Characterization of Five Collagenous Biomaterials by SEM Observations, TG-DTA, Collagenase Dissolution Tests and Subcutaneous Implantation Tests
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. SEM Observations
2.2.2. TG-DTA
2.2.3. Collagenase Dissolution Tests
2.2.4. Subcutaneous Implantation Tests
2.2.5. Statistical Analysis
3. Results
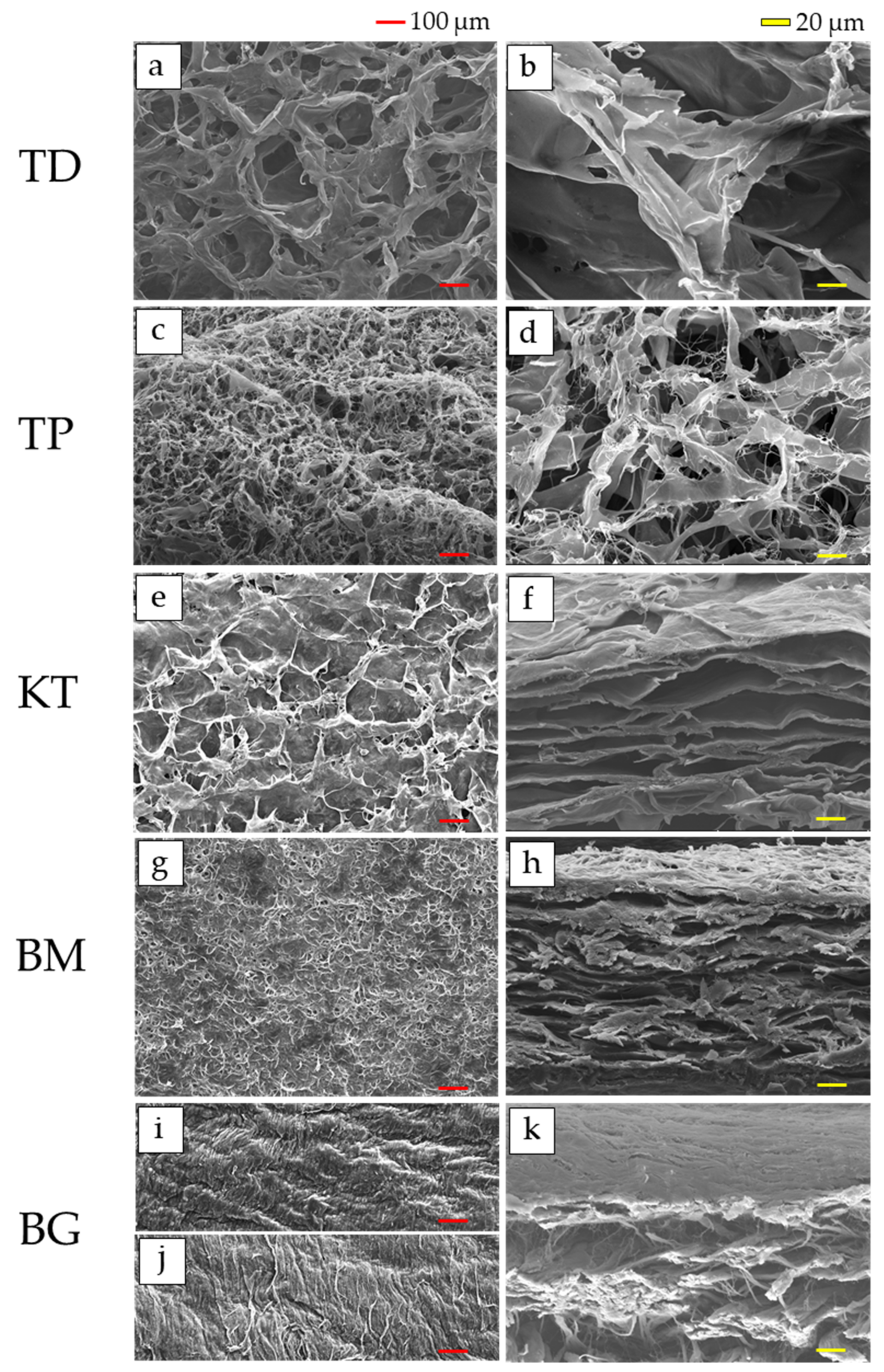
3.1. SEM Observations
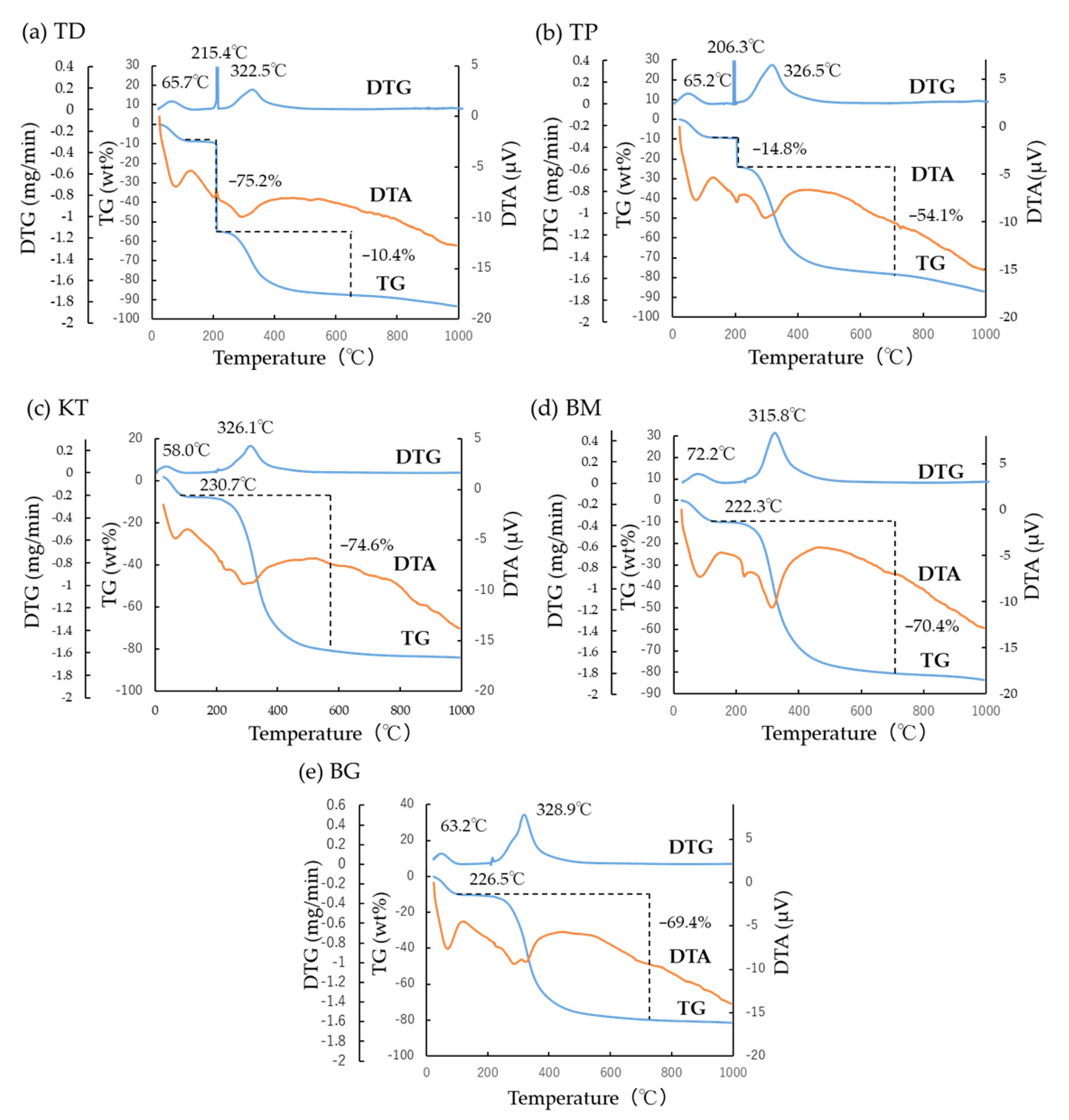
3.2. TG-DTA
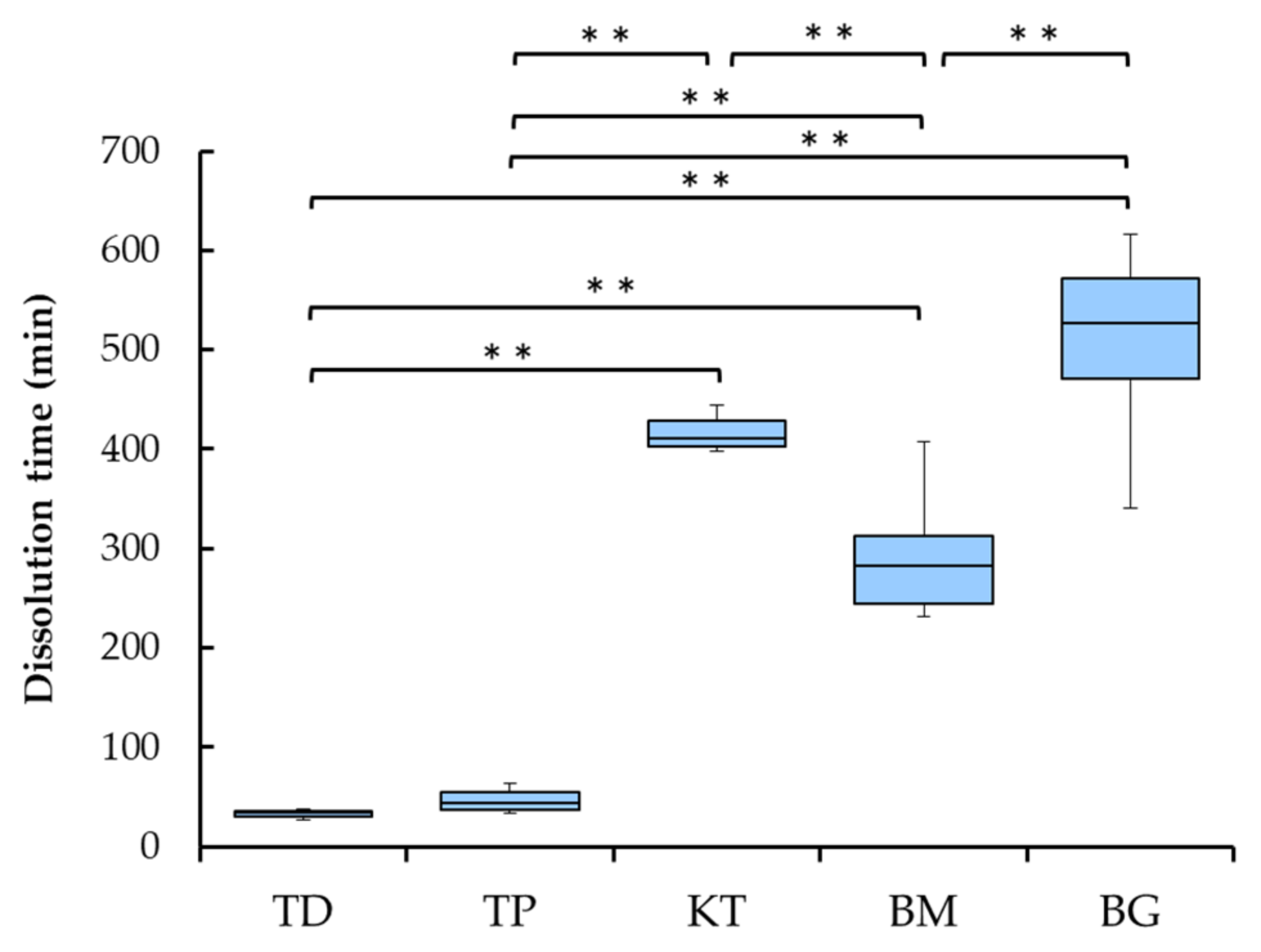
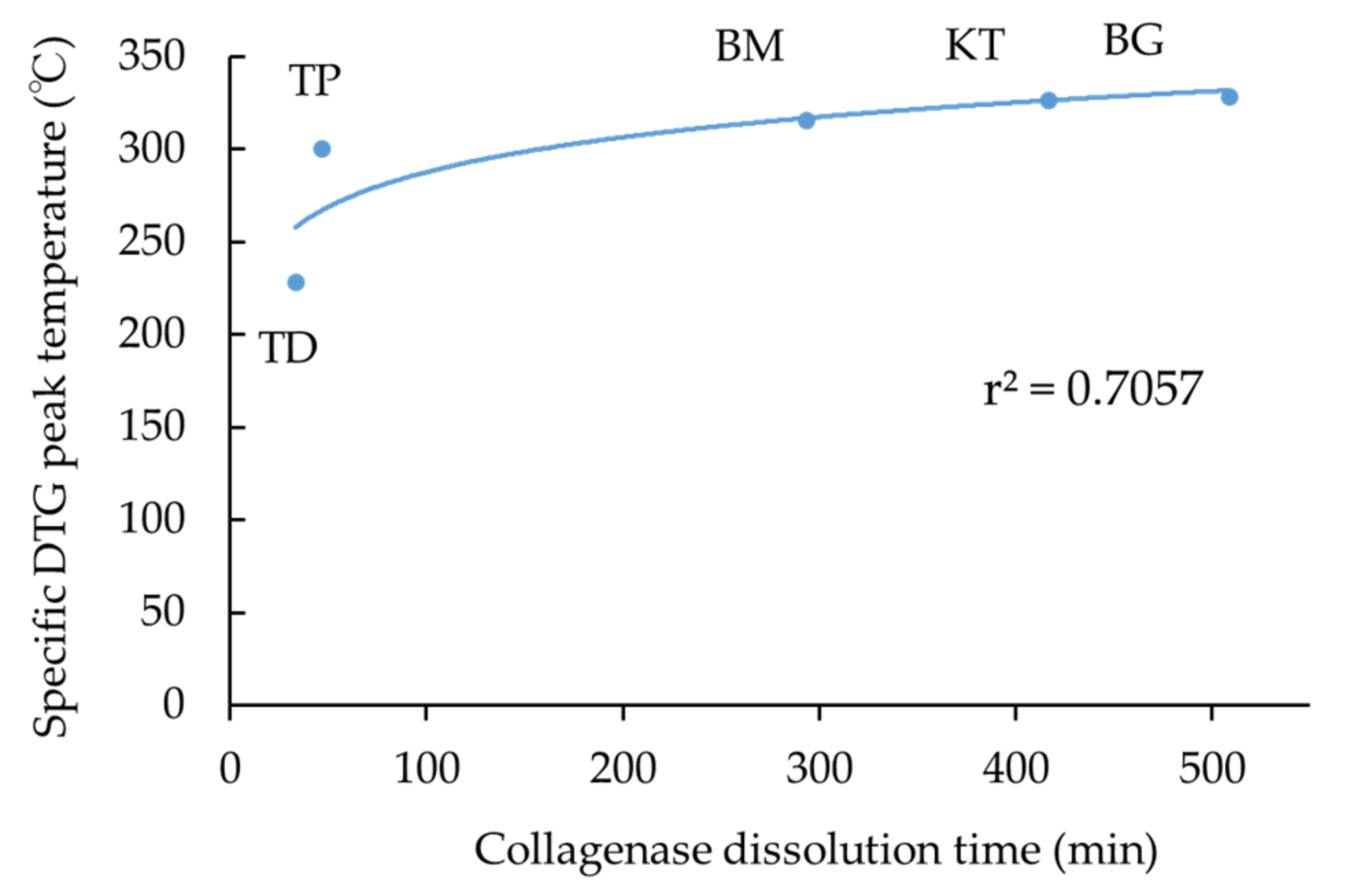
3.3. Collagenase Dissolution Tests
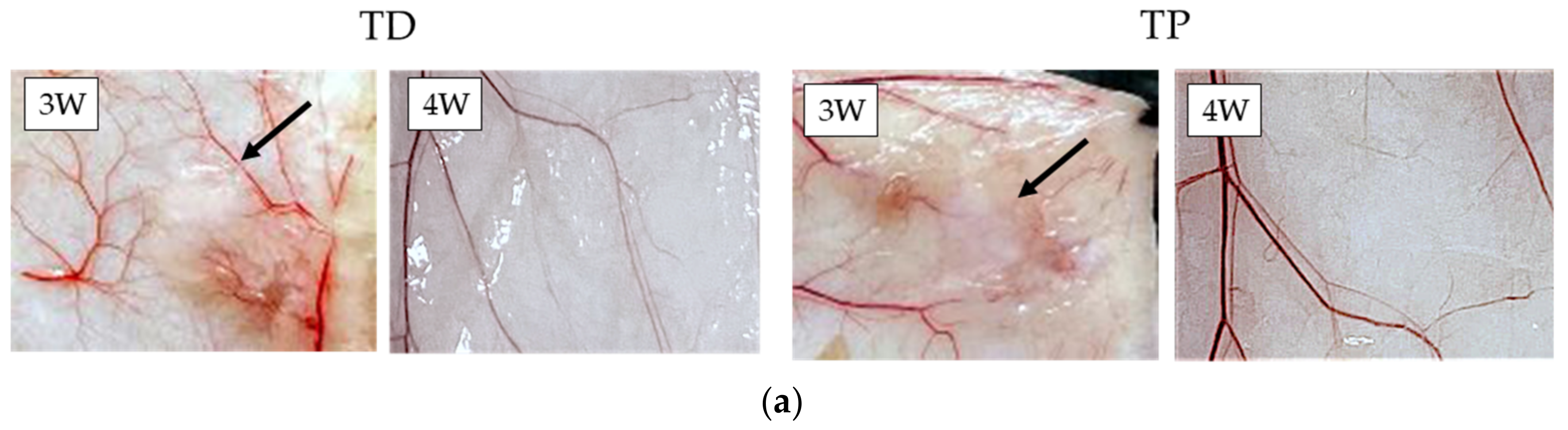
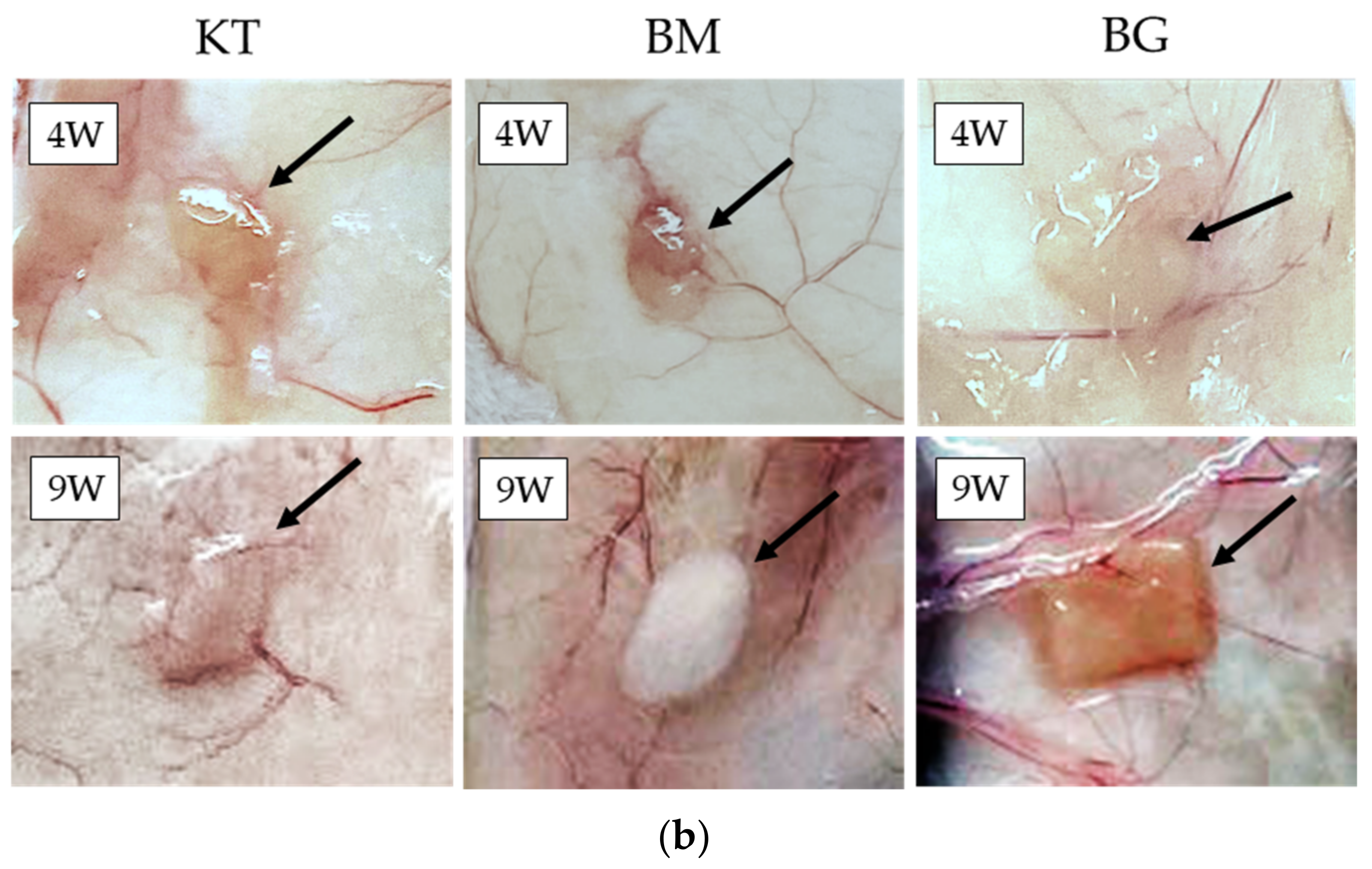
3.4. Subcutaneous Implantations Tests
4. Discussion
5. Conclusions
- SEM observations confirmed that the dermis-type and membranous-type materials were fibrous and porous;
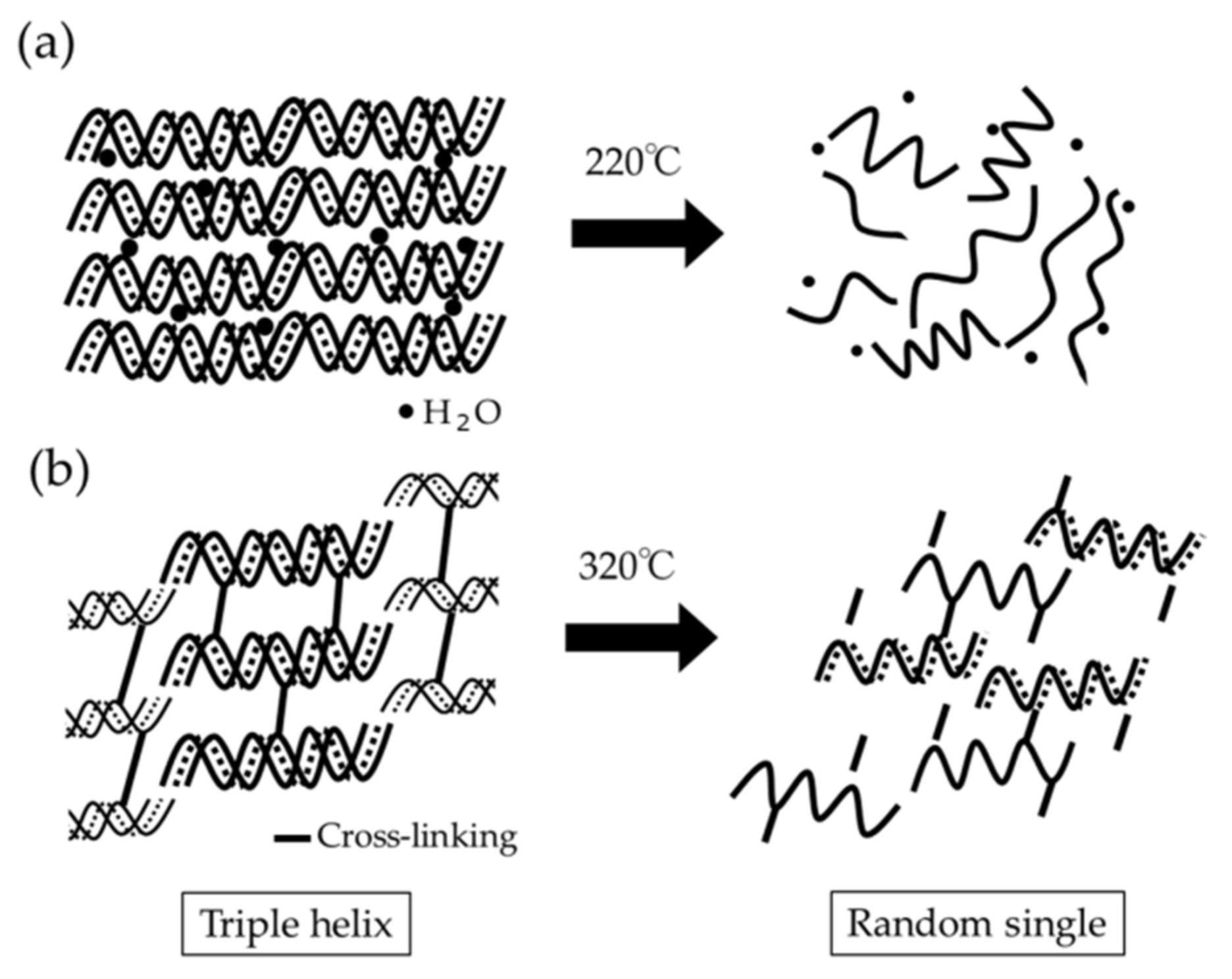
- TG-DTA revealed two characteristic DTG peak temperatures at about 220 °C and 320 °C. The combined specific DTG peak temperature appeared to imply the degree of cross-linking of collagen products;
- Collagenase dissolution tests directly indicated the level of cross-linking of collagen products, ranging from about 40 to 500 min;
- Subcutaneous implantation tests showed direct in vivo longevity of collagenous biomaterials; and
