Review on Micro-Alloying and Preparation Method of 7xxx Series Aluminum Alloys: Progresses and Prospects
Abstract
:1. Introduction
2. Micro-Alloying
2.1. Main Alloy Elements
2.2. Microalloying Elements
2.2.1. Zr and Mn Transition Elements
2.2.2. Cr and Ag Microalloying Elements
2.3. Rare Earth Elements
2.3.1. Er
2.3.2. Sc
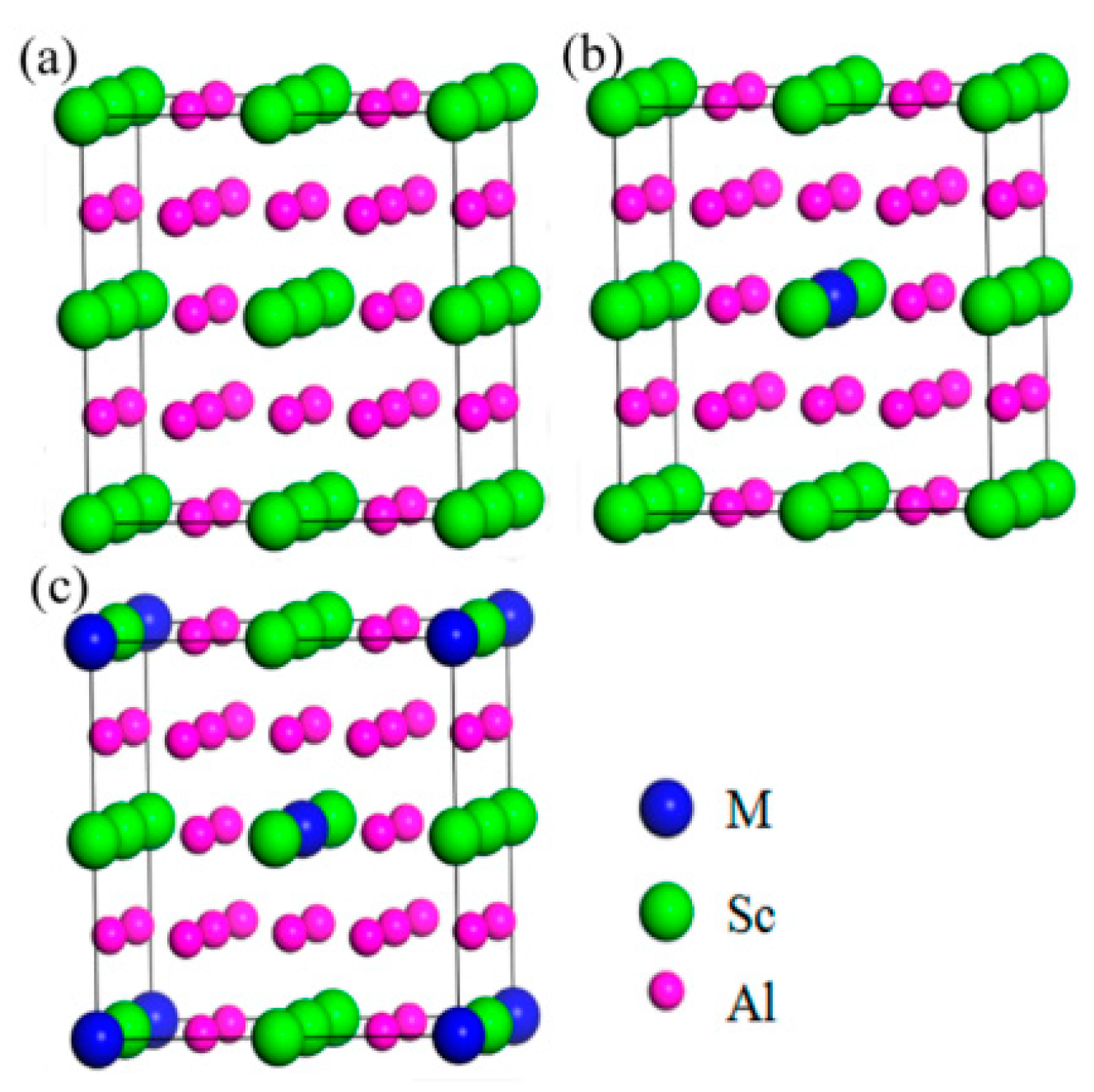
2.3.3. Sc and Zr
2.3.4. Y
2.3.5. Gd
2.4. Non-Metallic Inclusions Element
3. Aging Precipitation Sequence and Strengthening-Toughening Mechanism of 7xxx Series Aluminum Alloy
3.1. Precipitated Sequence
3.2. Strengthening and Toughening Mechanism
3.2.1. Strengthening
3.2.2. Strengthening and Toughening Method
3.2.3. First-Principle Calculation of Precipitation Strengthening Phase
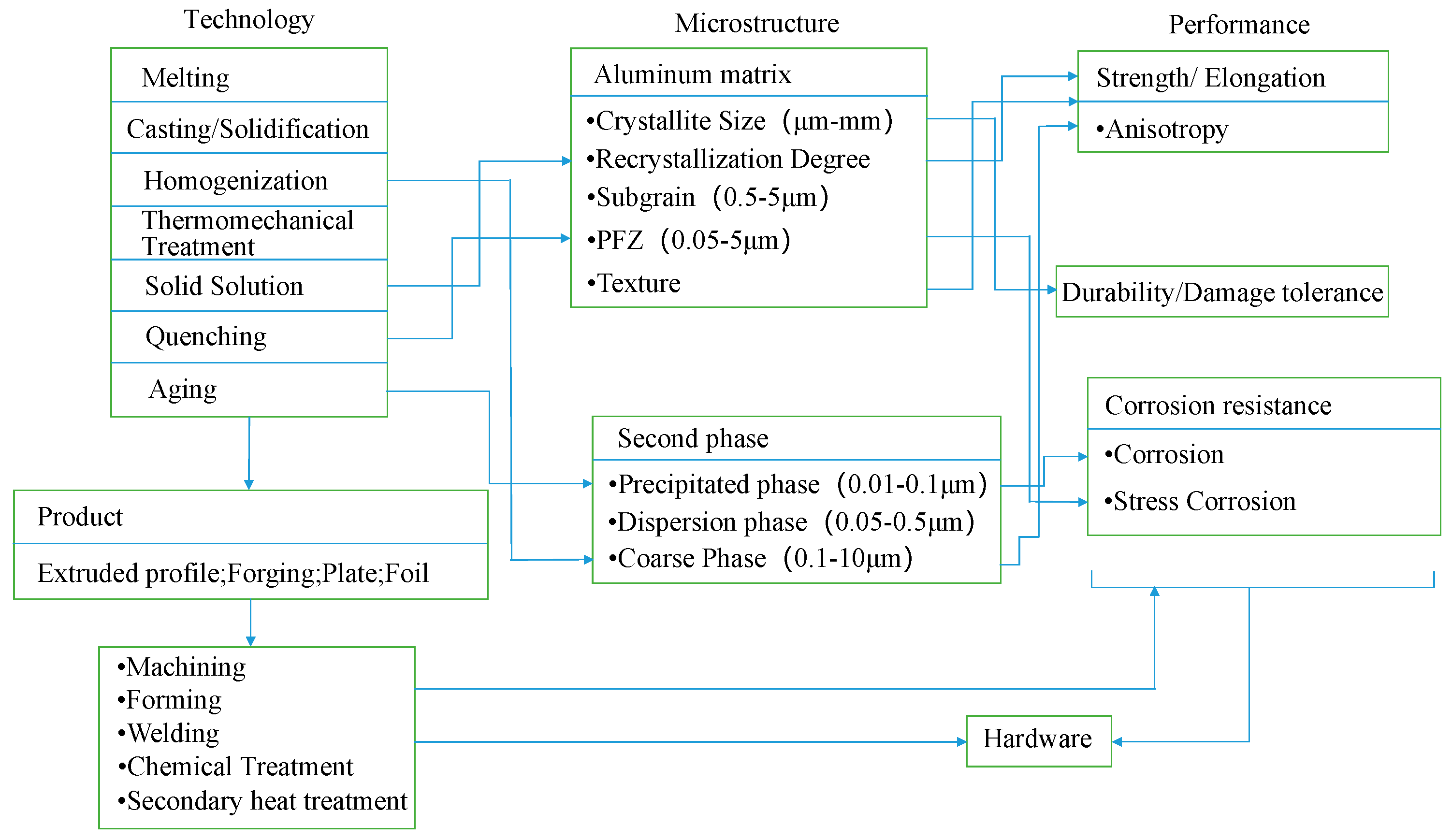
4. Preparation Method of 7xxx Series Aluminum Alloy
4.1. Casting Processes
4.2. Multistage Homogenization Heat Treatment of Ingot
4.3. Forming Process
4.4. Heat Treatment Method
4.4.1. Solution Treatment
4.4.2. Aging Treatment
5. Challenges and Conclusions
- To investigate the total amount and proportion of the main alloy elements and the action law of microalloying elements, Thermo-Calc, VASP, Factsage, Materials Studio and other software can be employed to integrate theoretical calculation, simulation and experiment. The research and development, performance improvement and product development period of alloys can be considerably shortened, and the efficiency can be improved immensely.
- Under the guidance of the calculation results of the first-principles theory, the microstructure-property characterization method of aluminum alloy was continuously improved according to the relationship among alloy composition, process, microstructure and property. The new principle and characterization method of the process-microstructure-property correlation of materials was developed to explore the characteristic microstructure 7xxx series aluminum alloy materials with high static strength, high strength, heat resistance, high toughness, damage resistance, low density, low quenching sensitivity, and high comprehensive performance, at the cutting edge.
- The η′ Phase is the only strengthening phase in the alloy, in order to increase the volume fraction of η′ precipitates and modify strength, rare earth elements should be added to the alloy, while controlling the Mg/Zn mass ratio in the range of 5:2–7:1 can refine the precipitates and improve the strength of the alloy and keep the alloy corrosion resistant.
- Sc rare earth element is currently the most significant alloying element, Al3(Sc,Zr) particles with L12 structure precipitated at any time into coherent Al3(Sc,Zr) particles with D023 structure can effectively hinder recrystallization, inhibit grain growth, and improve the mechanical properties of the alloy. Er and Sc rare earth elements are relatively low, and the eutectic point composition of Sc is the lowest, w (Sc)% ≈ 0.3%, w (Er) ≈ 1.0%. Therefore, it can be perceived that the properties of 7xxx series aluminum alloy with Sc addition will be remarkably improved. Er is much cheaper than Sc; consequently, Er is a potential rare earth element to replace Sc.
- The width, GBPs sum, distribution and continuity of the average PFZ determine the overall performance of the material; the purpose of different homogenization, multi-stage aging and RRA heat treatment is to adjust the method suitably to achieve material properties, according to grain size, solute atoms, GBPs and microstructure of precipitates.
- Although the heat treatment process of 7xxx series aluminum alloy requires controlling time lapses in the process of heating, holding and cooling, and there are many species precipitates in the crystal and the evolution mechanism is heterogeneous, the precipitated strengthening phase is a single type of equilibrium η′ phase. Therefore, for the heat treatment process, the grain boundary precipitate can be selected as the object, and the non-isothermal regression kinetic model was established to guide the accurate regulation of aging process parameters. More attention should be paid to how to increase the volume fraction of η′ precipitates and modify the comprehensive performance of the material by the regression re-aging method.
