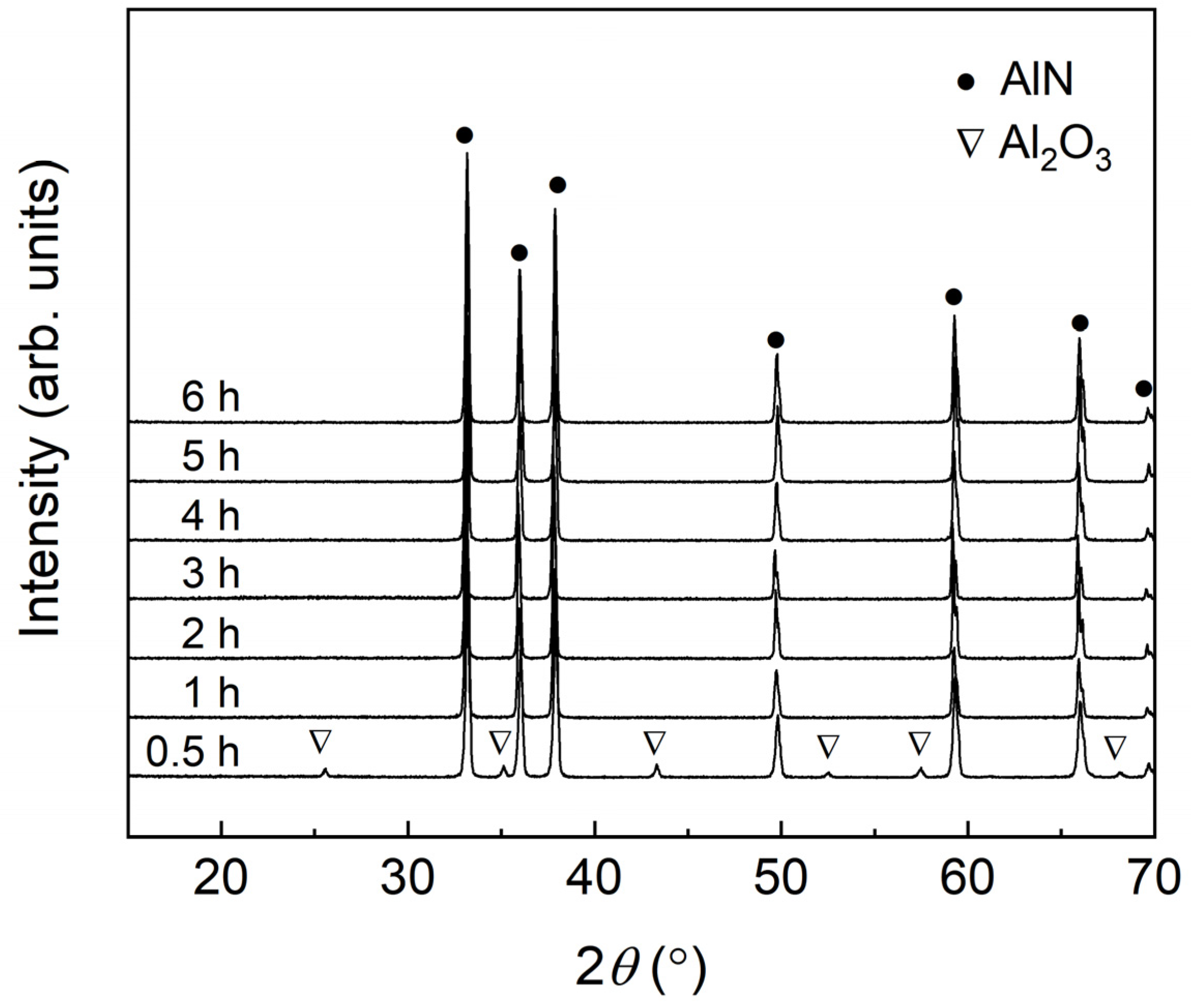
3.2.1. X-ray Diffraction
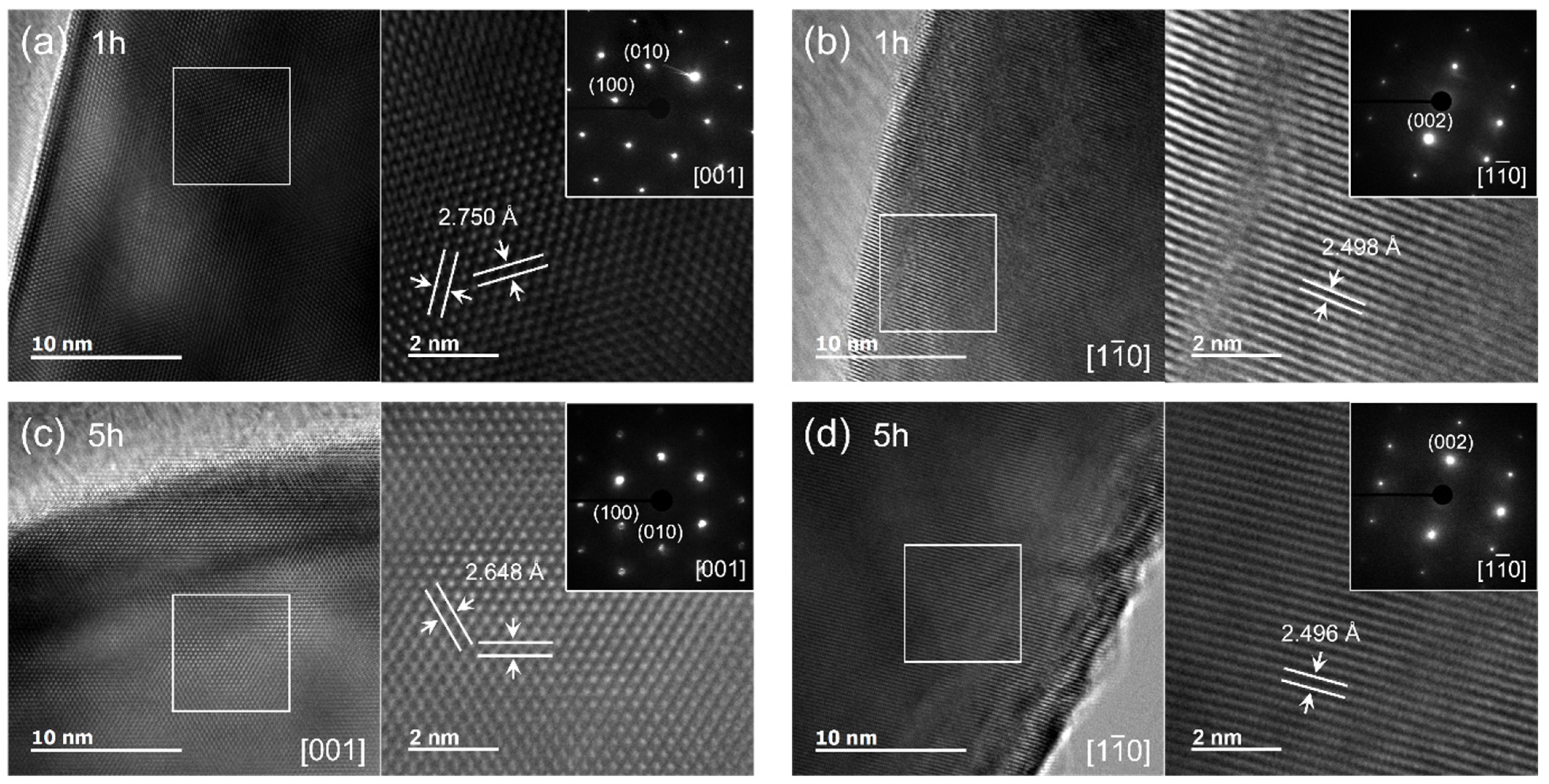
3.2.2. HRTEM Analysis
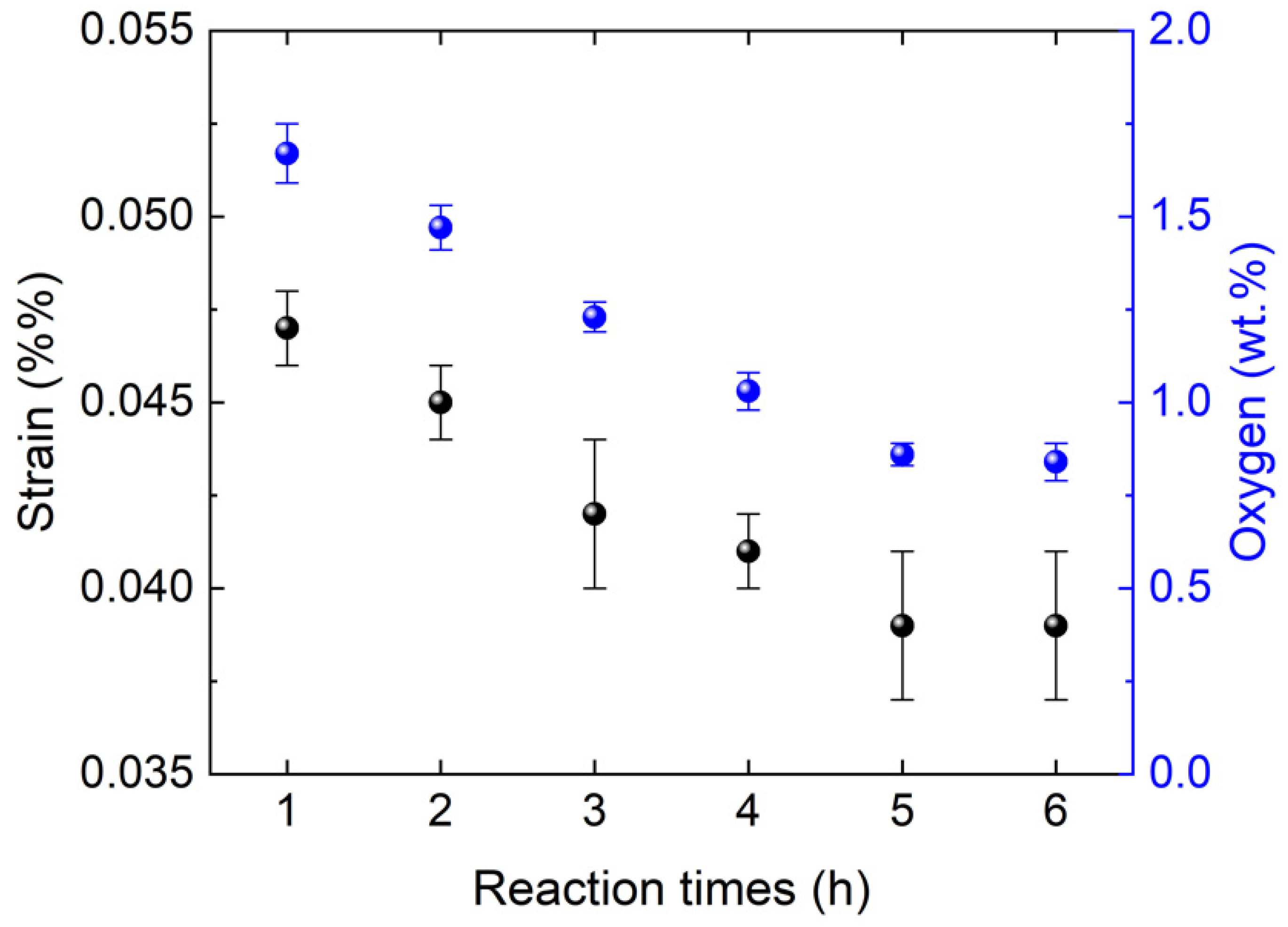
3.3. Micro-Strain Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Baik, Y.; Drew, R.A. Aluminum nitride: Processing and applications. Key Eng. Mater. 1996, 122, 553. [Google Scholar] [CrossRef]
- Kurokawa, Y.; Utsumi, K.; Takamizawa, H. Development and microstructural characterization of high-thermal-conductivity aluminum nitride ceramics. J. Am. Ceram. Soc. 1988, 71, 588–594. [Google Scholar] [CrossRef]
- Shang, Q.; Wang, Z.; Li, J.; Zhou, G.; Zhang, H.; Wang, S. Gel-tape-casting of aluminum nitride ceramics. J. Adv. Ceram. 2017, 6, 67–72. [Google Scholar] [CrossRef] [Green Version]
- Ide, T.; Komeya, K.; Meguro, T.; Tatami, J. Synthesis of AlN powder by carbothermal reduction-nitridation of various Al2O3 powders with CaF2. J. Am. Ceram. Soc. 1999, 82, 2993–2998. [Google Scholar] [CrossRef]
- Molisani, A.L.; Yoshimura, H.L. Low-temperature synthesis of AlN powder with multicomponent additive systems by carbothermal reduction–nitridation method. Mater. Res. Bull. 2010, 45, 733–738. [Google Scholar] [CrossRef]
- Slack, G.A. Nonmetallic crystals with high thermal conductivity. J. Phys. Chem. Solids 1973, 34, 321–335. [Google Scholar] [CrossRef]
- Virkar, A.V.; Jackson, T.B.; Cutler, R.A. Thermodynamic and kinetic effects of oxygen removal on the thermal conductivity of aluminum nitride. J. Am. Ceram. Soc. 1989, 72, 2031–2042. [Google Scholar] [CrossRef]
