Review on Graphene-, Graphene Oxide-, Reduced Graphene Oxide-Based Flexible Composites: From Fabrication to Applications
Abstract
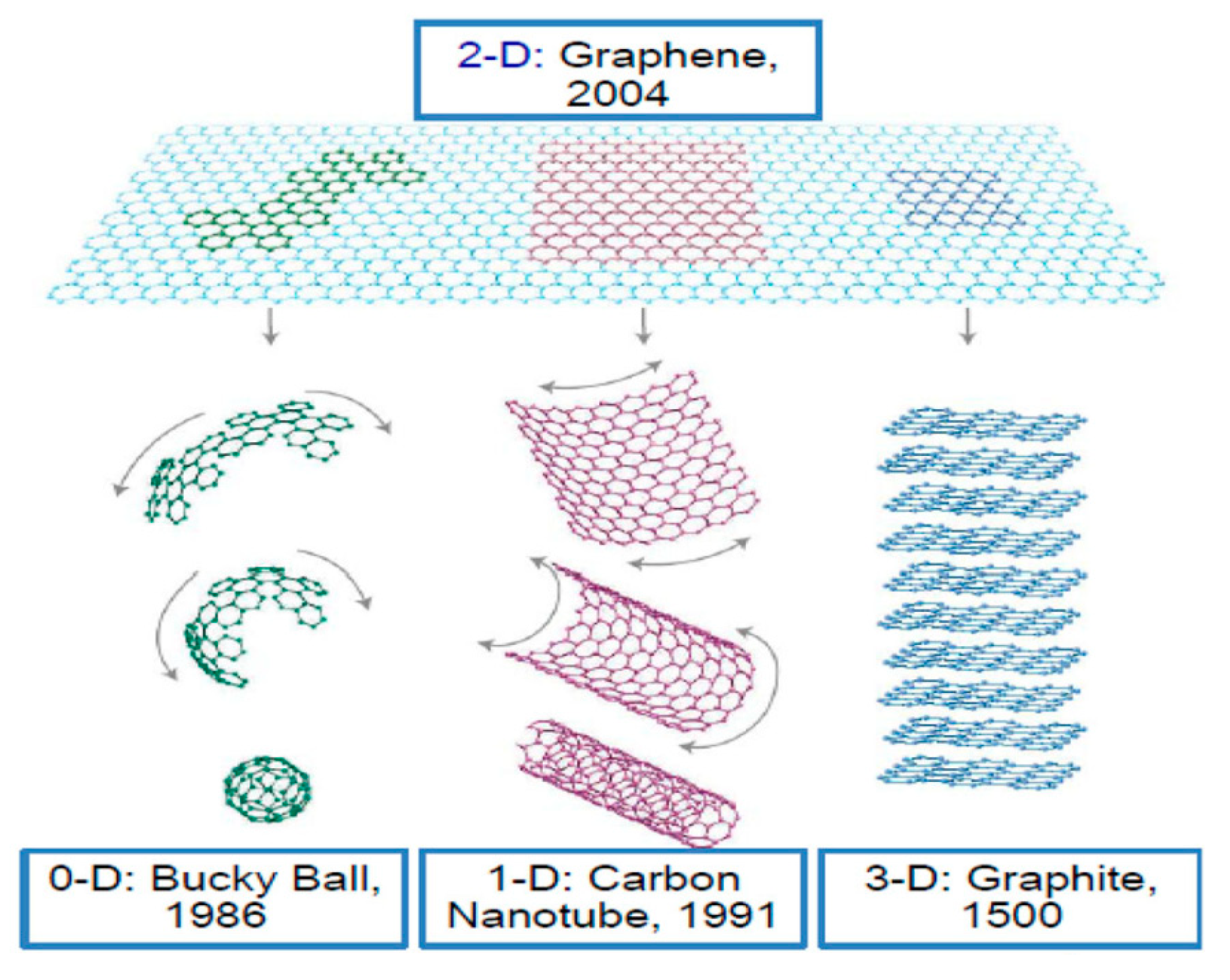
:1. Introduction
2. Synthesis of Graphene and Its Derivatives
2.1. GO
2.2. Graphene
2.2.1. Exfoliation and Cleavage
2.2.2. Thermal CVD Techniques
2.3. rGO
3. Flexible Graphene Composites: From Fabrication to Applications
4. Flexible Composites and Applications of GO/rGO
5. Conclusions and Perspective
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jalili, R.; Aboutalebi, S.H.; Esrafilzadeh, D.; Shepherd, R.L.; Chen, J.; Aminorroaya–Yamini, S.; Konstantinov, K.; Minett, A.I.; Razal, J.M.; Wallace, G.G. Scalable one–step wet–spinning of graphene fibers and yarns from liquid crystalline dispersions of graphene oxide: Towards multifunctional textiles. Adv. Funct. Mater. 2013, 23, 5345–5354. [Google Scholar] [CrossRef]
- Lundstedt, A.; Papadakis, R.; Li, H.; Han, Y.; Jorner, K.; Bergman, J.; Leifer, K.; Grennberg, H.; Ottosson, H. White–Light Photoassisted Covalent Functionalization of Graphene Using 2–Propanol. Small Methods 2017, 1, 1700214. [Google Scholar] [CrossRef]
- Li, H.; Papadakis, R.; Jafri, S.H.M.; Thersleff, T.; Michler, J.; Ottosson, H.; Leifer, K. Superior adhesion of graphene nanoscrolls. Commun. Phys. 2018, 1, 44. [Google Scholar] [CrossRef]
- Huang, W.; Xia, X.; Zhu, C.; Steichen, P.; Quan, W.; Mao, W.; Yang, J.; Chu, L.; Li, X. Memristive Artificial Synapses for Neuromorphic Computing. Nanomicro. Lett. 2021, 13, 85. [Google Scholar] [CrossRef]
- Chabot, V.; Higgins, D.; Yu, A.; Xiao, X.; Chen, Z.; Zhang, J. A review of graphene and graphene oxide sponge: Material synthesis and applications to energy and the environment. Energy Environ. Sci. 2014, 7, 1564–1596. [Google Scholar] [CrossRef]
- Khan, Z.U.; Kausar, A.; Ullah, H.; Badshah, A.; Khan, W.U. A review of graphene oxide, graphene buckypaper, and polymer/graphene composites: Properties and fabrication techniques. J. Plast. Film Sheeting 2016, 32, 336–379. [Google Scholar] [CrossRef]
- Geim, A.; Novoselov, K. The Rise of Graphene, Nanoscience and Technology: A Collection of Reviews from Nature Journals; World Scientific: Singapore, 2009; pp. 11–19. [Google Scholar]
- Eda, G.; Chhowalla, M. Chemically derived graphene oxide: Towards large-area thin-film electronics and optoelectronics. Adv. Mater. 2010, 22, 2392–2415. [Google Scholar] [CrossRef]
- Sajibul, M.; Bhuyan, A.; Uddin, M.N.; Islam, M.M.; Bipasha, F.A.; Hossain, S.S. Synthesis of graphene. Int. Nano Lett. 2016, 6, 65–83. [Google Scholar]
- Choi, W.; Lahiri, I.; Seelaboyina, R.; Kang, Y.S. Synthesis of graphene and its applications: A review. Crit. Rev. Solid State Mater. Sci. 2010, 35, 52–71. [Google Scholar] [CrossRef]
- Tsou, C.-H.; An, Q.-F.; Lo, S.-C.; De Guzman, M.; Hung, W.-S.; Hu, C.-C.; Lee, K.-R.; Lai, J.-Y. Effect of microstructure of graphene oxide fabricated through different self-ssembly techniques on 1-butanol dehydration. J. Membr. Sci. 2015, 477, 93–100. [Google Scholar] [CrossRef]