- Asynchronous rolling introduces shear strain and increases the deformation of the core plate, which can effectively solve the serious uneven strain distribution of aluminum alloy plate in the symmetric rolling process. The snake rolling method can not only refine the grain, but also change the crystal structure, therefore, it can be popularized for use.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Azarniya, A.; Abolfazl, T.; Karimi, T.; Kourosh, K. Recent advances in ageing of 7xxx series aluminum alloys: A physical metallurgy perspective. J. Alloys Compd. 2019, 781, 945–983. [Google Scholar] [CrossRef]
- Wu, H.; Wen, S.P.; Lu, J.T.; Mi, Z.P.; Zeng, X.L.; Huang, H.; Nie, Z.R. Microstructural evolution of new type Al-Zn-Mg-Cu alloy with Er and Zr additions during homogenization. Trans. Nonferr. Met. Soc. China 2017, 7, 1476–1482. [Google Scholar] [CrossRef]
- Zhou, B.; Liu, B.; Zhang, S.G. The advancement of 7xxx series aluminum alloys for aircraft structures: A review. Metals 2021, 11, 718. [Google Scholar] [CrossRef]
- Imran, M.; Khan, A.A. Characterization of Al-7075 metal matrix composites: A review. J. Mater. Res. Technol. 2019, 8, 3347–3356. [Google Scholar] [CrossRef]
- Wen, K.; Xiong, B.Q.; Zhang, Y.A.; Wang, G.J.; Li, X.W.; Li, Z.H.; Huang, S.H.; Liu, H.W. Microstructure Evolution of a High Zinc Containing Al-Zn-Mg-Cu Alloy during Homogenization. Rare Met. Mat Eng. 2017, 4, 928–934. [Google Scholar]
- Prabhu, T.R. An overview of high-performance aircraft structural Al alloy-AA7085. Acta Met. Sin. 2015, 28, 909–921. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Dong, L.; Wang, H.D.; Zhou, Y.X.; Gao, C. Research progress on corrosion fatigue of aerospace aluminum alloy. Surf. Technol. 2021, 7, 106–118. [Google Scholar]
- UmamaheshweRrao, A.C.; Vasu, M.; Govindaraju, K.V.; SaiSrinadh, K. Stress corrosion cracking behaviour of 7xxx series aluminum alloys: A literature review. Trans. Nonferr. Met. Soc. China 2016, 26, 1447–1471. [Google Scholar]
- Wagner, J.A.; Shenoy, R.N. The effect of copper, chromium, and zirconium on the microstructure and mechanical properties of Al-Zn-Mg-Cu alloy. Met. Trans. A 1991, 22, 2809. [Google Scholar] [CrossRef]
- Yuan, S.; Jia, L.N.; Zhang, H. Effects of Er on the microstructure and eutectic phase morphology of directionally solidified Al-Zn-Mg-Cu-Zr alloys. Rare Met. Mater. Eng. 2021, 50, 3477–3484. [Google Scholar]
- Pang, X.Z. Research on the Effect of Alloying on Some Important Phases, Interfaces and Microstructure Evolution and Performance of Al-Zn-Mg-Cu Alloy. Ph.D. Thesis, South China University of Technology, Guangzhou, China, 2017. [Google Scholar]
- Li, H.; Tang, K.; Wang, H.; Wang, K.S.; Li, Y.K.; Wu, Y.C. Precipitation behavior of Al-Zn-Mg-Cu-Sc-Zr alloy during homogenization process. Chin. J. Nonferr. Met. 2021, 31, 2403–2411. [Google Scholar]
- Lee, S.H.; Jung, J.G.; Bail, S.I.; Seidman, D.N.; Kim, M.S.; Lee, Y.K.; Euh, K.J. Precipitation strengthening in naturally aged Al–Zn–Mg–Cu alloy. Mater. Sci. Eng. A 2021, 803, 140719. [Google Scholar] [CrossRef]
- Senkov, O.N.; Bhat, R.B.; Senkova, S.V.; Schloz, J.D. Microstructure and properties of cast ingots of Al-Zn-Mg-Cu alloys modified with Sc and Zr. Met. Mater. Trans. A 2005, 36, 2115–2126. [Google Scholar] [CrossRef]
- Xu, D.; Li, Z.H.; Wang, G.J.; Li, X.W.; Lv, X.Y.; Zhang, Y.A.; Fan, Y.Q.; Xiong, B.Q. Phase transformation and microstructure evolution of an ultra-high strength Al-Zn-Mg-Cu alloy during homogenization. Mater. Charact. 2017, 131, 285–297. [Google Scholar] [CrossRef]
- Xia, P.; Wang, S.C.; Huang, H.L.; Zhou, N.; Song, D.F.; Jia, Y.W. Effect of Sc and Zr additions on recrystallization behavior and intergranular corrosion resistance of Al-Zn-Mg-Cu alloys. Materials 2021, 14, 5516. [Google Scholar] [CrossRef]
- Gu, D.D.; Zhang, H.; Liu, G.; Yang, B.Q. Process optimization of additive manufactured sandwich panel structure using rare earth element modified high-performance Al alloy. Acta Aeronaut. Astronaut. Sin. 2021, 10, 454–466. [Google Scholar]
- Xu, X.F.; Zhao, Y.G.; Zhang, M.; Ning, Y.H.; Wang, X.D. Effect of extrusion ratio on the microstructure and mechanical properties in an Al-Cu-Mg-Ag alloy. J. Wuhan Univ. Technol. 2018, 33, 710–714. [Google Scholar] [CrossRef]
- Zhang, X.L.; Chen, S.Y.; Zhou, L.; Fan, S.M.; Chen, K.H. Effect of composition on quenching sensitivity and microstructures-properties of super-strength Al-Zn-Mg-Cu aluminum alloys. Rare Met. 2019, 43, 561–570. [Google Scholar]