- Buhr, H.; Müller, G.; Wiggers, H.; Aldinger, F.; Foley, P.; Roosen, A. Phase composition, oxygen content, and thermal conductivity of AIN(Y2O3) ceramics. J. Am. Ceram. Soc. 1991, 74, 718–723. [Google Scholar] [CrossRef]
- Harris, J.; Youngman, R.; Teller, R. On the nature of the oxygen-related defect in aluminum nitride. J. Mater. Res. 1990, 5, 1763–1773. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, H.; Zhang, S.; Wang, Y.; Zhang, Y.; Liu, C.; Zhang, D.; Jia, B.; Guo, D.; Chu, A.; et al. The Quantitative investigation of the lattice oxygen and grain edge oxygen on the thermal conductivity of aluminum nitride ceramics. J. Eur. Ceram. Soc. 2023, 43, 313–320. [Google Scholar] [CrossRef]
- De Baranda, P.S.; Knudsen, A.K.; Ruh, E. Effect of yttria on the thermal conductivity of aluminum nitride. J. Am. Ceram. Soc. 1994, 77, 1846–1850. [Google Scholar] [CrossRef]
- Van de Walle, C.G. Effects of impurities on the lattice parameters of GaN. Phys. Rev. B 2003, 68, 165209. [Google Scholar] [CrossRef] [Green Version]
- Krysko, M.; Sarzynski, M.; Domagała, J.; Grzegory, I.; Łucznik, B.; Kamler, G.; Porowski, S.; Leszczyński, M. The influence of lattice parameter variation on microstructure of GaN single crystals. J. Alloys Compd. 2005, 401, 261–264. [Google Scholar] [CrossRef]
- Pandey, A.; Raman, R.; Dalal, S.; Kaur, D.; Kapoor, A.K. Structural and optical characteristics investigations in oxygen ion implanted GaN epitaxial layers. Mater. Sci. Semicond. Process. 2020, 107, 104833. [Google Scholar] [CrossRef]