- Naficy, S.; Jalili, R.; Aboutalebi, S.H.; Gorkin III, R.A.; Konstantinov, K.; Innis, P.C.; Spinks, G.M.; Poulin, P.; Wallace, G.G. Graphene oxide dispersions: Tuning rheology to enable fabrication. Mater. Horiz. 2014, 1, 326–331. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Feng, J. An attempt towards fabricating reduced graphene oxide composites with traditional polymer processing techniques by adding chemical reduction agents. Compos. Sci. Technol. 2017, 140, 16–22. [Google Scholar] [CrossRef]
- Liu, J.; Chen, S.; Papadakis, R.; Li, H. Nanoresolution patterning of hydrogenated graphene by electron beam induced C-H dissociation. Nanotechnology 2018, 29, 415304. [Google Scholar] [CrossRef]
- Liu, J.; Papadakis, R.; Li, H. Experimental observation of size-dependent behavior in surface energy of gold nanoparticles through atomic force microscope. Appl. Phys. Lett. 2018, 113, 083108. [Google Scholar] [CrossRef]
- Hummers, W.S., Jr.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Dikin, D.A.; Stankovich, S.; Zimney, E.J.; Piner, R.D.; Dommett, G.H.; Evmenenko, G.; Nguyen, S.T.; Ruoff, R.S. Preparation and characterization of graphene oxide paper. Nature 2007, 448, 457–460. [Google Scholar] [CrossRef]
- Paulchamy, B.; Arthi, G.; Lignesh, B. A simple approach to stepwise synthesis of graphene oxide nanomaterial. J. Nanomed. Nanotechnol. 2015, 6, 1. [Google Scholar]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved synthesis of graphene oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef]
- Chowdhury, D.R.; Singh, C.; Paul, A. Role of graphite precursor and sodium nitrate in graphite oxide synthesis. RSC Adv. 2014, 4, 15138–15145. [Google Scholar] [CrossRef]
- Song, J.; Wang, X.; Chang, C.-T. Preparation and characterization of graphene oxide. J. Nanomater. 2014, 2014, 276143. [Google Scholar] [CrossRef]
- Boehm, H.; Clauss, A.; Fischer, G.O.; Hofmann, U. In Surface Properties of Extremely Thin Graphite Lamellae. In Proceedings of the Fifth Conference on Carbon, University Park, PA, USA, 19–23 June 1961; pp. 73–80. [Google Scholar]
- McNaught, A.D.; Wilkinson, A. Compendium of Chemical Terminology. IUPAC Recommendations; Blackwell Science: Hoboken, NJ, USA, 1997. [Google Scholar]
- Kumar, V.; Kumar, A.; Lee, D.J.; Park, S.S. Estimation of Number of Graphene Layers Using Different Methods: A Focused Review. Materials 2021, 14, 4590. [Google Scholar] [CrossRef]
- Kamedulski, P.; Ilnicka, A.; Lukaszewicz, J.P. Selected Aspects of Graphene Exfoliation as an Introductory Step Towards 3D Structuring of Graphene Nano-Sheets. Curr. Graphene Sci. 2019, 2, 106–117. [Google Scholar] [CrossRef]