- The membranous collagenous biomaterials were more cross-linked, leading to higher specific DTG peak temperature, larger collagenase dissolution time, and longer in vivo durability, compared with dermis-type ones because of intensified degree of cross-linking in collagen fibrils.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| DSC | Differential Scanning Calorimetry |
| DTA | Differential Thermal Analysis |
| DTG | Derivative Thermal Gravimetry |
| GBR | Guided Bone Regeneration |
| GTR | Guided Tissue Regeneration |
| SEM | Scanning Electron Microscopy |
| TG-DTA | Thermogravimetry-Differential Thermal Analysis |
| 3D | Three-Dimensional |
References
- Ghomi, E.R.; Nourbakhsh, N.; Kenari, M.A.; Zare, M.; Ramakrishna, S. Collagen-based biomaterials for biomedical applications. J. Biomed. Mater. Res. B Appl. Biomater. 2021, 109, 1986–1999. [Google Scholar] [CrossRef] [PubMed]
- Wang, H. A review of the effects of collagen treatment in clinical studies. Polymers 2021, 13, 3868. [Google Scholar] [CrossRef] [PubMed]
- Ruszczak, Z. Effect of collagen matrices on dermal wound healing. Adv. Drug Deliv. Rev. 2003, 55, 1595–1611. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Levi-Polyanchenko, N.; Morykwas, M.; Argenta, L.; Wagner, W.D. Novel nanofiber-based material for endovascular scaffolds. J. Biomed. Mater. Res. A 2015, 103, 1150–1158. [Google Scholar] [CrossRef]