- Wang, Y.C.; Cao, L.F.; Wu, X.D.; Zou, Y.; Huang, G.J. Research progress on microstructure and properties of 7xxx series aluminum alloys for oil drill Pipes. Mater. Rev. 2019, 33, 1190–1197. [Google Scholar]
- Li, Y. Effect of Alloy Elements on Microstructure and Hot Tearing Susceptibility in Direct-Chill Casting of 7xxx Series Aluminum Alloys. Ph.D. Thesis, University of Science and Technology Beijing, Beijing, China, 2019. [Google Scholar]
- Dai, X.Y.; Li, N.; Xiong, L. Research progress of hardenability of Al-Cu-Mg-Ag alloys. Mater. Rev. 2015, 29, 75–80. [Google Scholar]
- Zhao, Q.; Wang, S.X. Selection and Design of Aluminum Alloy, 1st ed.; Chemical Industry Press: Beijing, China, 2017; pp. 34–35. [Google Scholar]
- Fang, H.C.; Yang, H.L.; Zhu, J.M.; Xiao, P.; Chen, Z.; Liu, T. Effect of minor Cr, Mn, Zr or Ti on recrystallization, secondary phases and fracture behaviour of Al-Zn-Mg-Cu-Yb Alloys. Rare Met. Mater. Eng. 2020, 49, 797–810. [Google Scholar]
- Pan, Y.L. Study on Compositonal Design, Microstructure and Properties of Novel High-Strenghness Al-Zn-Mg-Cu Aluminum Alloy with Good Weldability and Corrosion Resistance. Ph.D. Thesis, University of Science and Technology Beijing, Beijing, China, 2021. [Google Scholar]
- Gao, Y.H.; Liu, G.; Sun, J. Recent progress in high-temperature resistant aluminum-based alloys: Microstructural design and precipitation strategy. Acta Met. Sin. 2021, 57, 129–149. [Google Scholar]
- Bhuiyan, M.S.; Toda, H.; Uesugi, K.; Takeuchi, A.; Watanabe, Y. Damage micromechanisms in high Mn and Zn content 7xxx series aluminum alloys. Mater. Sci. Eng. A 2020, 793, 139423. [Google Scholar] [CrossRef]
- Valeev, I.S.; Barykin, N.P.; Trifonov, V.G.; Valeeva, A.K. Effect of powerful current pulses on the structure and mechanical properties of the aluminum alloy Al-6%Mg-0.6%Mn. J. Mater. Eng. Perform. 2014, 14, 236–240. [Google Scholar] [CrossRef]
- Wang, Z.P.; Wang, M.L.; Li, Y.G.; Xiao, H.Y.; Chen, H.; Geng, J.W.; Li, X.F.; Chen, D.; Wang, H.W. Effect of pretreatment on microstructural stability and mechanical property in a spray formed Al-Zn-Mg-Cu alloy. Mater. Des. 2021, 203, 109618. [Google Scholar] [CrossRef]
- Hu, G.Y.; Zhu, C.Z.; Xu, D.F.; Dong, P.X.; Chen, K.H. Effect of cerium on microstructure, mechanical properties and corrosion properties of Al-Zn-Mg alloy. J. Rare Earth 2021, 39, 208–216. [Google Scholar] [CrossRef]
- Chai, W.R.; Chen, J.C.; Liu, S.D.; Ye, L.Y.; Lin, H.Q.; Zhang, X.M. Effect of minor Zr addition on exfoliation corrosion resistance of Al-Zn-Mg-Mn alloy sheet. Chin. J. Mater. Res. 2019, 33, 488–496. [Google Scholar]
- Fang, H.C.; Chao, H.; Chen, K.H.; Zhang, Z. Microstructures fracture and localized corrosion behaviors of Al-Zn-Mg-Cu alloy with Zr, Yb and Cr additions. Rare Met. 2015, 39, 686–695. [Google Scholar]
- Feng, C.; Shou, W.B.; Liu, H.Q.; Yi, D.Q.; Feng, Y.R. Microstructure and mechanical properties of high strength Al−Zn−Mg−Cu alloys used for oil drill pipes. Trans. Nonferr. Met. Soc. China 2015, 25, 3515–3522. [Google Scholar] [CrossRef]
- Xu, X.F. Study on the Microstructure Evolution and Toughening-Strengthening in Al-Cu-Mg-(Ag)/Al-Zn-Mg-Cu-(Ag) Alloys. Ph.D. Thesis, Jilin University, Jilin, China, 2015. [Google Scholar]
- Yu, C.; Chen, L.P.; Zhou, Q. Research progress in effects of rare earth elements on microstructure and properties of aluminum alloy. Spec. Cast Nonferr. Alloy. 2021, 41, 241–246. [Google Scholar]
- Wang, D.W.; Fu, Y.D.; Li, T.; Cong, F.G. Role of rare earth elements in wrought aluminum alloys and their development trend. Light Alloy. Fabr. Technol. 2020, 48, 19–24. [Google Scholar]
- Xiao, J.M.; Huo, M.Y. Theoretical and Applied Researches on Rare Earths in China, 1st ed.; Higher Education Press: Beijing, China, 1992; pp. 81–83. [Google Scholar]
- Liu, G.H. Rare Earth Materials and Application Technology, 1st ed.; Chemical Industry Press: Beijing, China, 2005; pp. 151–152. [Google Scholar]
- Liu, L.H.; Liu, L.F.; Cao, F.H.; Yang, T.; Yin, P.Z. Effect of Er element on impact deformation and failure behaviors of 7xxx series aluminum alloy. Ordnance Mater. Sci. Eng. 2020, 43, 125–130. [Google Scholar]
- Huang, Y.C.; Zhang, C.C.; Ren, X.W.; Liu, Y.; Chen, S.Z.; Wang, Y.L. Existence form of trace Er in Al-Zn-Mg-Cu alloy and its “genetic effect”. Rare Metal Mater. Eng. 2019, 48, 2848–2856. [Google Scholar]
- Li, Z.M.; Jiang, H.C.; Wang, Y.L.; Zhang, D.; Yan, D.S.; Rong, L.J. Effect of minor Sc addition on microstructure and stress corrosion cracking behavior of medium strength Al-Zn-Mg alloy. J. Mater. Sci. Technol. 2018, 34, 1172–1179. [Google Scholar] [CrossRef]