- Prekodravac, J.R.; Kepić, D.P.; Colmenares, J.C.; Giannakoudakis, D.A.; Jovanović, S.P. A comprehensive review on selected graphene synthesis methods: From electrochemical exfoliation through rapid thermal annealing towards biomass pyrolysis. J. Mater. Chem. C 2021, 9, 6722–6748. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Jiang, Z.; Zhang, Y.; Morozov, S.; Stormer, H.L.; Zeitler, U.; Maan, J.; Boebinger, G.; Kim, P.; Geim, A.K. Room-temperature quantum Hall effect in graphene. Science 2007, 315, 1379. [Google Scholar] [CrossRef] [Green Version]
- Hong, Y.; Wang, Z.; Jin, X. Sulfuric acid intercalated graphite oxide for graphene preparation. Sci. Rep. 2013, 3, 3439. [Google Scholar] [CrossRef]
- Alam, S.N.; Sharma, N.; Kumar, L. Synthesis of graphene oxide (GO) by modified hummers method and its thermal reduction to obtain reduced graphene oxide (rGO). Graphene 2017, 6, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Ray, S. Applications of Graphene and Graphene-Oxide Based Nanomaterials; William Andrew: Norwich, NY, USA, 2015. [Google Scholar]
- Abdolhosseinzadeh, S.; Asgharzadeh, H.; Kim, H.S. Fast and fully–scalable synthesis of reduced graphene oxide. Sci. Rep. 2015, 5, 10160. [Google Scholar] [CrossRef] [Green Version]
- Chu, C.-Y.; Tsai, J.-T.; Sun, C.-L. Synthesis of PEDOT-modified graphene composite materials as flexible electrodes for energy storage and conversion applications. Int. J. Hydrogen Energy 2012, 37, 13880–13886. [Google Scholar] [CrossRef]
- Wu, Q.; Xu, Y.; Yao, Z.; Liu, A.; Shi, G. Supercapacitors based on flexible graphene/polyaniline nanofiber composite films. ACS Nano 2010, 4, 1963–1970. [Google Scholar] [CrossRef]
- Tang, P.; Han, L.; Zhang, L. Facile synthesis of graphite/PEDOT/MnO2 composites on commercial supercapacitor separator membranes as flexible and high–performance supercapacitor electrodes. ACS Appl. Mater. Interfaces 2014, 6, 10506–10515. [Google Scholar] [CrossRef]
- Yoo, D.; Kim, J.; Kim, J.H. Direct synthesis of highly conductive poly (3,4-ethylenedioxythiophene): Poly (4-styrenesulfonate) (PEDOT: PSS)/graphene composites and their applications in energy harvesting systems. Nano Res. 2014, 7, 717–730. [Google Scholar] [CrossRef]
- Bao, L.; Zang, J.; Li, X. Flexible Zn2SnO4/MnO2 core/shell nanocable-carbon microfiber hybrid composites for high-performance supercapacitor electrodes. Nano Lett. 2011, 11, 1215–1220. [Google Scholar] [CrossRef]
- Chang, H.; Wang, G.; Yang, A.; Tao, X.; Liu, X.; Shen, Y.; Zheng, Z. A transparent, flexible, low-temperature, and solution-processible graphene composite electrode. Adv. Funct. Mater. 2010, 20, 2893–2902. [Google Scholar] [CrossRef]
- Cheng, Y.; Lu, S.; Zhang, H.; Varanasi, C.V.; Liu, J. Synergistic effects from graphene and carbon nanotubes enable flexible and robust electrodes for high-performance supercapacitors. Nano Lett. 2012, 12, 4206–4211. [Google Scholar] [CrossRef]
- Cong, H.-P.; Ren, X.-C.; Wang, P.; Yu, S.-H. Flexible graphene-polyaniline composite paper for high-performance supercapacitor. Energy Environ. Sci. 2013, 6, 1185–1191. [Google Scholar] [CrossRef]
