Recent Advances in Materials and Flexible Sensors for Arrhythmia Detection
Abstract
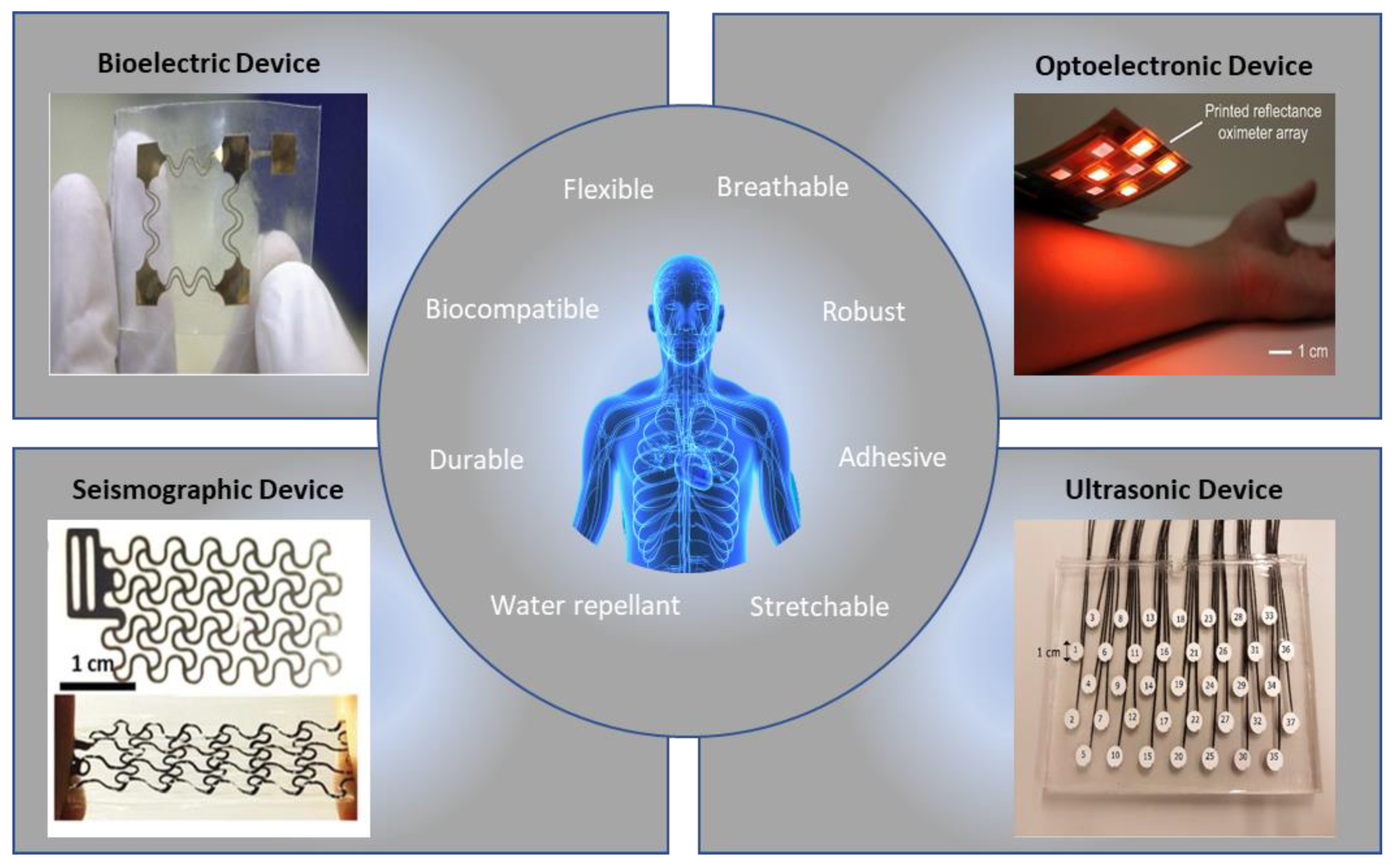
:1. Introduction
2. Bioelectric Signals
2.1. Mechanics of ECG
2.2. Materials for Flexible ECG Devices
3. Optoelectronic Signals
3.1. Mechanics of PPG
3.2. Materials for Flexible PPG Devices
4. Other Signals
4.1. Ultrasonic Signals
4.2. Mechanoelectric Signals
4.3. Electrochemical Signals
5. Arrhythmia Detection
| Reference | Device | Target Signal | Arrhythmia Type | Detection Methodology | Accuracy |
|---|---|---|---|---|---|
| [12] | iRhythm Zio monitor | ECG | 10 types | Deep neural network | ROC = 0.97 F1 = 0.837 |
| [89] | Apple watch | PPG, ACC | Atrial fibrillation | Deep neural network | Sens = 0.98 Spec = 0.90 |
| [90] | 2-lead Holter monitor | ECG | Atrial fibrillation, atrial flutter, AV junctional rhythm | Hybrid CNN-LSTM | Sens = 0.9787 Spec = 0.9929 |
| [91] | Fingertip pulse oximeter | PPG | Atrial fibrillation | CNN, RNN | AOC = 0.998 AOC = 0.996 |
| [73] | MIT-BIH arrhythmia database | ECG | Ventricular fibrillation | CNN | Acc = 0.9318 |
| [92] | Point-of-care ultrasound | Ultrasound images | Atrial fibrillation | Semi-supervised deep learning network | Acc = 0.79 |
6. Substrate Materials and Skin Interfaces
7. Wearable Devices
8. Limitations
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Virani, S.S.; Alonso, A.; Aparicio, H.J.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Cheng, S.; Delling, F.N.; et al. Heart disease and stroke statistics—2021 update. Circulation 2021, 143, e254–e743. [Google Scholar] [CrossRef]
- Mou, L.; Norby, F.L.; Chen, L.Y.; O’Neal, W.T.; Lewis, T.T.; Loehr, L.R.; Soliman, E.Z.; Alonso, A. Lifetime risk of atrial fibrillation by race and socioeconomic status. Circ. Arrhythm. Electrophysiol. 2018, 11, e006350. [Google Scholar] [CrossRef] [PubMed]
- Richards, K.J.C.; Cohen, A.T. Cardiac arrhythmias in the critically ill. Anaesth. Intensive Care Med. 2006, 7, 289–293. [Google Scholar] [CrossRef]
- Kléber, A.G.; Rudy, Y. Basic mechanisms of cardiac impulse propagation and associated arrhythmias. Physiol. Rev. 2004, 84, 431–488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sardana, H.K.; Kanwade, R.; Tewary, S. Arrhythmia detection and classification using ECG and PPG techniques: A review. Phys. Eng. Sci. Med. 2021, 44, 1027–1048. [Google Scholar] [CrossRef]