- Liu, L. Sc and Zr Microalloying Effects and Microscopic Mechanisms in Al Alloys. Ph.D. Thesis, Harbin Institute of Technology, Harbin, China, 2020. [Google Scholar]
- Xiao, Q.F.; Huang, J.W.; Jiang, Y.G.; Jiang, F.Q.; Wu, Y.F.; Xu, G.F. Effects of minor Sc and Zr additions on mechanical properties and microstructure evolution of Al-Zn-Mg-Cu alloys. Trans. Nonferr. Met. Soc. China 2020, 30, 1429–1438. [Google Scholar] [CrossRef]
- Li, G.F.; Zhang, X.M.; Zhu, H.F. Effect of minor Er and Y additions to Al-Zn-Mg-Cu-Zr alloy on homogenizing behavior. J. Aeronaut Mater. 2010, 30, 1–6. [Google Scholar]
- Chen, K.H.; Jian, S.S.; Zhou, L.; Chen, S.Y.; Huang, L.P. Effect of trace rare earth element Gd on microstructureand corrosion resistance of 7056 aluminum alloy. J. Hunan Univ. Nat. Sci. Ed. 2020, 47, 96–107. [Google Scholar]
- Mei, F.Q.; Wang, S.H.; Fang, C.F.; Meng, L.G.; Jia, F.; Hao, H.; Zhang, X.G. Effect of Gd content on microstructures and mechanical properties of Al-Zn-Mg-Cu-Zr alloys. Chin. J. Nonferr. Met. 2012, 22, 2439–2447. [Google Scholar]
- Rokhlin, L.L.; Dobatkina, T.V.; Bochvar, N.R. Investigation of phase equilibria in alloys of the Al-Zn-Mg-Cu-Zr-Sc system. J. Alloys Compd. 2004, 367, 10–16. [Google Scholar] [CrossRef]
- Zhang, X.M.; Deng, Y.L. China’s Strategic Emerging Industry-New Alloy Materials, 1st ed.; China Railway Publishing House: Beijing, China, 2018; pp. 25–26. [Google Scholar]
- Won, S.J.; So, H.K.; Leeseung, O.; Soong, J.K.; Kyou, H. Development of a high-strength Al-Zn-Mg-Cu-based alloy via multi-strengthening mechanisms. Scr. Mater. 2021, 205, 114216. [Google Scholar] [CrossRef]
- Yu, X.W. Study on the Relationship between 3D Microstructure and Properties of Al-Zn-Mg-(Cu) Alloy During Pre-Deformation and Aging. Ph.D. Thesis, Hunan University, Hunan, China, 2020. [Google Scholar]
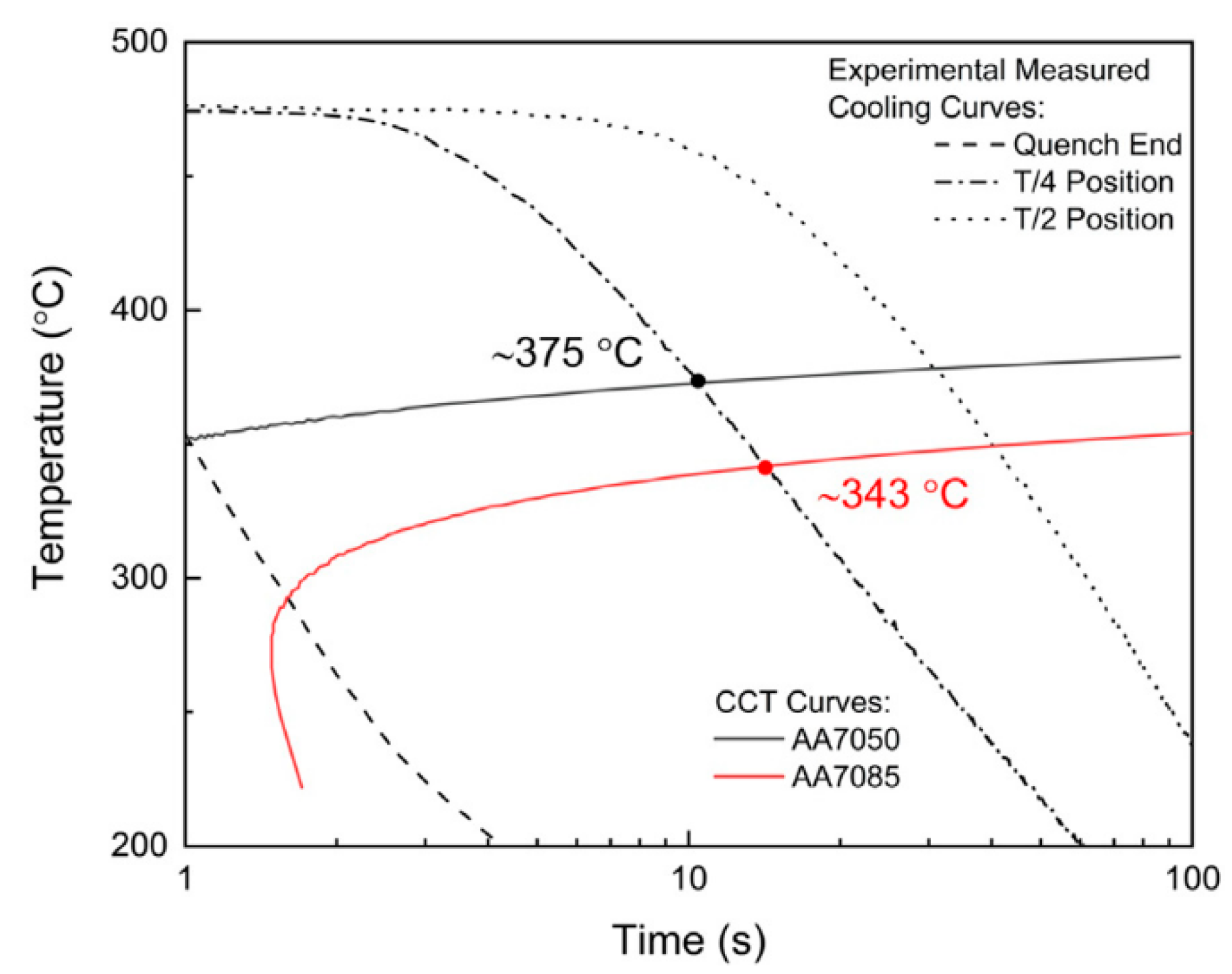
- Priya, P.; Johnson, D.R.; Krane, M.J.M. Precipitation during cooling of 7xxx series aluminum alloys. Comput. Mater. Sci. 2017, 139, 273–284. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, H.P.; Kang, W.; Zhang, X.M. Aging microstructure evolution in high strength aluminum alloys and performance controlling. Chin. J. Nonferr. Met. 2017, 27, 1323–1336. [Google Scholar]
- Yu, X.W.; Chen, J.H.; Ming, W.Q.; Yang, X.B.; Zhao, T.T.; Shen, R.H.; He, Y.T.; Wu, C.L. Revisiting the Hierarchical Microstructures of an Al-Zn-Mg Alloy fabricated by pre-deformation and aging. Acta Met. Sin. 2020, 33, 1518–1526. [Google Scholar] [CrossRef]
- Bakhshi, R.; Farshidi, M.H.; Sajjadi, S.A. Strengthening of aluminium alloy 7005 through the imposition of severe plastic deformation supplemented by different ageing treatments. Chin. J. Nonferr. Met. 2021, 31, 2909–2921. [Google Scholar] [CrossRef]
- Zang, J.X.; Dai, P.; Yang, Y.Q.; Liu, S.; Huang, B.; Ru, J.G.; Luo, X. Study on the Relationship between High Temperature Mechanical Properties and Precipitates Evolution of 7085 Al Alloy after Long Time Thermal Exposures. Metals 2021, 11, 1483. [Google Scholar] [CrossRef]
- Khalfallah, A.; Raho, A.A.; Amzert, S.; Djemli, A. Precipitation kinetics of GP zones, metastable η′ phase and equilibrium η phase in Al-5.46wt.%Zn-1.67wt.%Mg alloy. Trans. Nonferr. Met. Soc. China 2019, 29, 233–241. [Google Scholar] [CrossRef]
- Berg, L.K.; Gjoønnes, J.; Hansen, V.; Li, X.Z.; Knutson-Wedel, M.; Waterloo, G.; Schryvers, D.; Wallenberg, L.R. GP-zones in Al-Zn-Mg alloys and their role in artificial aging. Acta Mater. 2001, 49, 3443–3451. [Google Scholar] [CrossRef]
- Bendo, A.; Matsuda, K.; Nishimura, K.; Nunomura, N.; Tsuchiya, T.; Lee, S.; Marioara, C.D.; Tsuru, T.; Yamaguchi, M.; Shimizu, K.; et al. The possible transition mechanism for the meta-stable phase in the 7xxx aluminium. Mater. Sci. Technol. Lond. 2020, 36, 1621–1627. [Google Scholar] [CrossRef]
- Chen, Y.X. Microstructure and Texture in the Surface Gradient Nanostructured Nickle and Aluminum Alloys. Ph.D. Thesis, Northwestern Polytechnical University, Xi’an, China, 2018. [Google Scholar]
- Garbiec, D.; Leshchynsky, V.; Garcia-Junceda, A.; Swadzba, R.; Siwak, P.; Adamek, G.; Radwanski, K. Microstructure and mechanical properties of spark plasma sintered and severely deformed AA7075 alloy. Metals 2021, 11, 1433. [Google Scholar] [CrossRef]
- Wang, Y.C.; Tong, X.; You, G.Q.; Cao, L.F. Research progress and prospects of the microstructures, properties, and forming techniques of Al-Li Alloy. Rare Met. Mater. Eng. 2021, 50, 1069–1083. [Google Scholar]
- Cui, Z.Q.; Tan, Y.C. Metallography and Heat Treatment, 2nd ed.; Machinery Industry Press: Beijing, China, 2008; pp. 176–178. [Google Scholar]