- Ding, X.; Zhao, Y.; Hu, C.; Hu, Y.; Dong, Z.; Chen, N.; Zhang, Z.; Qu, L. Spinning fabrication of graphene/polypyrrole composite fibers for all-solid-state, flexible fibriform supercapacitors. J. Mater. Chem. A 2014, 2, 12355–12360. [Google Scholar] [CrossRef]
- Zhang, Z.; Xiao, F.; Qian, L.; Xiao, J.; Wang, S.; Liu, Y. Facile Synthesis of 3D MnO2-Graphene and Carbon Nanotube-Graphene Composite Networks for High-Performance, Flexible, All-Solid-State Asymmetric Supercapacitors. Adv. Energy Mater. 2014, 4, 1400064. [Google Scholar] [CrossRef]
- Guo, M.-X.; Bian, S.-W.; Shao, F.; Liu, S.; Peng, Y.-H. Hydrothermal synthesis and electrochemical performance of MnO2/graphene/polyester composite electrode materials for flexible supercapacitors. Electrochim. Acta 2016, 209, 486–497. [Google Scholar] [CrossRef]
- Jiang, L.-L.; Lu, X.; Xie, C.-M.; Wan, G.-J.; Zhang, H.-P.; Youhong, T. Flexible, free-standing TiO2-graphene-polypyrrole composite films as electrodes for supercapacitors. J. Phys. Chem. C 2015, 119, 3903–3910. [Google Scholar] [CrossRef]
- Kuilla, T.; Bhadra, S.; Yao, D.; Kim, N.H.; Bose, S.; Lee, J.H. Recent advances in graphene based polymer composites. Prog. Polym. Sci. 2010, 35, 1350–1375. [Google Scholar] [CrossRef]
- Sun, F.; Li, H.; Leifer, K.; Gamstedt, E.K. Rate effects on localized shear deformation during nanosectioning of an amorphous thermoplastic polymer. Int. J. Solids Struct. 2017, 129, 40–48. [Google Scholar] [CrossRef]
- Li, Z.; Mi, Y.; Liu, X.; Liu, S.; Yang, S.; Wang, J. Flexible graphene/MnO2 composite papers for supercapacitor electrodes. J. Mater. Chem. 2011, 21, 14706–14711. [Google Scholar] [CrossRef]
- Li, X.; Zang, X.; Li, Z.; Li, X.; Li, P.; Sun, P.; Lee, X.; Zhang, R.; Huang, Z.; Wang, K. Large-area flexible core-shell graphene/porous carbon woven fabric films for fiber supercapacitor electrodes. Adv. Funct. Mater. 2013, 23, 4862–4869. [Google Scholar] [CrossRef]
- Li, L.; Bi, H.; Gai, S.; He, F.; Gao, P.; Dai, Y.; Zhang, X.; Yang, D.; Zhang, M.; Yang, P. Uniformly dispersed ZnFe2O4 nanoparticles on nitrogen-modified graphene for high-performance supercapacitor as electrode. Sci. Rep. 2017, 7, 43116. [Google Scholar] [CrossRef] [Green Version]
- Lin, T.-W.; Dai, C.-S.; Hung, K.-C. High energy density asymmetric supercapacitor based on NiOOH/Ni3S2/3D graphene and Fe3O4/graphene composite electrodes. Sci. Rep. 2014, 4, 7274. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.-W.; Yan, X.-B.; Lang, J.-W.; Peng, C.; Xue, Q.-J. Flexible and conductive nanocomposite electrode based on graphene sheets and cotton cloth for supercapacitor. J. Mater. Chem. 2012, 22, 17245–17253. [Google Scholar] [CrossRef]
- Liu, L.; Niu, Z.; Zhang, L.; Zhou, W.; Chen, X.; Xie, S. Nanostructured graphene composite papers for highly flexible and foldable supercapacitors. Adv. Mater. 2014, 26, 4855–4862. [Google Scholar] [CrossRef]