- Dabrowski, A.; Lepère, M.; Zaranis, C.; Coelio, C.; Hauters, P. Efficacy and safety of a resorbable collagen membrane COVA+™ for the prevention of postoperative adhesions in abdominal surgery. Surg. Endosc. 2016, 30, 2358–2366. [Google Scholar] [CrossRef]
- Kemp, S.W.K.; Syed, S.; Walsh, W.; Zochodne, D.W.; Midha, R. Collagen nerve conduits promote enhanced axonal regeneration, schwann cell association, and neovascularization compared to silicone conduits. Tissue Eng. A 2009, 15, 1975–1988. [Google Scholar] [CrossRef]
- Samarawickrama, C.; Samanta, A.; Liszka, A.; Fagerholm, P.; Buznyk, O.; Griffith, M.; Allan, B. Collagen-based fillers as alternatives to cyanoacrylate glue for the sealing of large corneal perforations. Cornea 2018, 37, 609–616. [Google Scholar] [CrossRef]
- De Mulder, E.L.W.; Hannink, G.; van Kuppevelt, T.H.; Daamen, W.F.; Buma, P. Similar hyaline-like cartilage repair of osteochondral defects in rabbits using isotropic and anisotropic collagen scaffolds. Tissue. Eng. A 2014, 20, 635–645. [Google Scholar] [CrossRef]
- Griebling, T.L.; Kreder, K.J.; Williams, R.D. Transurethral collagen injection for treatment of postprostatectomy urinary incontinence in men. Urology 1997, 49, 907–912. [Google Scholar] [CrossRef]
- Sheikh, Z.; Qureshi, J.; Alshahrani, A.M.; Nassar, H.; Ikeda, Y.; Glogauer, M.; Ganss, B. Collagen based barrier membranes for periodontal guided bone regeneration applications. Odontology 2017, 105, 1–12. [Google Scholar] [CrossRef]