- Shu, W.X. Solidification Characteristics and Strengthening-Thoughening Mechanisms of 7xxx al Alloys with Tailored Mg and Cu Elements. Ph.D. Thesis, University of Science and Technology Beijing, Beijing, China, 2015. [Google Scholar]
- Dai, X.Y.; Xia, C.Q.; Liu, C.B.; Gu, Y. Effects of solution treatment and aging process on microstructure and mechanical properties of 7xxx aluminium alloy. Trans. Mater. Heat Treat. 2007, 4, 59–63. [Google Scholar]
- Yu, H.Q. Principle of Metal Plastic Forming, 1st ed.; Machinery Industry Press: Beijing, China, 2018; pp. 12–13. [Google Scholar]
- Huang, K.F.; Qin, Z.; Zhou, W.B.; Ren, Y.L.; Huang, L.; Xiang, J.; Tang, H.Q.; Zhu, Y.T.; Wei, Y.Z. Enhancement of strength mechanical and corrosion resistance of 7055 alloy with minor Sc and Y addition. Mater. Res. Express 2021, 8, 016524. [Google Scholar] [CrossRef]
- Yan, Y.C.; Huang, B.; Li, W.J.; Qing, P.L.; He, B. Research progress of Al-Zn-Mg-Cu Ultra-high Strength aluminum alloy. Mater. Rev. 2018, 32, 358–364. [Google Scholar]
- Zhang, X.G.; Mei, F.Q.; Zhang, H.Y.; Wang, S.H.; Fang, C.F.; Hao, H. Effects of Gd and Y additions on microstructure and properties of Al-Zn-Mg-Cu-Zr alloys. Mater. Sci. Eng. A 2012, 552, 230–235. [Google Scholar] [CrossRef]
- Huang, L.P.; Chen, K.H.; Li, S. Influence of grain-boundary pre-precipitation and corrosion characteristics of inter-granular phases on corrosion behaviors of an Al-Zn-Mg-Cu alloy. Mater. Sci. Eng. B 2012, 177, 862–868. [Google Scholar] [CrossRef]
- Guan, R.G.; Tie, D. A Review on Grain Refinement of Aluminum Alloys: Progresses, Challenges and Prospects. Acta Met. Sin. 2017, 30, 409–432. [Google Scholar] [CrossRef]
- Charit, I.; Mishra, R. Effect of friction stir processed microstructure on tensile properties of an Al-Zn-Mg-Sc alloy upon subsequent aging heat treatment. J. Mater. Sci. Technol. 2018, 34, 214–218. [Google Scholar] [CrossRef]
- Garner, A.; Euesden, R.; Yao, Y.; Aboura, Y.; Zhao, H.; Donoghue, J.; Curioni, M.; Gault, B.; Shanthraj, P.; Barrett, Z.; et al. Multiscale analysis of grain boundary microstructure in high strength 7xxx Al alloys. Acta Mater. 2021, 202, 190–210. [Google Scholar] [CrossRef]
- Menzemer, C.C.; Lam, D.F.; Srivatsan, T.S. An investigation of strain concentration in high-strength Al-Zn-Mg-Cu alloy 7085 subjected to tensile deformation. J. Mater. Eng. Perform. 2010, 5, 705–713. [Google Scholar] [CrossRef]
- Deschamps, A.; Hutchinson, C.R. Precipitation kinetics in metallic alloys: Experiments and modeling. Acta Mater. 2021, 220, 117338. [Google Scholar] [CrossRef]
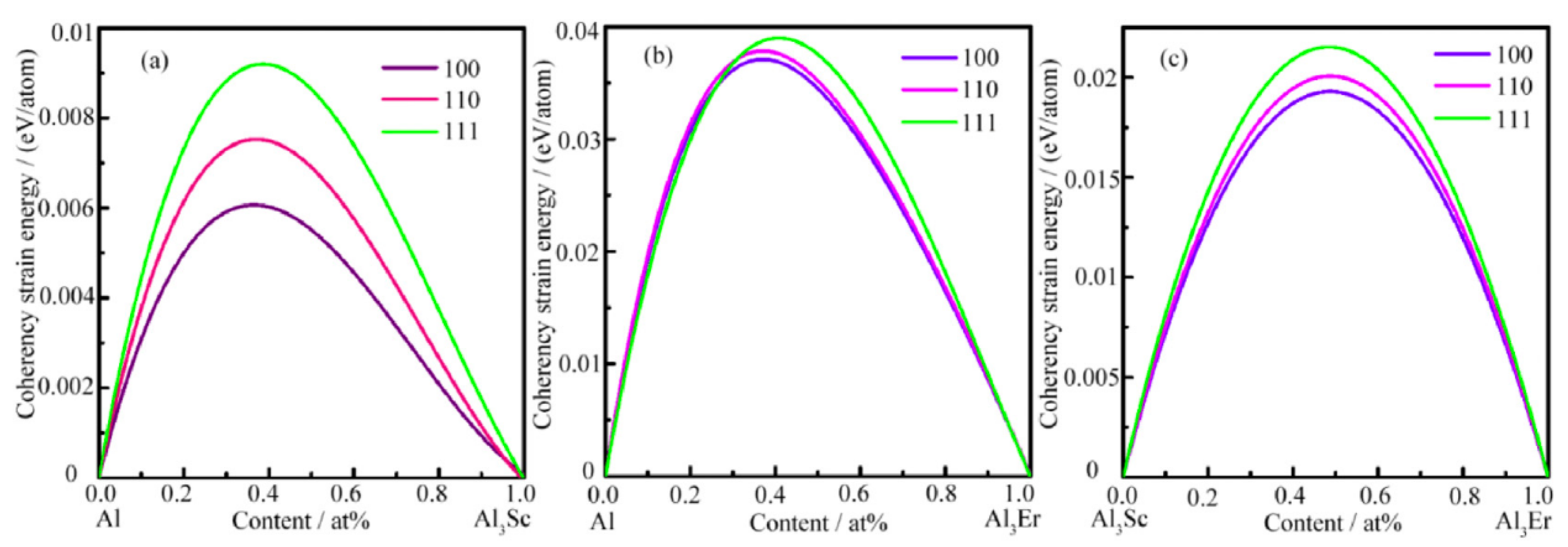
- Liu, X.M.; Wang, Q.; Zhao, C.; Li, H.P.; Wang, M.L.; Chen, D.; Wang, H.W. Formation of ordered precipitates in Al-Sc-Er-(Si/Zr) alloy from first-principles study. J. Rare Earths 2021, 39, 609–620. [Google Scholar] [CrossRef]
- Chen, D.; Xia, C.J.; Liu, X.M.; Wu, Y.; Wang, M.L. The effect of alloying elements on the structural stability, and mechanical and electronic properties of Al3Sc: A first-principles study. Materials 2019, 12, 1539. [Google Scholar] [CrossRef] [Green Version]
- Sun, S.P.; Li, X.P.; Lei, W.N.; Wang, H.J.; Wang, X.C.; Jiang, H.F.; Li, R.X.; Jiang, Y.; Yi, D.Q. Calculation of point defect structures and bonding behavior of L12-Al3Sc intermetallic based on first-principles. Chin. J. Nonferr. Met. 2013, 23, 2147–2155. [Google Scholar]
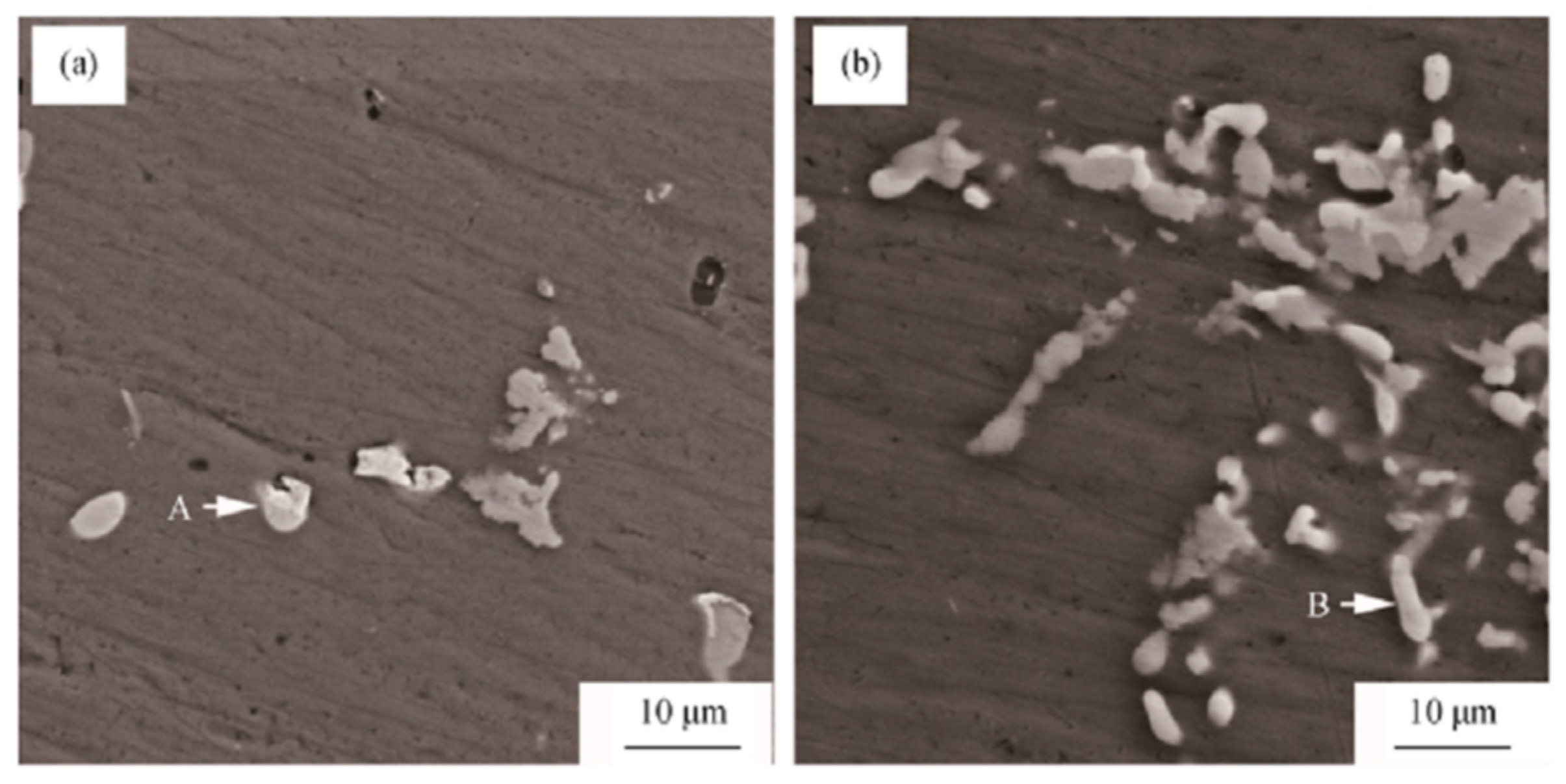
- Peng, G.S.; Gu, Y.C.; Chen, S.Y.; Chen, K.H.; Fang, H.C.; Song, G.S. Research progress of relationship between multi-scale second phase particles and properties of Al-Zn-Mg-Cu alloys. Rare Met. Mater. Eng. 2021, 50, 775–786. [Google Scholar]
- Deng, Y.L.; Zhang, X.M. Development of aluminium and aluminium alloy. Chin. J. Nonferr. Met. 2019, 29, 2115–2141. [Google Scholar]
- Rometsch, P.A.; Zhang, Y.; Knight, S. Heat treatment of 7xxx series aluminium alloys-Some recent developments. Trans. Nonferr. Met. Soc. China 2014, 24, 2003–2017. [Google Scholar] [CrossRef]
- Ye, Z.Y. Corrosion Behavior of New Aluminum Alloy and the Effect of Surface Modification. Ph.D. Thesis, Northwestern Polytechnical University, Xi’an, China, 2015. [Google Scholar]
- Hu, H.Q. Principle of Metal Solidification, 2nd ed.; Machinery Industry Press: Beijing, China, 2000; pp. 190–192. [Google Scholar]
- Bai, Q.L. Study on the Cracking Defects during Direct-Chill Casting of 7xxx Series Aluminum Alloys. Ph.D. Thesis, University of Science and Technology Beijing, Beijing, China, 2016. [Google Scholar]