- Perera, S.D.; Liyanage, A.D.; Nijem, N.; Ferraris, J.P.; Chabal, Y.J.; Balkus, K.J., Jr. Vanadium oxide nanowire-Graphene binder free nanocomposite paper electrodes for supercapacitors: A facile green approach. J. Power Sources 2013, 230, 130–137. [Google Scholar] [CrossRef]
- Tai, Z.; Yan, X.; Lang, J.; Xue, Q. Enhancement of capacitance performance of flexible carbon nanofiber paper by adding graphene nanosheets. J. Power Sources 2012, 199, 373–378. [Google Scholar] [CrossRef]
- Vadukumpully, S.; Paul, J.; Mahanta, N.; Valiyaveettil, S. Flexible conductive graphene/poly(vinyl chloride) composite thin films with high mechanical strength and thermal stability. Carbon 2011, 49, 198–205. [Google Scholar] [CrossRef]
- Wang, D.-W.; Li, F.; Zhao, J.; Ren, W.; Chen, Z.-G.; Tan, J.; Wu, Z.-S.; Gentle, I.; Lu, G.Q.; Cheng, H.-M. Fabrication of graphene/polyaniline composite paper via in situ anodic electropolymerization for high-performance flexible electrode. ACS Nano 2009, 3, 1745–1752. [Google Scholar] [CrossRef]
- Wang, R.; Bian, H.; Ji, H.; Yang, R. Preparation of lignocellulose/graphene composite conductive paper. Cellulose 2018, 25, 6139–6149. [Google Scholar] [CrossRef]
- Pan, L.; Xia, Y.; Qiu, B.; Zhao, H.; Guo, H.; Jia, K.; Gu, Q.; Liu, Z. Synthesis and electrochemical performance of micro-sized Li-rich layered cathode material for Lithium-ion batteries. Electrochim. Acta 2016, 211, 507–514. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Hong, W.; Bai, H.; Li, C.; Shi, G. Strong and ductile poly (vinyl alcohol)/graphene oxide composite films with a layered structure. Carbon 2009, 47, 3538–3543. [Google Scholar] [CrossRef]
- Xu, J.; Wang, K.; Zu, S.-Z.; Han, B.-H.; Wei, Z. Hierarchical nanocomposites of polyaniline nanowire arrays on graphene oxide sheets with synergistic effect for energy storage. ACS Nano 2010, 4, 5019–5026. [Google Scholar] [CrossRef]
- Wang, L.; Ye, Y.; Lu, X.; Wen, Z.; Li, Z.; Hou, H.; Song, Y. Hierarchical nanocomposites of polyaniline nanowire arrays on reduced graphene oxide sheets for supercapacitors. Sci. Rep. 2013, 3, 3568. [Google Scholar] [CrossRef]
- Xu, C.; Cui, A.; Xu, Y.; Fu, X. Graphene oxide–TiO2 composite filtration membranes and their potential application for water purification. Carbon 2013, 62, 465–471. [Google Scholar] [CrossRef]
- Yu, C.; Ma, P.; Zhou, X.; Wang, A.; Qian, T.; Wu, S.; Chen, Q. All-solid-state flexible supercapacitors based on highly dispersed polypyrrole nanowire and reduced graphene oxide composites. ACS Appl. Mater. Interfaces 2014, 6, 17937–17943. [Google Scholar] [CrossRef]
- Yun, Y.J.; Hong, W.G.; Kim, W.J.; Jun, Y.; Kim, B.H. A novel method for applying reduced graphene oxide directly to electronic textiles from yarns to fabrics. Adv. Mater. 2013, 25, 5701–5705. [Google Scholar] [CrossRef]