- Sorushanova, A.; Delgado, L.M.; Wu, Z.; Shologu, N.; Kshirsagar, A.; Raghunath, R.; Mullen, A.M.; Bayon, Y.; Pandit, A.; Raghunath, M.; et al. The collagen suprafamily: From biosynthesis to advanced biomaterial development. Adv. Mater. 2019, 31, e1801651. [Google Scholar] [CrossRef] [Green Version]
- Lee, A.; Hudson, A.R.; Shiwarski, D.J.; Tashman, J.W.; Hinton, T.J.; Yerneni, S.; Bliley, J.M.; Campbell, P.G.; Feinberg, A.W. 3D bioprinting of collagen to rebuild components of the human heart. Science 2019, 365, 482–487. [Google Scholar] [CrossRef]
- Hotta, T.; Yokoo, S.; Terashi, H.; Komori, T. Clinic al and histopathological analysis of healing process of intraoral reconstruction with ex vivo produced oral mucosa equivalent. Kobe J. Med. Sci. 2007, 53, 1–14. [Google Scholar]
- Lin, H.-C.; Chen, C.-M.; Tung, C.-L. Postextraction ridge preservation using an artificial collagen sponge for implant site development: A case report. Taiwan J. Oral Med. Sci. 2015, 30, 68–73. [Google Scholar]
- Romagna-Genon, C. Comparative clinical study of guided tissue regeneration with a bioabsorbable bilayer collagen membrane and subepithelial connective tissue graft. J. Periodontol. 2001, 72, 1258–1264. [Google Scholar] [CrossRef]