- Guo, F.B.; Zhu, B.H.; Jin, L.B.; Wang, G.J.; Yan, H.W.; Li, Z.H.; Zhang, Y.A.; Xiong, B.Q. Microstructure and mechanical properties of 7A56 aluminum alloy after solution treatment. Rare Met. 2021, 40, 168–175. [Google Scholar] [CrossRef]
- Zhang, J.L.; Song, B.; Wei, Q.S.; Bourell, D.; Shi, Y.S. A review of selective laser melting of aluminum alloys: Processing, microstructure, property and developing trends. J. Mater. Sci. Technol. 2019, 35, 270–284. [Google Scholar] [CrossRef]
- Wang, T.; Yin, Z.M.; Sun, Q. Effect of homogenization treatment on microstructure and hot workability of high strength 7B04 aluminium alloy. Chin. J. Nonferr. Met. 2007, 2, 335–339. [Google Scholar] [CrossRef]
- Liu, C.Y.; Teng, G.B.; Ma, Z.Y.; Wei, L.L.; Zhang, B.; Chen, Y. Effects of Sc and Zr microalloying on the microstructure and mechanical properties of high Cu content 7xxx Al alloy. Int. J. Min. Met. Mater. 2019, 26, 1559–1569. [Google Scholar] [CrossRef]
- Jia, M.; Zheng, Z.Q.; Gong, Z. Microstructure evolution of the 1469 Al-Cu-Li-Sc alloy during homogenization. J. Alloys Compd. 2014, 614, 131–139. [Google Scholar] [CrossRef]
- Gazizov, M.; Teleshov, V.; Zakharov, V.; Kaibyshev, R. Solidification behaviour and the effects of homogenisation on the structure of an Al–Cu–Mg–Ag–Sc alloy. J. Alloys Compd. 2011, 509, 9497–9507. [Google Scholar] [CrossRef]
- Manfredi, D.; Bidulský, R. Laser powder bed fusion of aluminum alloys. Acta Metall. Slovaca. 2017, 23, 276–282. [Google Scholar] [CrossRef] [Green Version]
- Bidulská, J.; Bidulský, R.; Grande, M.A.; Kvačkaj, T. Different formation routes of pore structure in aluminum powder metallurgy alloy. Materials 2019, 12, 3724. [Google Scholar] [CrossRef] [Green Version]
- Rai, R.; De, A.; Bhadeshia, H.K.D.H.; DebRoy, T. Review: Friction stir welding tools. Sci. Technol. Weld. Joi. 2011, 16, 325–342. [Google Scholar] [CrossRef]
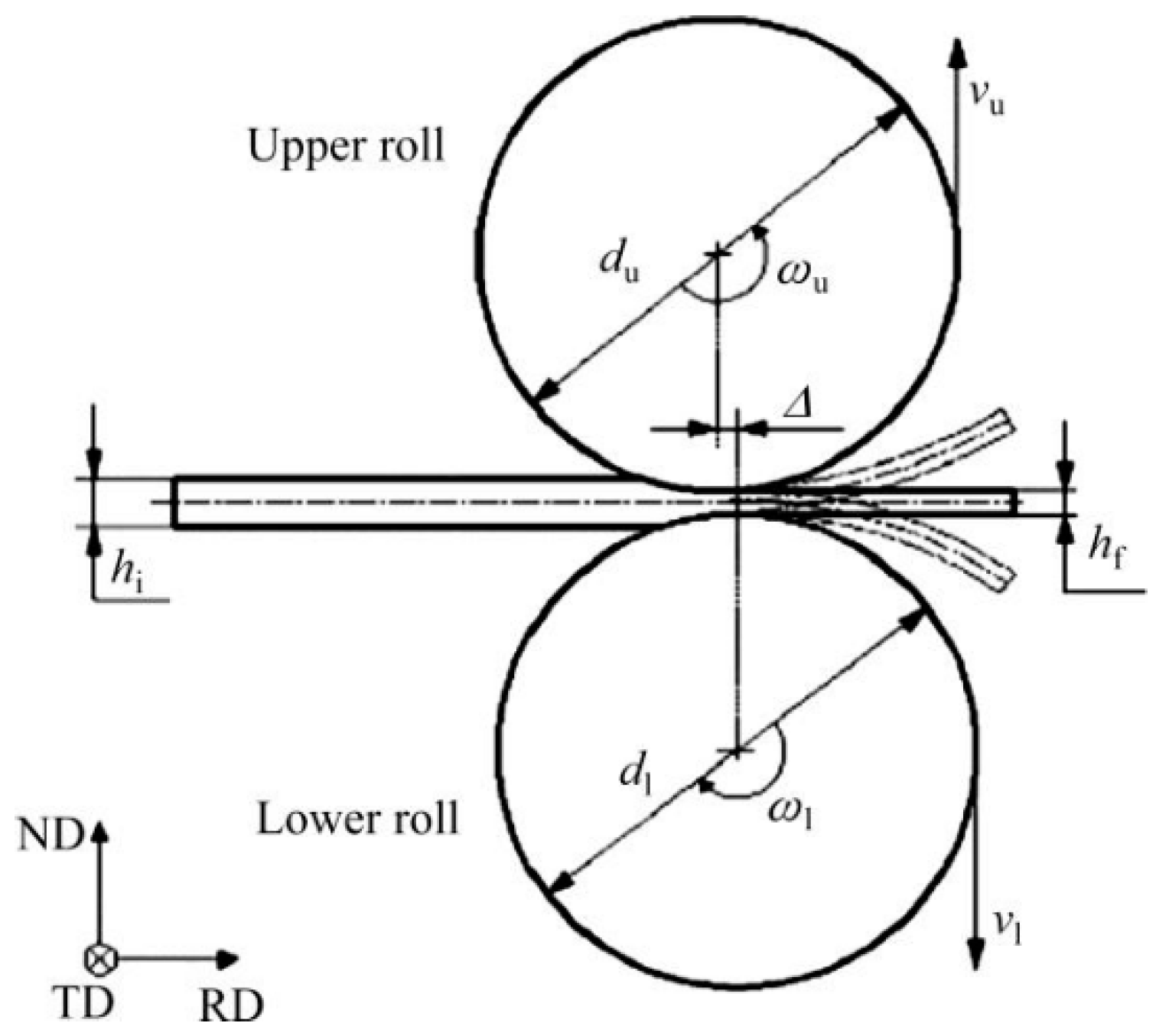
- Xu, F.S.; Zhang, J.; Deng, Y.L.; Zhang, X.M. Effect of snake rolling on strength, toughness and microstructure of Al-Cu-Mg alloy plate. Chin. J. Nonferr. Met. 2017, 27, 2005–2011. [Google Scholar]
- Xia, W.J. Researeh on Special Rolling Techniques of Wrought Magnesium Alloy Sheet. Ph.D. Thesis, Hunan University, Hunan, China, 2010. [Google Scholar]
- Li, S.; Qin, N.; Liu, J.; Zhang, X. Microstructure, texture and mechanical properties of AA1060 aluminum plate processed by snake rolling. Mater. Des. 2016, 90, 1010–1017. [Google Scholar] [CrossRef]
- Zhang, T.; Wu, Y.X.; Gong, H.; Zheng, X.Z.; Jiang, S.S. Effects of rolling parameters of snake hot rolling on strain distribution of aluminum alloy 7075. Trans. Nonferr. Met. Soc. China 2014, 24, 2150–2156. [Google Scholar] [CrossRef]
- Qin, G.H.; Yang, Y.; Li, Q.; Lin, F. Analysis and prediction of muti-pass snake hot rolling for 7075 aluminum alloy thick plate. Opt. Precis. Eng. 2017, 25, 437–446. [Google Scholar]
- Song, M.; Liu, X.H.; Liu, X.; Liu, L.Z. Ultrafine microstructure and texture evolution of aluminum foil by asymmetric rolling. J. Ccent. S. Univ. 2017, 24, 2783–2792. [Google Scholar] [CrossRef]
- Liang, S.B. Extrusion and Heat Treatment of Aluminum Alloy, 1st ed.; Central South University Press: Changsha, China, 2015; pp. 470–472. [Google Scholar]
- Feng, D.; Liu, S.D.; Han, N.M.; Chen, H.M.; Cao, W.K.; Han, Z.J. Multifactorial effects on microstructure, properties and through-thickness inhomogeneity of 7A55-RRA treated aluminum alloy thick plate. Chin. J. Nonferr. Met. 2019, 29, 1150–1160. [Google Scholar]
- Wang, J.Y. The Mechanism Research of Strength, Thoughness and Aging Stability of AA7021 Aluminum Alloy Thin Plates. Ph.D. Thesis, University of Science and Technology Beijing, Beijing, China, 2019. [Google Scholar]
- Wang, F.F.; Meng, W.; Zhang, H.W.; Han, Z.Q. Effects of under-ageing treatment on microstructure and mechanical properties of squeeze-cast Al-Zn-Mg-Cu alloy. Trans. Nonferr. Met. Soc. China 2018, 28, 1920–1927. [Google Scholar] [CrossRef]
- Wang, X.G.; Ma, L.Y. Microstructure and property Evolution of Al-Zn-Mg-Cu alloy with Ho addition during homogenization. Rare Met. Mater. Eng. 2021, 50, 2771–2776. [Google Scholar]
- Zhang, K.R.; Xu, X.J.; Zhang, J. Effect of multistage solution and aging treatment on microstructure and properties of 7xxx series ultra-high strength cold extruded aluminum alloy. Heat Treat Metal. 2021, 46, 165–168. [Google Scholar]
- Zhang, C.S.; Zhang, Z.G.; Liu, M.P.; Bao, E.C.; Chen, L.; Zhao, G.Q. Effects of single- and multi-stage solid solution treatments on microstructure and properties of as-extruded AA7055 helical profile. Trans. Nonferr. Met. Soc. China 2021, 31, 1885–1901. [Google Scholar] [CrossRef]