- Zhang, X.-J.; Wang, G.-S.; Cao, W.-Q.; Wei, Y.-Z.; Liang, J.-F.; Guo, L.; Cao, M.-S. Enhanced microwave absorption property of reduced graphene oxide (RGO)-MnFe2O4 nanocomposites and polyvinylidene fluoride. ACS Appl. Mater. Interfaces 2014, 6, 7471–7478. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, Q.; Wang, A.; Xu, J.; Wu, S.; Shen, J. Bamboo–like composites of V2O5/polyindole and activated carbon cloth as electrodes for all-solid-state flexible asymmetric supercapacitors. ACS Appl. Mater. Interfaces 2016, 8, 3776–3783. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, L.; Cao, P.; Cai, C.; Fu, Y.; Ma, X. Mesoporous composite nickel cobalt oxide/graphene oxide synthesized via a template-assistant co-precipitation route as electrode material for supercapacitors. J. Power Sources 2016, 306, 742–752. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, Q.; Zhang, H.; Passerini, S.; Qian, X. Two-dimensional titanium carbide/RGO composite for high-performance supercapacitors. ACS Appl. Mater. Interfaces 2016, 8, 15661–15667. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.J.; Seo, D.H.; Yick, S.; Chen, J.H.; Ostrikov, K.K. MnOx/carbon nanotube/reduced graphene oxide nanohybrids as high-performance supercapacitor electrodes. NPG Asia Mater. 2014, 6, e140. [Google Scholar] [CrossRef] [Green Version]
- Cao, X.; Zheng, B.; Shi, W.; Yang, J.; Fan, Z.; Luo, Z.; Rui, X.; Chen, B.; Yan, Q.; Zhang, H. Reduced graphene oxide-wrapped MoO3 composites prepared by using metal–organic frameworks as precursor for all-solid-state flexible supercapacitors. Adv. Mater. 2015, 27, 4695–4701. [Google Scholar] [CrossRef]
- Dong, X.; Wang, K.; Zhao, C.; Qian, X.; Chen, S.; Li, Z.; Liu, H.; Dou, S. Direct synthesis of RGO/Cu2O composite films on Cu foil for supercapacitors. J. Alloys Compd. 2014, 586, 745–753. [Google Scholar] [CrossRef] [Green Version]
- Ghorbani, M.; Golobostanfard, M.R.; Abdizadeh, H. Flexible freestanding sandwich type ZnO/rGO/ZnO electrode for wearable supercapacitor. Appl. Surf. Sci. 2017, 419, 277–285. [Google Scholar] [CrossRef]
- Hu, Y.; Guan, C.; Ke, Q.; Yow, Z.F.; Cheng, C.; Wang, J. Hybrid Fe2O3 nanoparticle clusters/rGO paper as an effective negative electrode for flexible supercapacitors. Chem. Mater. 2016, 28, 7296–7303. [Google Scholar] [CrossRef]
- Ali, G.A.M.; Yusoff, M.M.; Algarni, H.; Chong, K.F. One-step electrosynthesis of MnO2/rGO nanocomposite and its enhanced electrochemical performance. Ceram. Int. 2018, 44, 7799–7807. [Google Scholar] [CrossRef]
- Thalji, M.R.; Ali, G.A.M.; Liu, P.; Zhong, Y.L.; Chong, K.F. W18O49 nanowires-graphene nanocomposite for asymmetric supercapacitors employing AlCl3 aqueous electrolyte. Chem. Eng. J. 2021, 409, 128216. [Google Scholar] [CrossRef]
- Kabiri, R.; Namazi, H. Nanocrystalline cellulose acetate (NCCA)/graphene oxide (GO) nanocomposites with enhanced mechanical properties and barrier against water vapor. Cellulose 2014, 21, 3527–3539. [Google Scholar] [CrossRef]