- Stoecklin-Wasmer, C.; Rutjes, A.W.S.; da Costa, B.R.; Salvi, G.E.; Jüni, P.; Sculean, A. Absorbable collagen membranes for periodontal regeneration: A systematic review. J. Dent. Res. 2013, 92, 773–781. [Google Scholar] [CrossRef]
- Lucaciu, O.; Apostu, D.; Mester, A.; Campian, R.S.; Gheban, D.; Miron, R.J. Atelo-collagen type I bovine bone substitute and membrane in guided bone regeneration: A series of clinical cases and histopathological assessments. Histol. Histopathol. 2019, 34, 1061–1071. [Google Scholar] [CrossRef]
- Sbricoli, L.; Guazzo, R.; Annunziata, M.; Gobbato, L.; Bressan, E.; Nastri, L. Selection of collagen membranes for bone regeneration: A literature review. Materials 2020, 13, 786. [Google Scholar] [CrossRef] [Green Version]
- EI Raouf, M.A.; Kobayashi, M.F.; AbdEl-Aal, A.B.M.; Zhang, Y.; Miron, R.J. Novel bioabsorbable bovine derived atelo-collagen type I membrane: Characterization into host tissues. Periodontics Prosthodont. 2017, 3, 100032. [Google Scholar] [CrossRef] [Green Version]
- Oh, T.-J.; Meraw, S.J.; Lee, E.J.; Giannobile, W.V.; Wang, H.L. Comparative analysis of collagen membranes for the treatment of implant dehiscence defects. Clin. Oral Implants Res. 2003, 14, 80–90. [Google Scholar] [CrossRef]
- Rothamel, D.; Schwarz, F.; Sager, M.; Herten, M.; Sculean, A.; Becker, J. Biodegradation of differently cross-linked collagen membranes: An experimental study in the rat. Clin. Oral Implants Res. 2005, 16, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Bunyaratavej, P.; Wang, H.L. Collagen membranes: A review. J. Periodontol. 2001, 72, 215–229. [Google Scholar] [CrossRef] [Green Version]