- Rho, R.W.; Page, R.L. Asymptomatic atrial fibrillation. Prog. Cardiovasc. Dis. 2005, 48, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.E.; O’Neill, M.; Kotadia, I.D. Supraventricular tachycardia: An overview of diagnosis and management. Clin. Med. 2020, 20, 43. [Google Scholar] [CrossRef]
- DiMarco, J.P.; Philbrick, J.T. Use of ambulatory electrocardiographic (Holter) monitoring. Ann. Intern. Med. 1990, 113, 53–68. [Google Scholar] [CrossRef]
- Fung, E.; Järvelin, M.-R.; Doshi, R.N.; Shinbane, J.S.; Carlson, S.K.; Grazette, L.P.; Chang, P.M.; Sangha, R.S.; Huikuri, H.V.; Peters, N.S. Electrocardiographic patch devices and contemporary wireless cardiac monitoring. Front. Physiol. 2015, 6, 149. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.H.; Op de Beeck, M.; Vanderheyden, L.; Carrette, E.; Mihajlović, V.; Vanstreels, K.; Grundlehner, B.; Gadeyne, S.; Boon, P.; van Hoof, C. Soft, comfortable polymer dry electrodes for high quality ECG and EEG recording. Sensors 2014, 14, 23758. [Google Scholar] [CrossRef] [Green Version]
- Ackermans, P.A.J.; Solosko, T.A.; Spencer, E.C.; Gehman, S.E.; Nammi, K.; Engel, J.; Russell, J.K. A user-friendly integrated monitor-adhesive patch for long-term ambulatory electrocardiogram monitoring. J. Electrocardiol. 2012, 45, 148–153. [Google Scholar] [CrossRef]
- Hannun, A.Y.; Rajpurkar, P.; Haghpanahi, M.; Tison, G.H.; Bourn, C.; Turakhia, M.P.; Ng, A.Y. Cardiologist-level arrhythmia detection and classification in ambulatory electrocardiograms using a deep neural network. Nat. Med. 2019, 25, 65–69. [Google Scholar] [CrossRef]
- Fayyaz Shahandashti, P.; Pourkheyrollah, H.; Jahanshahi, A.; Ghafoorifard, H. Highly conformable stretchable dry electrodes based on inexpensive flex substrate for long-term biopotential (EMG/ECG) monitoring. Sens. Actuators A Phys. 2019, 295, 678–686. [Google Scholar] [CrossRef]
- Khan, Y.; Han, D.; Pierre, A.; Ting, J.; Wang, X.; Lochner, C.M.; Bovo, G.; Yaacobi-Gross, N.; Newsome, C.; Wilson, R.; et al. A flexible organic reflectance oximeter array. Proc. Natl. Acad. Sci. USA 2018, 115, E11015–E11024. [Google Scholar] [CrossRef] [Green Version]
- Hamelmann, P.; Mischi, M.; Kolen, A.F.; van Laar, J.O.E.H.; Vullings, R.; Bergmans, J.W.M. Fetal heart-rate monitoring Implemented by dynamic adaptation of transmission power of a flexible ultrasound transducer array. Sensors 2019, 19, 1195. [Google Scholar] [CrossRef] [Green Version]