- Kafy, A.; Sadasivuni, K.K.; Kim, H.-C.; Akther, A.; Kim, J. Designing flexible energy and memory storage materials using cellulose modified graphene oxide nanocomposites. Phys. Chem. Chem. Phys. 2015, 17, 5923–5931. [Google Scholar] [CrossRef]
- Kim, H.; Cho, M.Y.; Kim, M.H.; Park, K.Y.; Gwon, H.; Lee, Y.; Roh, K.C.; Kang, K. A novel high-energy hybrid supercapacitor with an anatase TiO2-reduced graphene oxide anode and an activated carbon cathode. Adv. Energy Mater. 2013, 3, 1500–1506. [Google Scholar] [CrossRef]
- Mianehrow, H.; Sabury, S.; Bazargan, A.; Sharif, F.; Mazinani, S. A flexible electrode based on recycled paper pulp and reduced graphene oxide composite. J. Mater. Sci. Mater. Electron. 2017, 28, 4990–4996. [Google Scholar] [CrossRef]
















| Carbon-Based Material | Composites | Preparation Methods | Applications |
|---|---|---|---|
| graphene | poly ethylenedioxythiophene-graphene | electrochemically deposition | energy storage devices [32] |
| (PEDOT:PSS)/graphene | in situ polymerization | energy harvesting systems [35] | |
| graphene/MnO2/CNTs | chemical co-precipitation method | energy storage devices as a robust electrode [38] | |
| graphene/PANI | electropolymerization | paper electrode [39] | |
| graphene/MnO2 | electrodeposition | asymmetric supercapacitor [41] | |
| TiO2/graphene/PPy | electrodeposition | supercapacitor [43]. | |
| GO/rGO | PANI/GO | polymerization | supercapacitor electrodes [59,60] |
| GO based TiO2 composite membrane | vacuum filtration | water purification system [61] | |
| nickel cobalt oxide/GO | coprecipitation | supercaps electrode [66] | |
| rGO/polypyrrole nanowires composite | in situ route | portable electronic devices [62] | |
| rGO/Cu2O | hydrothermal technique | supercaps [70] | |
| paper pulp/rGO | drop casting technique | flexible electrode [78] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Razaq, A.; Bibi, F.; Zheng, X.; Papadakis, R.; Jafri, S.H.M.; Li, H. Review on Graphene-, Graphene Oxide-, Reduced Graphene Oxide-Based Flexible Composites: From Fabrication to Applications. Materials 2022, 15, 1012. https://doi.org/10.3390/ma15031012
Razaq A, Bibi F, Zheng X, Papadakis R, Jafri SHM, Li H. Review on Graphene-, Graphene Oxide-, Reduced Graphene Oxide-Based Flexible Composites: From Fabrication to Applications. Materials. 2022; 15(3):1012. https://doi.org/10.3390/ma15031012
Chicago/Turabian StyleRazaq, Aamir, Faiza Bibi, Xiaoxiao Zheng, Raffaello Papadakis, Syed Hassan Mujtaba Jafri, and Hu Li. 2022. "Review on Graphene-, Graphene Oxide-, Reduced Graphene Oxide-Based Flexible Composites: From Fabrication to Applications" Materials 15, no. 3: 1012. https://doi.org/10.3390/ma15031012
APA StyleRazaq, A., Bibi, F., Zheng, X., Papadakis, R., Jafri, S. H. M., & Li, H. (2022). Review on Graphene-, Graphene Oxide-, Reduced Graphene Oxide-Based Flexible Composites: From Fabrication to Applications. Materials, 15(3), 1012. https://doi.org/10.3390/ma15031012