- León-Mancilla, B.H.; Araiza-Téllez, M.A.; Flores-Flores, J.O.; Pina-Barba, M.C. Physico-chemical characterization of collagen scaffolds for tissue engineering. J. Appl. Res. Technol. 2006, 14, 77–85. [Google Scholar] [CrossRef]
- Qian, J.; Okada, Y.; Ogura, T.; Tanaka, K.; Hattori, S.; Ito, S.; Satoh, J.; Takita, T.; Yasukawa, K. Kinetic analysis of the digestion of bovine type I collagen telopeptides with porcine pepsin. J. Food. Sci. 2016, 81, C27–C34. [Google Scholar] [CrossRef]
- Khor, E. Methods for the treatment of collagenous tissues for bioprostheses. Biomaterials 1997, 18, 95–105. [Google Scholar] [CrossRef]
- Meyer, M. Processing of collagen based biomaterials and the resulting materials properties. Biomed. Eng. Online 2019, 18, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adamiak, K.; Sionkowska, A. Current methods of collagen cross-linking: Review. Int. J. Biol. Macromol. 2020, 161, 550–560. [Google Scholar] [CrossRef]
- Li, S.; Chen, H.; Lee, N.; Ringshia, R.; Yuen, D. A ComparativeS of Zimmer BioMend® and BioMend® Extend™ Membranes Made at Two Different Manufacturing Facilities. Available online: https://www.zimmerbiometdental.com/en (accessed on 12 December 2021).