- Liu, P.; Hu, L.L.; Zhang, Q.H.; Yang, C.P.; Yu, Z.S.; Zhang, J.Q.; Hu, J.M.; Cao, F.H. Effect of aging treatment on microstructure and corrosion behavior of Al-Zn-Mg aluminum alloy in aqueous solutions with different aggressiveions. J. Mater. Sci. Technol. 2021, 64, 85–98. [Google Scholar] [CrossRef]
- Qu, M.; Tang, J.G.; Ye, L.Y.; Li, C.B.; Li, J.X.; Zhou, W.; Deng, Y.L. Comparative study on the effect of over-aging and addition of Zr on the corrosion resistance of Al-Zn-Mg alloy. Mater. Rev. 2020, 34, 2083–2087. [Google Scholar]
- Zheng, X.; Tang, J.G.; Zhang, Y.; Chen, M.Y.; Liu, S.D.; Zhu, Y.T.; He, K.H.; Zhang, X.M. Effect of intermittent aging on mechanical properties and local corrosion properties of Al-Zn-Mg-Cu aluminum alloy thick plate. Chin. J. Nonferr. Met. 2022. Available online: http://kns.cnki.net/kcms/detail/43.1238.tg.20211022.1156.003.html (accessed on 20 January 2022).
- Ding, Q.W. Microstructure and Properties of Age-Hardenable Al-Mg-Zn Aluminum Alloy and the Process Optimization. Ph.D. Thesis, University of Science and Technology Beijing, Beijing, China, 2019. [Google Scholar]
- Gokhan, O.; Ahmet, K. Properties of AA7075 aluminum alloy in aging and retrogression and reaging process. Trans. Nonferr. Met. Soc. China 2017, 27, 2357–2362. [Google Scholar]
- Liao, B. Basic Research on Deformation Behavior and Heat Treatment Characteristics of 7055 Aluminum Alloy Thick Plate. Ph.D. Thesis, Chongqing University, Chongqing, China, 2019. [Google Scholar]
- Jaburek, N.; Merklein, M. Influence of a retrogression and reaging (RRA)-treatment on the mechanical and microstructural characteristics of the aluminium alloy AlZn4.5Mg1. Prod. Eng. Res. Devel. 2015, 9, 161–166. [Google Scholar] [CrossRef]
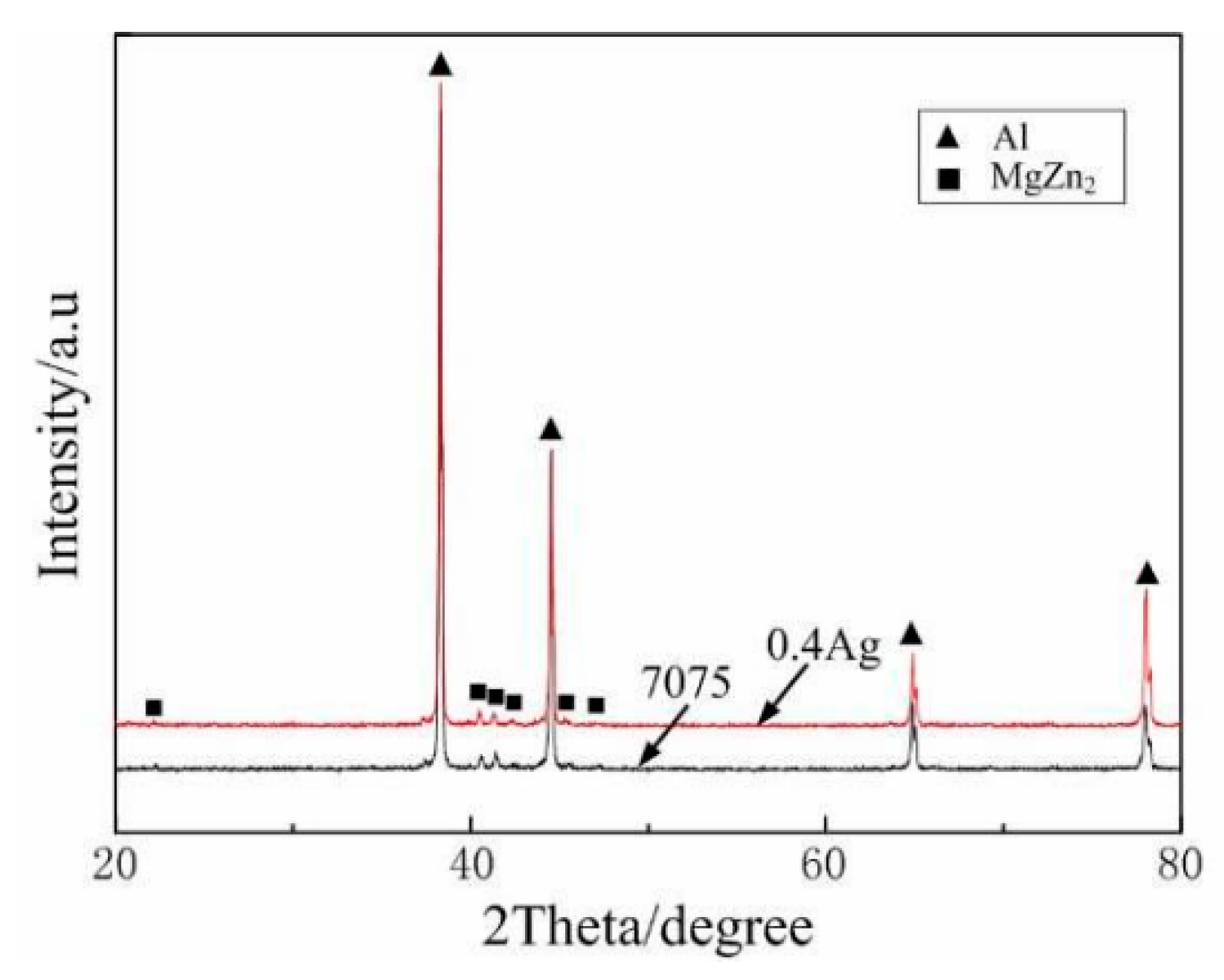
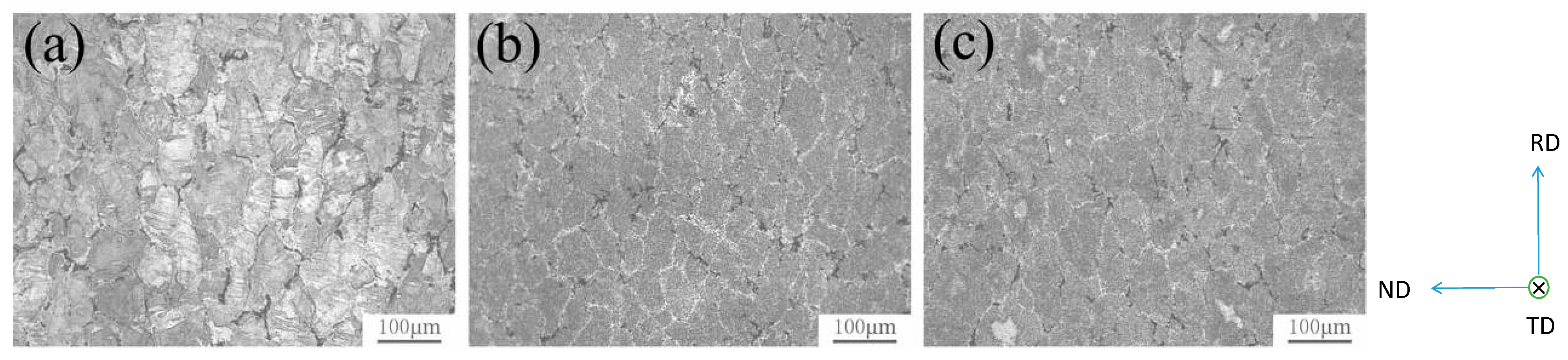
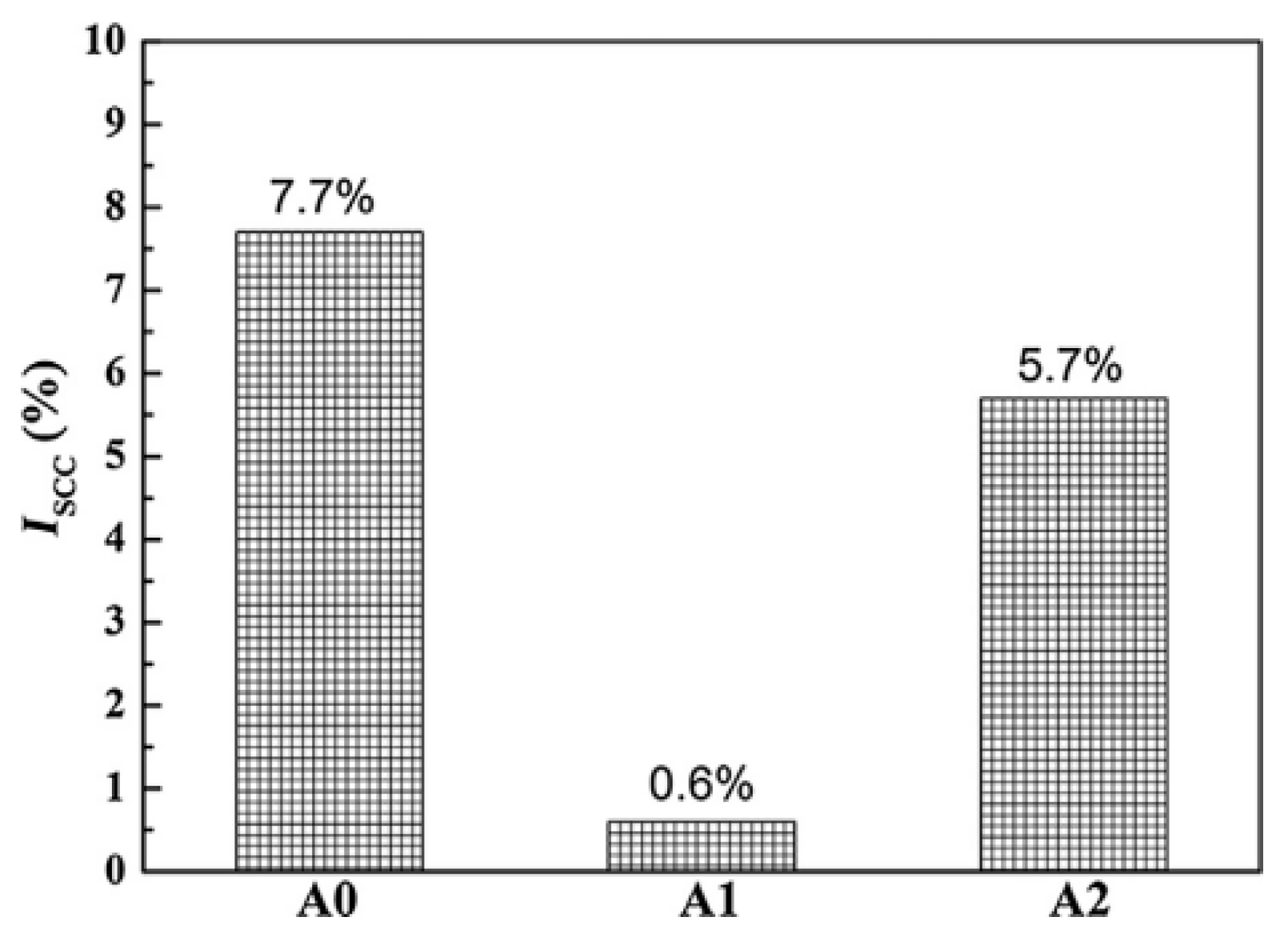

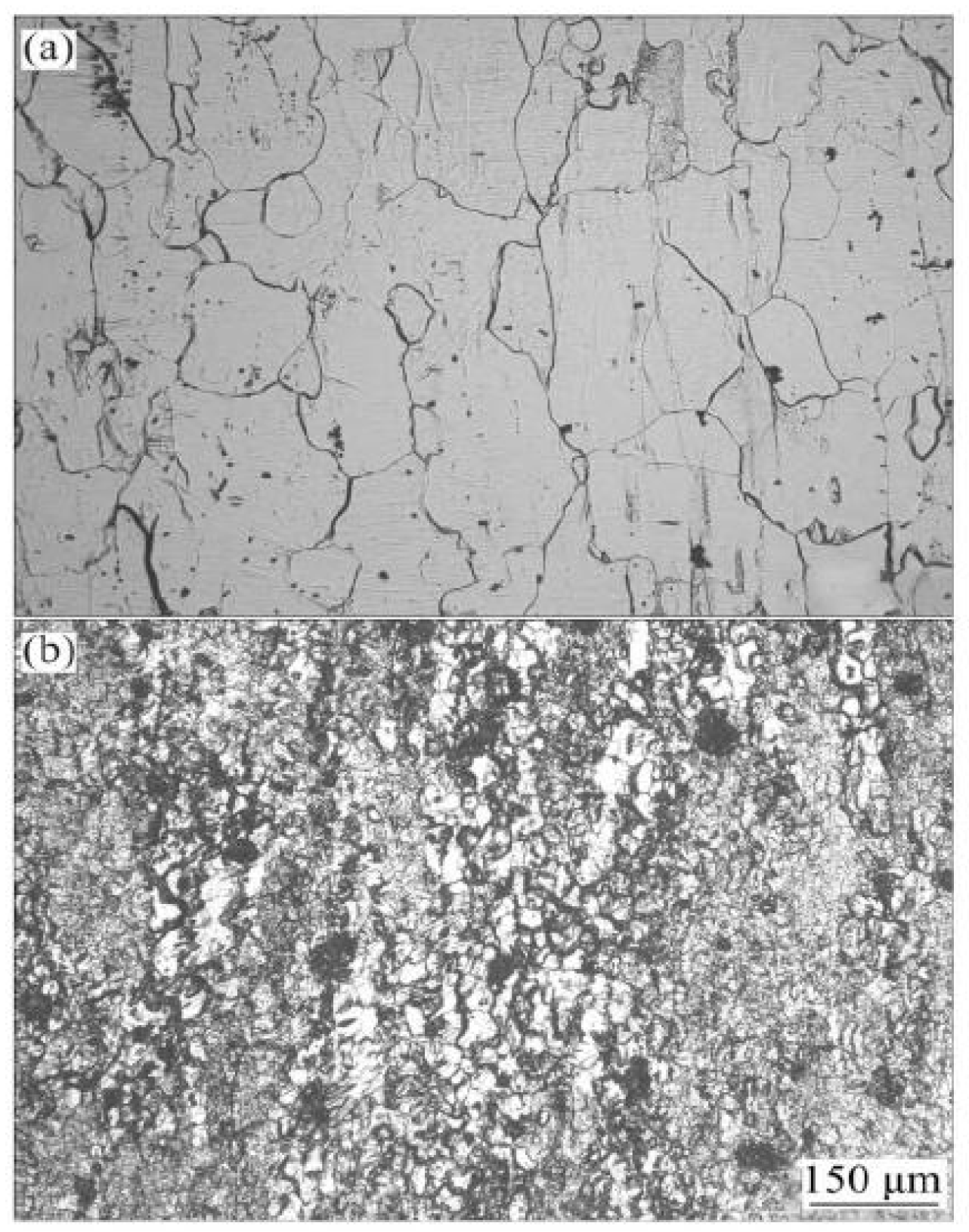
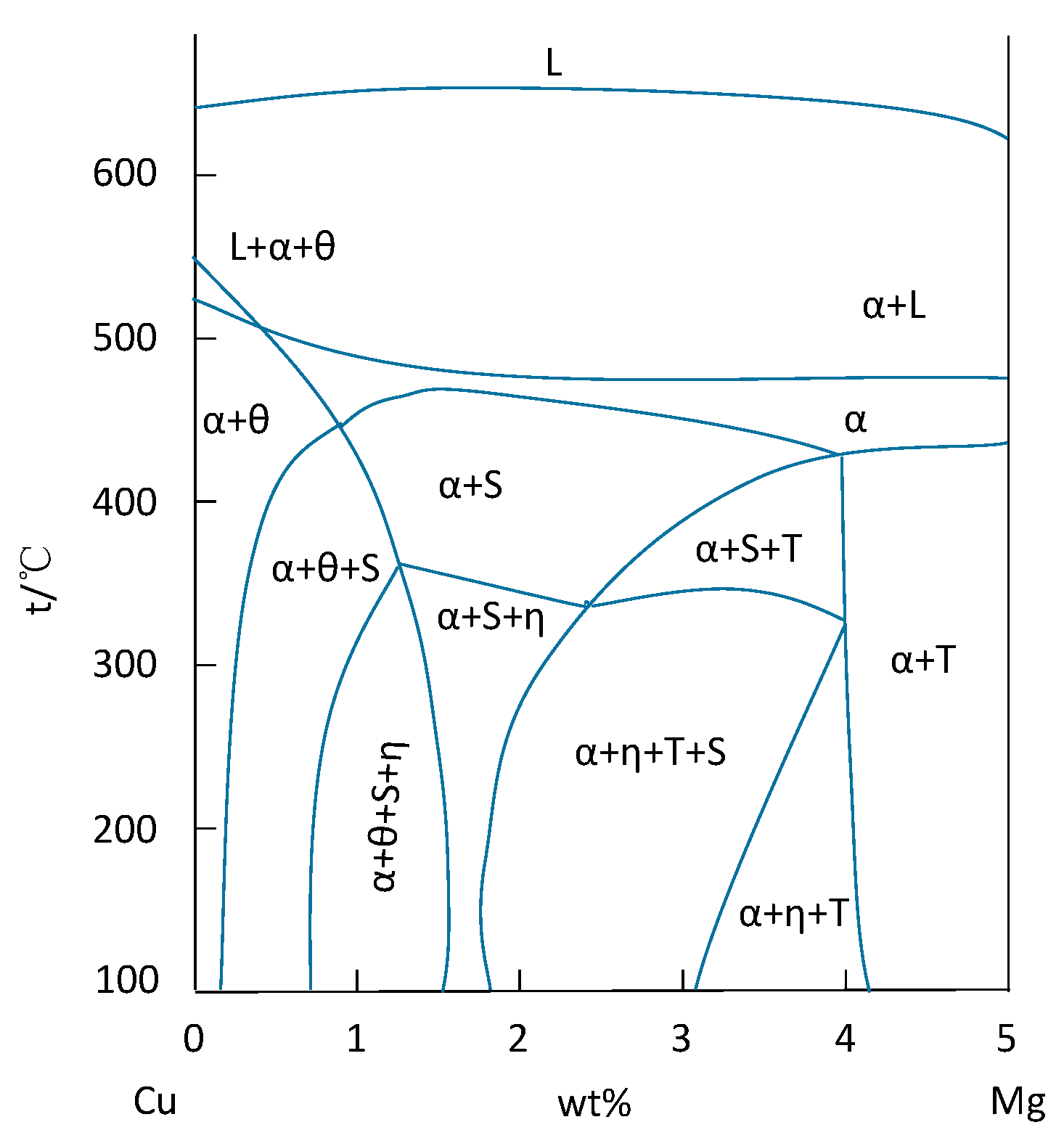

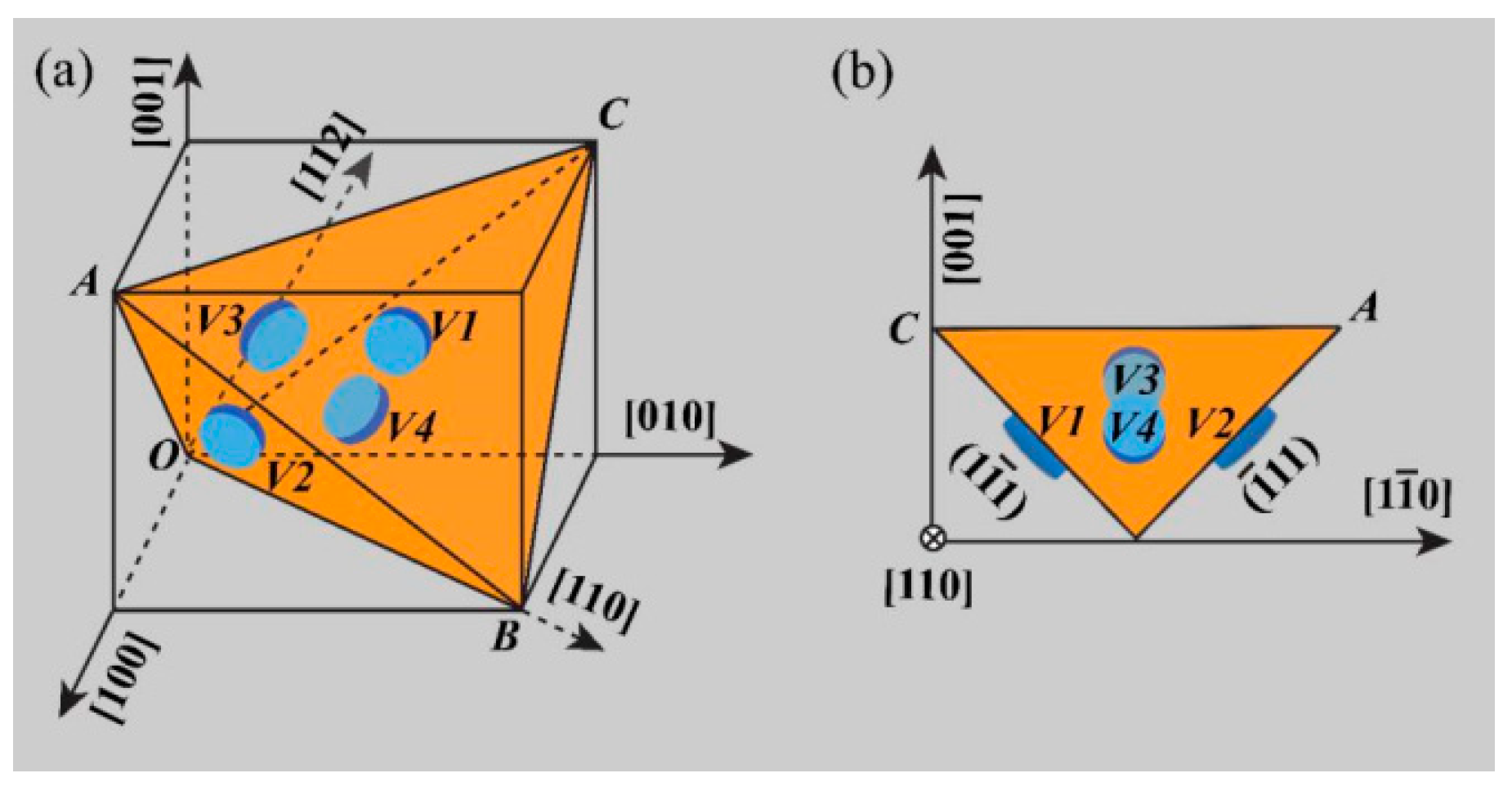

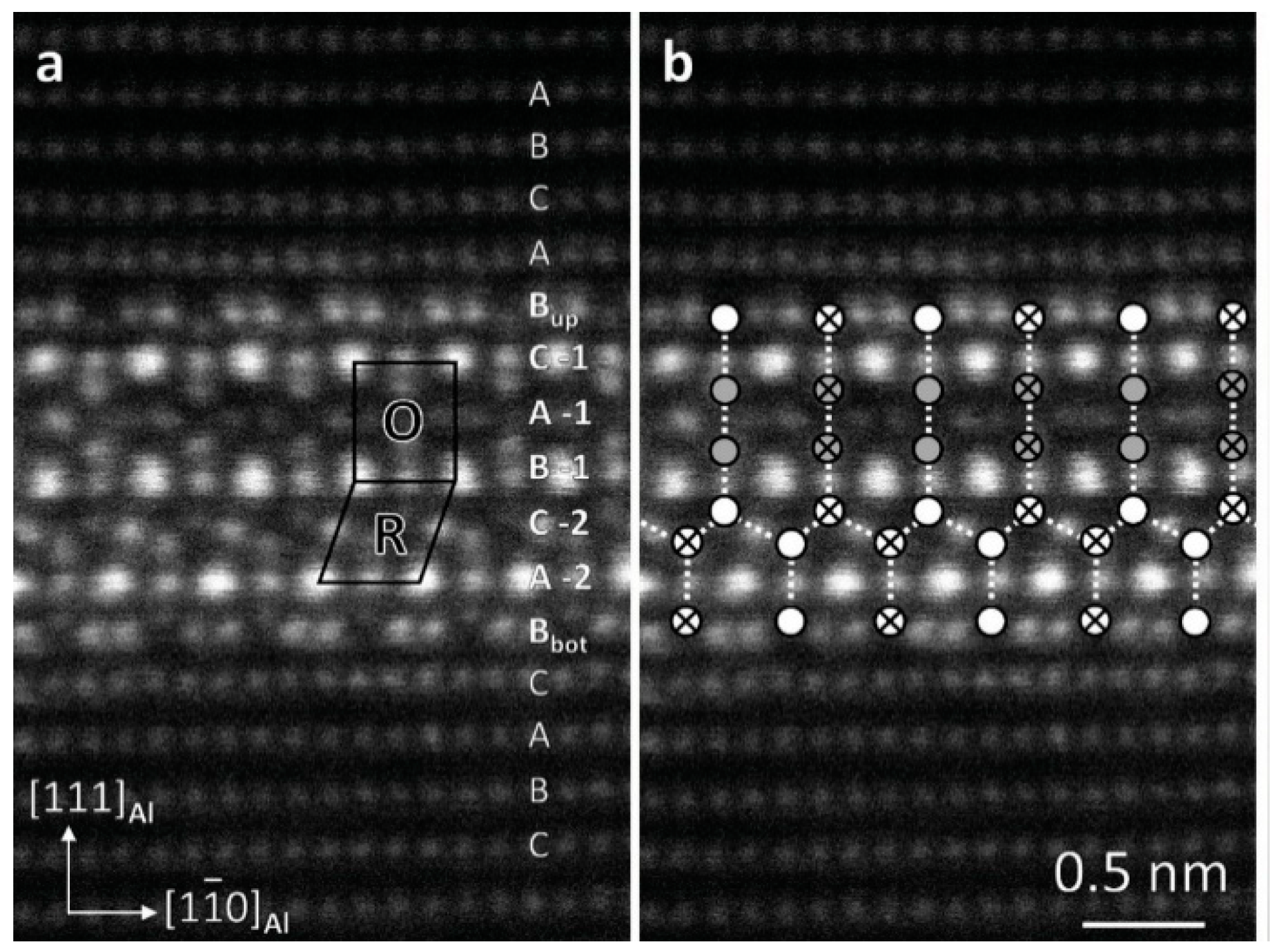
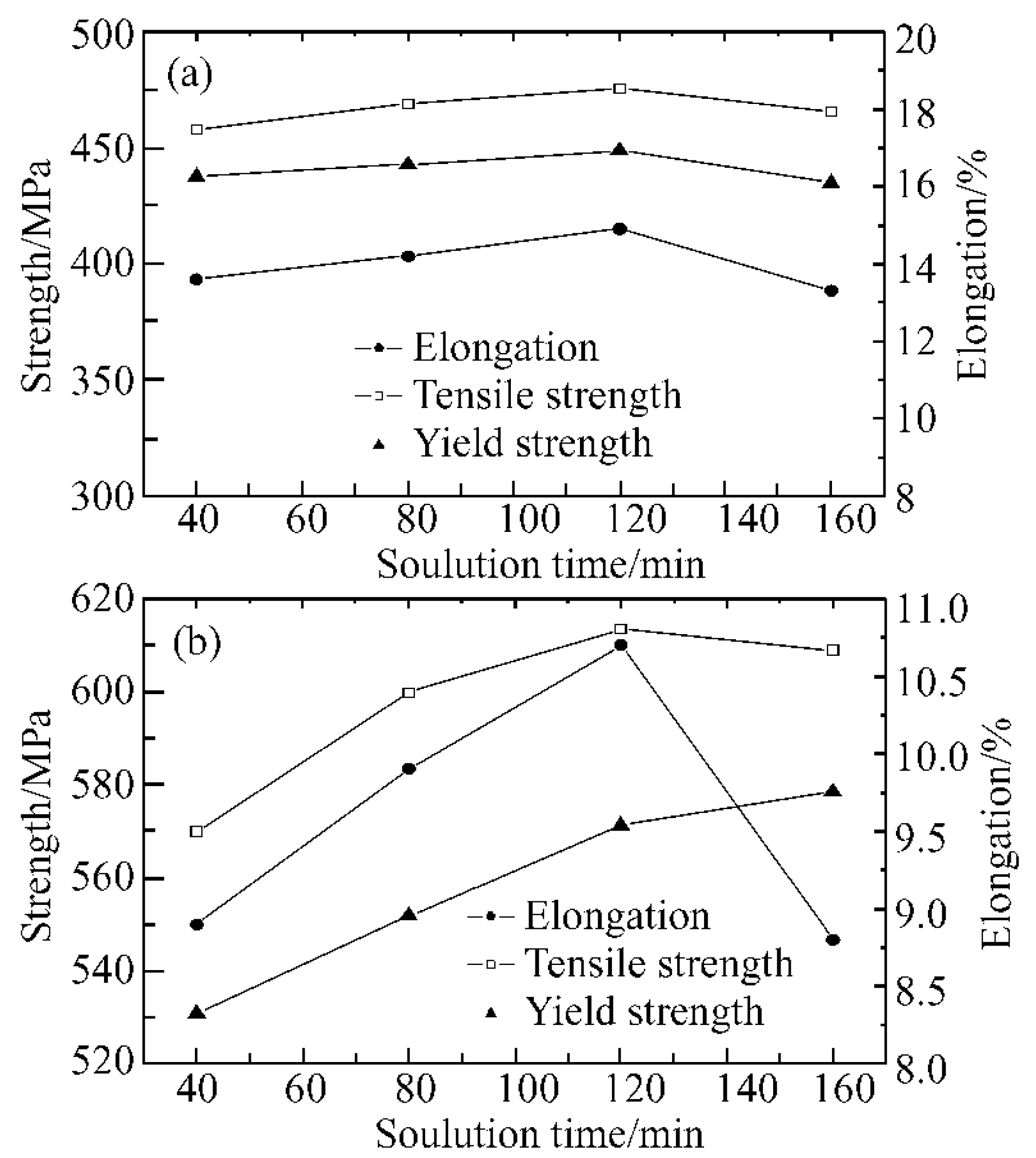
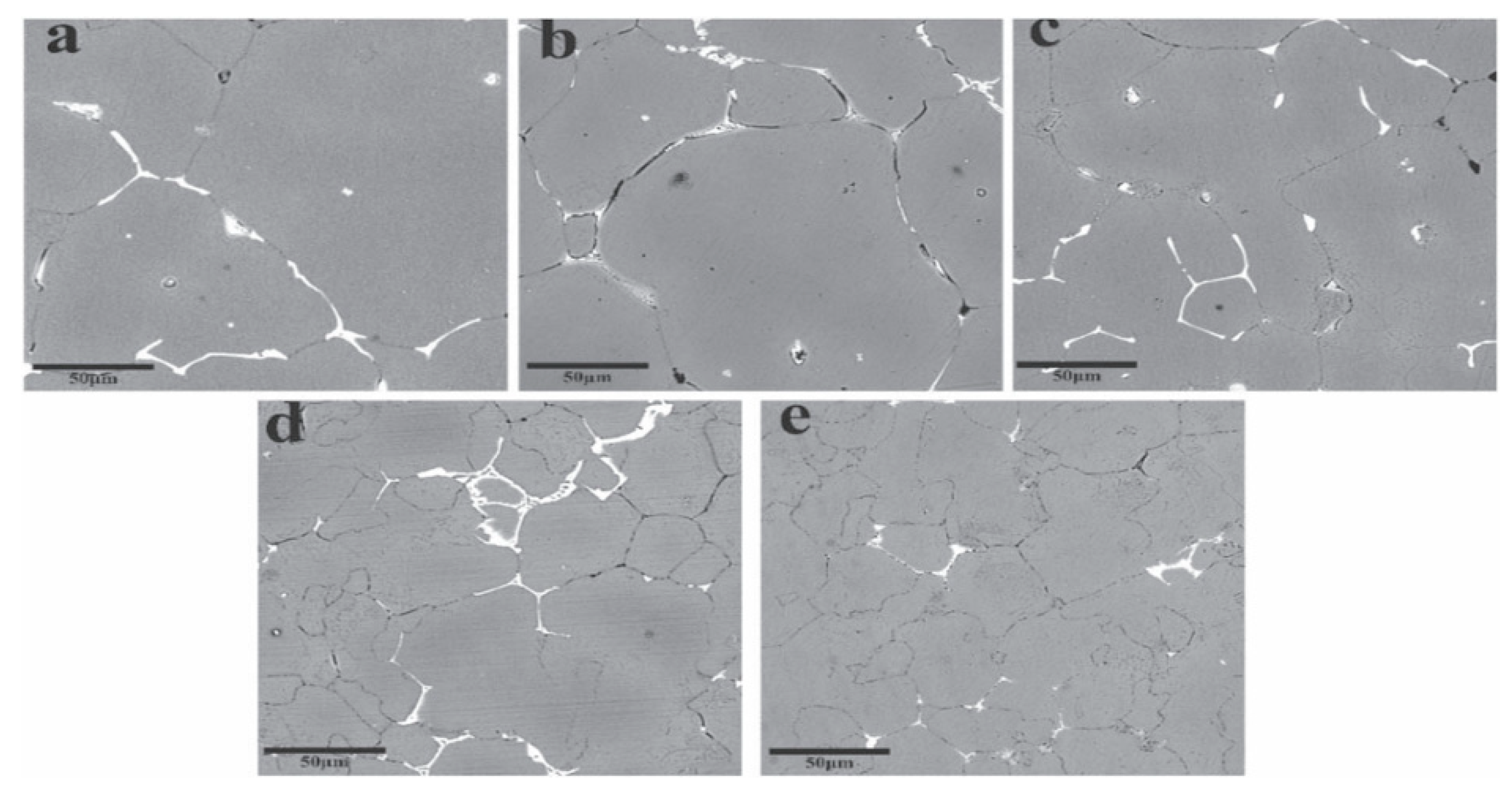
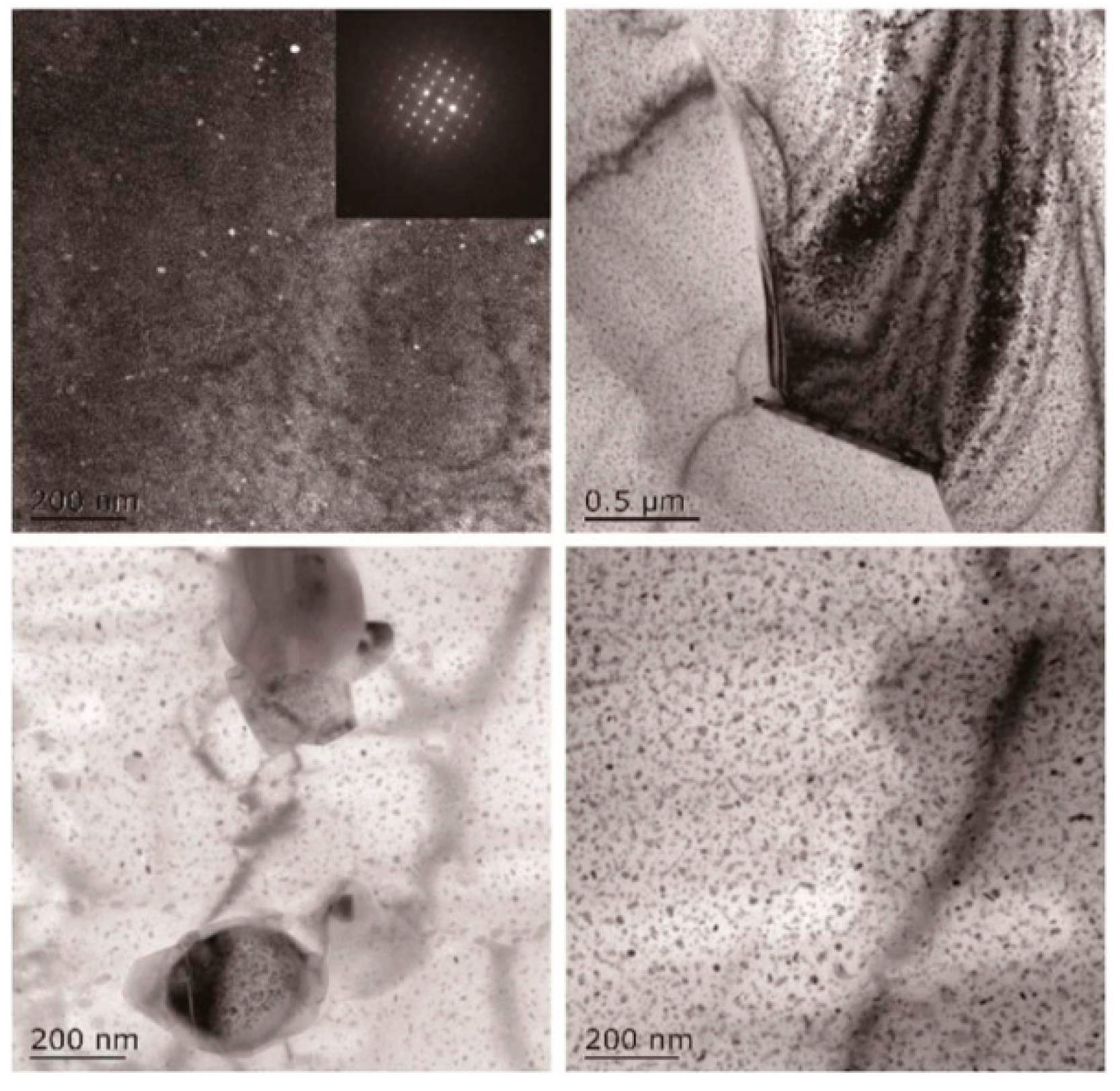

| Element | Precipitate | Content (ω%) | Main Effect |
|---|---|---|---|
| Zn + Mg | η (MgZn2); T (Al2Mg2Zn3) | 0.0–10 (Zn ≥ 0.9) | Increased tensile strength; heat treatment effect |
| Zn + Mg ≥ 10% | Decreased conductivity, fracture toughness, stress-corrosion resistance, and spalling-corrosion resistance | ||
| Mg | β (Al8Mg5) | 0.0–4.0 (Mg ≥ 0.0) | Reduced welding crack tendency |
| Mg + C | S (CuMgAl2) | Zn/Mg > 2.2 Cu > Mg | Improved alloy strength |
| Cu | θ (CuAl2) | ≤3 | Improved corrosion resistance |
| Expanded the stable temperature range of GP zone, improved the tensile strength, plasticity and fatigue strength | |||
| Cr | Incoherent E (Al18Cr2Mg3) | ≤0.35 | Nucleation and precipitation of coarse equilibrium phase |
| (CrMn)Al12; (CrFe)Al7 | 0.1–0.2 | Fine grain strengthening; inhibits recrystallization nucleation and growth; Improve anti SCC ability | |
| Mn | Al6Mn | 0.2–0.4 | Improved maximum tensile strength and fracture toughness, performance of low cycle fatigue, quenching sensitivity |
| Al20Cu2Mn3 | >0.4 | Reduced the number of strengthening phases | |
| Zr | Al3Zr | 0.05–0.16 | Improved the strength, toughness, aging effect and corrosion resistance of the alloy |
| Ag | 0.16 | Promoted the formation of GP region and transition phase; delayed the over-aging of the alloy | |
| Co | (Co,Fe)Al9, Co2Al9 | 0.05≤ ≥0.2 | Improved the hardenability; retention of subcrystalline structure |
| Ti | 0.01–0.08 | Refined grain, improved casting properties | |
| Er | Al3Er; Al8Cu4Er | 0.1–0.15 | Improved the toughness; hardenability; dimples appear |
| Sc | Al3Sc | 0.1–0.4 | Grain refinement; recrystallization inhibition |
| Sc + Zr | Al3(Sc,Zr) | 0.6 | Improved anti SCC ability |
| Y | Al3Y | 0.3 | Improved the hardness, tensile strength, elongation |
| Gd | Al3(Gd,Zr) | 0.11 | Hindered dislocation; grain boundary movement |
| Si | Mg2Si; AlFeMnSi | ≥0.15 | Reduced plasticity and fracture toughness |
| Fe | Al6FeMn | ≥0.15 | Reduced plasticity and fracture toughness |
| Allloys | Si | Fe | Cu | Mn | Mg | Cr | Zn | Ti | Zr | Other | Al | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Each | Total | |||||||||||
| 7A01 | 0.30 | 0.30 | 0.01 | - | - | - | 0.9–1.3 | - | - | 0.03 | - | Bal. |
| 7A03 | 0.20 | 0.20 | 1.8–2.4 | 0.10 | 1.2–1.6 | 0.05 | 6.0–6.7 | 0.02–0.08 | - | 0.05 | 0.10 | Bal. |