- Lefort, P.; Billy, M. Mechanism of AlN formation through the carbothermal reduction of Al2O3 in a flowing N2 atmosphere. J. Am. Ceram. Soc. 1993, 76, 2295–2299. [Google Scholar] [CrossRef]
- Kobayashi, R.; Moriya, Y.; Imamura, M.; Oosawa, K.; Oh-Ishi, K. Relation between oxygen concentration in AlN lattice and thermal conductivity of AlN ceramics sintered with various sintering additives. J. Ceram. Soc. Jpn. 2011, 119, 291–294. [Google Scholar] [CrossRef] [Green Version]
- Potter, G.E.; Knudsen, A.F.; Tou, J.C.; Choudhury, A. Measurement of the Oxygen and Impurity Distribution in Polycrystalline Aluminum Nitride with Secondary Ion Mass Spectrometry. J. Am. Ceram. Soc. 1992, 75, 3215–3224. [Google Scholar] [CrossRef]
- Kasori, M.; Ueno, F. Thermal Conductivity Improvement of YAG Added AlN Ceramics in the Grain Boundary Elimination Process. J. Eur. Ceram. Soc. 1995, 15, 435–443. [Google Scholar] [CrossRef]
- Schulz, H.; Tiemann, K.H. Crystal structure refinement of AlN and GaN. Solid State Commun. 1977, 23, 815–819. [Google Scholar] [CrossRef]
- Kumagai, Y.; Kubota, Y.; Nagashima, T.; Kinoshita, T.; Dalmau, R.; Schlesser, R.; Moody, B.; Xie, J.; Murakami, H.; Koukitu, A. Preparation of a Freestanding AlN Substrate from a Thick AlN Layer Grown by Hydride Vapor Phase Epitaxy on a Bulk AlN Substrate Prepared by Physical Vapor Transport. Appl. Phys. Exp. 2012, 5, 055504. [Google Scholar] [CrossRef]