- Aprile, P.; Letourneur, D.; Simon-Yarza, T. Membranes for guided bone regeneration: A road from bench to bedside. Adv. Healthc. Mater. 2020, 9, e2000707. [Google Scholar] [CrossRef]
- Abraham, L.C.; Zuena, E.; Perez-Ramirez, B.; Kaplan, D.L. Guide to collagen characterization for biomaterial studies. J. Biomed. Mater. Res. B Appl. Biomater. 2008, 87B, 264–285. [Google Scholar] [CrossRef]
- Caputo, I.; Lepretti, M.; Scarabino, C.; Esposito, C.; Proto, A. An acetic acid-based extraction method to obtain high quality collagen from archeological bone remains. Anal. Biochem. 2012, 421, 92–96. [Google Scholar] [CrossRef]
- Nimptsch, A.; Schibur, S.; Ihling, C.; Sinz, A.; Riemer, T.; Huster, D.; Schiller, J. Quantitative analysis of denatured collagen by collagenase digestion and subsequent MALDI-TOF mass spectrometry. Cell Tissue Res. 2011, 343, 605–617. [Google Scholar] [CrossRef]
- Drzewiecki, K.E.; Grisham, D.R.; Parmar, A.S.; Nanda, V.; Shreiber, D.I. Circular dichroism spectroscopy of collagen fibrillogenesis: A new use for an old technique. Biophys. J. 2016, 111, 2377–2386. [Google Scholar] [CrossRef] [Green Version]
- Flandin, F.; Buffevant, C.; Herbage, D. A differential scanning calorimetry analysis of the age-related changes in the thermal stability of rat skin collagen. Biochim. Biophys. Acta BBA Protein Struct. Mol. Enzymol. 1984, 791, 205–211. [Google Scholar] [CrossRef]
- Scarano, A.; Lorusso, F.; Orsini, T.; Morra, M.; Iviglia, G.; Valbonetti, L. Biomimetic surfaces coated with covalently immobilized collagen type I: An x-ray photoelectron spectroscopy, atomic force microscopy, micro-CT and histomorphometrical study in rabbits. Int. J. Mol. Sci. 2019, 20, 724. [Google Scholar] [CrossRef] [Green Version]
- Ber, S.; Köse, G.T.; Hasirci, V. Bone tissue engineering on patterned collagen films: An in vitro study. Biomaterials 2005, 26, 1977–1986. [Google Scholar] [CrossRef]
- Liang, H.; Russell, S.J.; Wood, D.J.; Tronci, G. A hydroxamic acid-methacrylated collagen conjugate for the modulation of inflammation-related MMP upregulation. J. Mater. Chem. B 2018, 6, 3703–3715. [Google Scholar] [CrossRef] [Green Version]
- Everaerts, F.; Torrianni, M.; Hendriks, M.; Feijen, J. Quantification of carboxyl groups in carbodiimide cross-linked collagen sponges. J. Biomed. Mater. Res. A 2007, 83A, 1176–1183. [Google Scholar] [CrossRef] [Green Version]
- Natarajan, V.; Krithica, N.; Madhan, B.; Sehgal, P.K. Preparation and properties of tannic acid cross-linked collagen scaffold and its application in wound healing. J. Biomed. Mater. Res. B Appl. Biomater. 2013, 101B, 560–567. [Google Scholar] [CrossRef]
- Bozec, L.; Odlyha, M. Thermal denaturation studies of collagen by microthermal analysis and atomic force microscopy. Biophys. J. 2011, 101, 228–236. [Google Scholar] [CrossRef] [Green Version]
- Tegza, M.; Andreyeva, O.; Maistrenko, L. Thermal analysis of collagen preparations. Chem. Technol. 2012, 59, 40–45. [Google Scholar] [CrossRef]
- Unsal, B.; Kurtiş, B.; Ozcan, G.; Ozdemir, A.; Karaöz, E. An investigation of resorption and tissue reaction after subcutaneous implantation of collagen based membrane materials in rats. J. Marmara Univ. Dent. Fac. 1997, 2, 609–615. [Google Scholar] [PubMed]
- De Kok, I.J.; Jere, D.; Padilla, R.J.; Cooper, L.F. Evaluation of a collagen scaffold for cell-based bone repair. Int. J. Oral Maxillofac. Implants 2014, 29, e122–e129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.H.C.; Guo, Q.; Li, B. Tendon biomechanics and mechanobiology--a minireview of basic concepts and recent advancements. J. Hand Ther. 2012, 25, 133–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flecknell, P. Replacement, reduction, and refinement. ALTEX Altern. Anim. Exp. 2002, 19, 73–78. [Google Scholar]
- Engeland, C.G.; Sabzehei, B.; Marucha, P.T. Sex hormones and mucosal wound healing. Brain. Behav. Immun. 2009, 23, 629–635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwata, S.; Matsuzaka, K.; Inoue, T. Effects of an atelocollagen sponge during the wound healing of tooth extraction sockets at an early stage. Oral Med. Pathol. 2010, 15, 15–20. [Google Scholar] [CrossRef]
- Wang, H.L.; O’Neal, R.B.; Thomas, C.L.; Shyr, Y.; MacNeil, R.L. Evaluation of an absorbable collagen membrane in treating Class II furcation defects. J. Periodontol. 1994, 65, 1029–1036. [Google Scholar] [CrossRef]
- Zhang, L.; Dong, Y.; Zhang, N.; Shi, J.; Zhang, X.; Qi, C.; Midgley, A.C.; Wang, S. Potentials of sandwich-like chitosan/polycaprolactone/gelatin scaffolds for guided tissue regeneration membrane. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 109, 110618. [Google Scholar] [CrossRef] [PubMed]
- Matsui, R. Development of TERUDERMIS, collagen-based artificial dermis and TRRUPLUG, collagen-based material for extraction sockets. J. Jpn. Assoc. Regener. Dent. 2008, 6, 9–20. (In Japanese) [Google Scholar] [CrossRef]
- Light, A.N.; Bailey, A.J. Collagen cross-links: Location of pyridinoline in type I collagen. FEBS Lett. 1985, 182, 503–508. [Google Scholar] [CrossRef] [Green Version]
- Olde Damink, L.H.H.; Dijkstra, P.J.; Van Luyn, M.J.A.; Van Wachem, P.B.; Nieuwenhuis, P.; Feijen, J. Crosslinking of dermal sheep collagen using hexamethylene diisocyanate. J. Mater. Sci. Mater. Med. 1995, 6, 429–434. [Google Scholar] [CrossRef]
- Sakae, T. Method of Analyzing Cross-Linked Collagen. JP Pantent 2003329626A, 19 November 2003. Available online: https://patents.google.com/patent/JP2003329626A/en (accessed on 12 December 2021).