| 7A04 | 0.50 | 0.50 | 1.4–2.0 | 0.20–0.60 | 1.8–2.8 | 0.10–0.25 | 5.0–7.0 | 0.10 | - | 0.05 | 0.10 | Bal. |
| 7A05 | 0.25 | 0.25 | 0.20 | 0.15–0.40 | 1.1–1.7 | 0.05–0.15 | 4.4–5.0 | 0.02–0.06 | 0.10–0.25 | 0.05 | 0.15 | Bal. |
| 7A09 | 0.50 | 0.50 | 1.2–2.0 | 0.15 | 2.0–3.0 | 0.16–0.30 | 5.1–6.1 | 0.10 | - | 0.05 | 0.10 | Bal. |
| 7A10 | 0.30 | 0.30 | 0.50–1.0 | 0.20–0.35 | 3.0–4.0 | 0.10–0.30 | 3.2–4.2 | 0.10 | - | 0.05 | 0.10 | Bal. |
| 7A19 | 0.30 | 0.40 | 0.08–0.30 | 0.30–0.50 | 1.3–1.9 | 0.10–0.20 | 1.5–5.3 | - | 0.08–0.20 | 0.05 | 0.15 | Bal. |
| 7A33 | 0.25 | 0.30 | 0.25–0.55 | 0.05 | 2.2–2.7 | 0.10–0.20 | 4.6–5.4 | 0.05 | - | 0.05 | 0.10 | Bal. |
| 7A52 | 0.25 | 0.30 | 0.05–0.20 | 0.20–0.50 | 2.0–2.8 | 0.15–0.25 | 4.0–4.8 | 0.05–0.18 | 0.05–0.15 | 0.05 | 0.15 | Bal. |
| 7003 | 0.30 | 0.35 | 0.20 | 0.30 | 0.50–1.0 | 0.20 | 5.0–6.5 | 0.20 | 0.05–0.25 | 0.05 | 0.15 | Bal. |
| 7020 | 0.35 | 0.20 | 0.20 | 0.05–0.50 | 1.0–1.4 | 0.10–0.35 | 4.0–5.0 | - | 0.08–0.20 | 0.05 | 0.15 | Bal. |
| 7022 | 0.50 | 0.50 | 0.50–1.0 | 0.10–0.40 | - | 0.10–0.30 | 4.3–5.2 | - | - | 0.05 | 0.15 | Bal. |
| 7050 | 0.12 | 0.15 | 2.0–2.6 | 0.10 | 1.9–2.6 | 0.04 | 5.7–6.7 | 0.06 | 0.08–0.15 | 0.05 | 0.15 | Bal. |
| 7075 | 0.40 | 0.50 | 1.2–2.0 | 0.03 | 2.1–2.9 | 0.18–0.28 | 5.1–6.1 | 0.02 | - | 0.05 | 0.15 | Bal. |
| 7475 | 0.10 | 0.12 | 1.2–1.9 | 0.06 | 1.9–2.6 | 0.18–0.25 | 5.2–6.2 | 0.06 | - | 0.05 | 0.15 | Bal. |
| Element | HV | Atomic Radius (nm) | Relative Difference with Al Atomic Radius (%) | Electronegativity | Electronegativity Difference with Al | Melting Point of Pure Aluminum and Eutectic Temperature Difference (°C) | Eutectic POINT Composition (w(RE)%) |
|---|---|---|---|---|---|---|---|
| Er | 700 | 0.1757 | 23 | 1.2 | 0.3 | 5 | 1 |
| Sc | 850 | 0.1641 | 14.8 | 1.3 | 0.2 | 5 | 0.3 |
| Y | 600 | 0.1803 | 26.2 | 1.2 | 0.3 | 10 | 3.3 |
| La | 400 | 0.1877 | 31.4 | 1.1 | 0.4 | 20 | 2.5 |
| Ce | 250 | 0.1824 | 27.6 | 1.05 | 0.45 | 20 | 2 |
| Nd | 350 | 0.1522 | 27.5 | 1.2 | 0.3 | 20 | 7.4 |
| Sm | 450 | 0.1802 | 26.1 | 1.2 | 0.3 | 28 | 1.5 |
| Eu | - | 0.2041 | 42.8 | 1.1 | 0.4 | - | - |
| Gd | 550 | 0.1801 | 26 | 1.2 | 0.3 | 17 | 2 |
| Tb | 600 | 0.1783 | 24.8 | 1.2 | 0.3 | 16 | 1.8 |
| Dy | 550 | 0.1775 | 24.2 | 1.2 | 0.3 | 24 | 8.2 |
| Ho | 600 | 0.1767 | 23.7 | 1.2 | 0.3 | 13 | 2.6 |
| Tm | 650 | 0.1747 | 22.3 | 1.2 | 0.3 | 16 | 1.7 |
| Yb | 250 | 0.1939 | 35.7 | 1.1 | 0.4 | 35 | 4 |
| Precipitation | Crystal Structure | Lattice Constant/nm | Morphology And Crystal Orientation | Precipitation Temperature/°C | |||
|---|---|---|---|---|---|---|---|
| a | b | c | |||||
| Metastable phase | H′ (MgZn2) | Hexagonal system (L12) | 0.496 | - | 1.403 | semi-Coherent, (0001)η′//{111}Al; (11–20)η′//<112>Al; (10–10)η′//(110)Al; habit plane(111)Al | 120–250 |
| ηp (Al2Mg2Zn8) | Hexagonal system | 0.496 | - | 0.935 | semi-Coherent, (0001)ηp//{111}Al; (11–20)ηp//<112>Al; (10–10)ηp//(110)Al | 300–400 | |
| T′ (Al2Mg2Zn3) | Cubic system | 500 | - | - | Incoherent; Equiaxed Polytopic | 471–476 | |
| S′ (Al2CuMg) | Orthorhombic system | 0.4012 | 0.9265 | 0.7124 | Incoherent; Bar shape | 456~482 | |
| Stable phase | η (MgZn2) | (D023) | 0.516–0.522 | - | 0.849–0.855 | Incoherent; 13 orientation relationship; classic orientation relationship (0001)η//{111}Al, (10–10)η//{110}Al | 150–476 |
| S (Al2CuMg) | Orthorhombic system | 0.4 | 0.025 | 0.715 | semi-Coherent | 400–494 | |
| T (Al2Mg2Zn3) | Cubic system | 1.416 | - | - | Coherent, (060)T//(111)Al;(103)//(2–20)Al; | Low temperature precipitation: 174 | |
| θ (CuAl2) | Tetragonal system | 0.607 | - | 0.487 | Coherent | 451; Low temperature precipitation: 80 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, Y.; Yan, L.; Hao, J. Review on Micro-Alloying and Preparation Method of 7xxx Series Aluminum Alloys: Progresses and Prospects. Materials 2022, 15, 1216. https://doi.org/10.3390/ma15031216
Dai Y, Yan L, Hao J. Review on Micro-Alloying and Preparation Method of 7xxx Series Aluminum Alloys: Progresses and Prospects. Materials. 2022; 15(3):1216. https://doi.org/10.3390/ma15031216
Chicago/Turabian StyleDai, Yuxin, Liangming Yan, and Jianpeng Hao. 2022. "Review on Micro-Alloying and Preparation Method of 7xxx Series Aluminum Alloys: Progresses and Prospects" Materials 15, no. 3: 1216. https://doi.org/10.3390/ma15031216
APA StyleDai, Y., Yan, L., & Hao, J. (2022). Review on Micro-Alloying and Preparation Method of 7xxx Series Aluminum Alloys: Progresses and Prospects. Materials, 15(3), 1216. https://doi.org/10.3390/ma15031216