- Wagner, J.-M.; Bechstedt, F. Phonon deformation potentials of α-GaN and -AlN: An ab initio calculation. Appl. Phys. Lett. 2000, 77, 346–348. [Google Scholar] [CrossRef]
- Rodríguez-Carvajal, J. Recent advances in magnetic structure determination by neutron powder diffraction. Phys. B Condens. Matter 1993, 192, 55–69. [Google Scholar] [CrossRef]
- Rodriguez-Carvajal, J. Commission on powder diffraction (IUCr). Newsletter 2001, 26, 12. [Google Scholar]
- Bagherian, S.; Zak, A.K. X-ray peak broadening and optical properties analysis of SnO2 nanosheets prepared by sol-gel method. Mater. Sci. Semicond. Process. 2016, 56, 52–58. [Google Scholar] [CrossRef]
- Williamson, G.; Hall, W. X-ray line broadening from filed aluminium and wolfram. Acta Metall. 1953, 1, 22–31. [Google Scholar] [CrossRef]
- Van de Walle, C.G. DX-center formation in wurtzite and zinc-blende AlxGa1−xN. Phys. Rev. B 1998, 57, R2033–R2036. [Google Scholar] [CrossRef] [Green Version]
- Hirata, K.; Mori, Y.; Yamada, H.; Uehara, M.; Anggraini, S.A.; Akiyama, M. Significant Enhancement of Piezoelectric Response in AlN by Yb Addition. Materials 2021, 14, 309. [Google Scholar] [CrossRef]
- Winiarski, M.J. Electronic Structure of Ternary Alloys of Group III and Rare Earth Nitrides. Materials 2021, 14, 4115. [Google Scholar] [CrossRef]
- Fitzgerald, J.J.; Kohl, S.D.; Piedra, G.P. Observation of Four-Coordinate Aluminum Oxynitride (AlO4−xNx) Environments in AlON Solids by MAS 27Al NMR at 14 T. Chem. Mater. 1994, 6, 1915–1917. [Google Scholar] [CrossRef]