- Perez-Puyana, V.; Ostos, F.J.; López-Cornejo, P.; Romero, A.; Guerrero, A. Assessment of the denaturation of collagen protein concentrates using different techniques. Biol. Chem. 2019, 400, 1583–1591. [Google Scholar] [CrossRef]
- Bozkurt, A.; Apel, C.; Sellhaus, B.; van Neerven, S.; Wessing, B.; Hilgers, R.-D.; Pallua, N. Differences in degradation behavior of two non-cross-linked collagen barrier membranes: An in vitro and in vivo study. Clin. Oral Implants Res. 2014, 25, 1403–1411. [Google Scholar] [CrossRef]
- Yussof, S.J.M.; Omar, E.; Pai, D.; Sood, S. Cellular events and biomarkers of wound healing. Indian J. Plast. Surg. 2012, 45, 220–228. [Google Scholar] [CrossRef]
- Abe, G.L.; Sasaki, J.; Katata, C.; Kohno, T.; Tsuboi, R.; Kitagawa, H.; Imazato, S. Fabrication of novel poly(lactic acid/caprolactone) bilayer membrane for GBR application. Dent. Mater. 2020, 36, 626–634. [Google Scholar] [CrossRef]
- Sasaki, J.; Abe, G.L.; Li, A.; Thongthai, P.; Tsuboi, R.; Kohno, T.; Imazato, S. Barrier membranes for tissue regeneration in dentistry. Biomater. Investig. Dent. 2021, 8, 54–63. [Google Scholar] [CrossRef]
- Wang, J.; Wang, L.; Zhou, Z.; Lai, H.; Xu, P.; Liao, L.; Wei, J. Biodegradable polymer membranes applied in guided bone/tissue regeneration: A review. Polymers 2016, 8, 115. [Google Scholar] [CrossRef]
- Doillon, C.J.; Drouin, R.; Côte, M.F.; Dallaire, N.; Pageau, J.F.; Laroche, G. Chemical inactivators as sterilization agents for bovine collagen materials. J. Biomed. Mater. Res. 1997, 37, 212–221. [Google Scholar] [CrossRef]
| Product Name | Tissue Source (Cross-Linking) | Main Usage | Lot No. | Code |
|---|---|---|---|---|
| Terudermis | Bovine dermis (Yes) | Artificial dermis | M10063 | TD |
| Teruplug | Bovine dermis (Yes) | Filling of tooth extraction socket | M1007F | TP |
| Koken Tissue Guide | Bovine dermis and tendon (Yes) | GTR membrane | 19050A | KT |
| Biomend | Bovine tendon (Yes) | GTR/GBR membrane | 1112022 | BM |
| Bio-gide | Porcine dermis (No) | GTR/GBR membrane | 81901547 | BG |
| Sample | Water Evaporation | Peak Temperature 1 | Peak Temperature 2 | Remained | Specific Peak Temperature |
|---|---|---|---|---|---|
| TD | 65.7 °C | 215.4 °C | 322.5 °C | 228.4 °C | |
| (7.0 wt%) | (75.2 wt%) | (10.4 wt%) | (7.4 wt%) | ||
| TP | 65.2 °C | 206.3 °C | 326.5 °C | 300.7 °C | |
| (9.0 wt%) | (14.8 wt%) | (54.1 wt%) | (22.1 wt%) | ||
| KT | 58.0 °C | 230.7 °C | 326.5 °C | 326.5 °C | |
| (7.5 wt%) | (virtually 0 wt%) | (74.6 wt%) | (17.9 wt%) | ||
| BM | 72.2 °C | 222.3 °C | 315.8 °C | 315.8 °C | |
| (9.8 wt%) | (virtually 0 wt%) | (70.4 wt%) | (19.8 wt%) | ||
| BG | 63.2 °C | 226.5 °C | 328.9 °C | 328.5 °C | |
| (9.7 wt%) | (0.3 wt%) | (69.1 wt%) | (20.9 wt%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoshi, M.; Sawada, T.; Hatakeyama, W.; Taira, M.; Hachinohe, Y.; Takafuji, K.; Kihara, H.; Takemoto, S.; Kondo, H. Characterization of Five Collagenous Biomaterials by SEM Observations, TG-DTA, Collagenase Dissolution Tests and Subcutaneous Implantation Tests. Materials 2022, 15, 1155. https://doi.org/10.3390/ma15031155
Hoshi M, Sawada T, Hatakeyama W, Taira M, Hachinohe Y, Takafuji K, Kihara H, Takemoto S, Kondo H. Characterization of Five Collagenous Biomaterials by SEM Observations, TG-DTA, Collagenase Dissolution Tests and Subcutaneous Implantation Tests. Materials. 2022; 15(3):1155. https://doi.org/10.3390/ma15031155
Chicago/Turabian StyleHoshi, Miki, Tomofumi Sawada, Wataru Hatakeyama, Masayuki Taira, Yuki Hachinohe, Kyoko Takafuji, Hidemichi Kihara, Shinji Takemoto, and Hisatomo Kondo. 2022. "Characterization of Five Collagenous Biomaterials by SEM Observations, TG-DTA, Collagenase Dissolution Tests and Subcutaneous Implantation Tests" Materials 15, no. 3: 1155. https://doi.org/10.3390/ma15031155