- Jung, W.; Chae, S. 27Al MAS NMR spectroscopic identification of reaction intermediates in the carbothermal reduction and nitridation of alumina. Mater. Chem. Phys. 2010, 123, 610–613. [Google Scholar] [CrossRef]
| Reaction Time (h) | O (wt%/at%) * | C (ppm) * | AlN3O NMR Peak Area (%) ** |
|---|---|---|---|
| 1 | 1.67(8)/4.28(21) | - | - |
| 2 | 1.47(6)/3.77(15) | 1691(16) | 11.37 |
| 3 | 1.23(4)/3.15(10) | 1527(14) | 8.63 |
| 4 | 1.03(5)/2.64(13) | 1412(13) | 7.17 |
| 5 | 0.86(3)/2.20(8) | 1087(10) | 4.72 |
| 6 | 0.84(5)/2.15(13) | 1132(10) | 4.75 |
| Commercial | 0.85(5)/2.17(13) | - | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Ahn, H.; Kim, S.-J.; Kim, J.-Y.; Pee, J.-H. Effect of Residual Oxygen Concentration on the Lattice Parameters of Aluminum Nitride Powder Prepared via Carbothermal Reduction Nitridation Reaction. Materials 2022, 15, 8926. https://doi.org/10.3390/ma15248926
Kim J, Ahn H, Kim S-J, Kim J-Y, Pee J-H. Effect of Residual Oxygen Concentration on the Lattice Parameters of Aluminum Nitride Powder Prepared via Carbothermal Reduction Nitridation Reaction. Materials. 2022; 15(24):8926. https://doi.org/10.3390/ma15248926
Chicago/Turabian StyleKim, Jaegyeom, Heewon Ahn, Seung-Joo Kim, Jong-Young Kim, and Jae-Hwan Pee. 2022. "Effect of Residual Oxygen Concentration on the Lattice Parameters of Aluminum Nitride Powder Prepared via Carbothermal Reduction Nitridation Reaction" Materials 15, no. 24: 8926. https://doi.org/10.3390/ma15248926