- Ha, T.; Tran, J.; Liu, S.; Jang, H.; Jeong, H.; Mitbander, R.; Huh, H.; Qiu, Y.; Duong, J.; Wang, R.L.; et al. A chest-laminated ultrathin and stretchable e-tattoo for the measurement of electrocardiogram, seismocardiogram, and cardiac time intervals. Adv. Sci. 2019, 6, 1900290. [Google Scholar] [CrossRef] [Green Version]
- Villegas, A.; McEneaney, D.; Escalona, O. Arm-ECG wireless sensor system for wearable long-term surveillance of heart arrhythmias. Electronics 2019, 8, 1300. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Luo, Y.; Nayak, S.; Liu, Z.; Chichvarina, O.; Zamburg, E.; Zhang, X.; Liu, Y.; Heng, C.H.; Thean, A.V.Y. A stretchable-hybrid low-power monolithic ECG patch with microfluidic liquid-metal interconnects and stretchable carbon-black nanocomposite electrodes for wearable heart monitoring. Adv. Electron. Mater. 2019, 5, 1800463. [Google Scholar] [CrossRef]
- Chen, E.; Jiang, J.; Su, R.; Gao, M.; Zhu, S.; Zhou, J.; Huo, Y. A new smart wristband equipped with an artificial intelligence algorithm to detect atrial fibrillation. Heart Rhythm 2020, 17, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhong, X.; Wang, W.; Yu, D. Flexible cellulose/polyvinyl alcohol/PEDOT:PSS electrodes for ECG monitoring. Cellulose 2021, 28, 4913–4926. [Google Scholar] [CrossRef]
- Lai, D.; Bu, Y.; Su, Y.; Zhang, X.; Ma, C.S. A flexible multilayered dry electrode and assembly to single-lead ECG patch to monitor atrial fibrillation in a real-life scenario. IEEE Sens. J. 2020, 20, 12295–12306. [Google Scholar] [CrossRef]
- Wang, C.; Li, X.; Hu, H.; Zhang, L.; Huang, Z.; Lin, M.; Zhang, Z.; Yin, Z.; Huang, B.; Gong, H.; et al. Monitoring of the central blood pressure waveform via a conformal ultrasonic device. Nat. Biomed. Eng. 2018, 2, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Hammad, M.; Maher, A.; Wang, K.; Jiang, F.; Amrani, M. Detection of abnormal heart conditions based on characteristics of ECG signals. Measurement 2018, 125, 634–644. [Google Scholar] [CrossRef]
- Chen, S.; Qi, J.; Fan, S.; Qiao, Z.; Yeo, J.C.; Lim, C.T. Flexible wearable sensors for cardiovascular health monitoring. Adv. Healthc. Mater. 2021, 10, 2100116. [Google Scholar] [CrossRef]
- Maršánová, L.; Němcová, A.; Smíšek, R.; Vítek, M.; Smital, L. Advanced P wave detection in ecg signals during pathology: Evaluation in different arrhythmia contexts. Sci. Rep. 2019, 9, 19053. [Google Scholar] [CrossRef] [Green Version]
- Pan, J.; Tompkins, W.J. A Real-Time QRS Detection Algorithm. IEEE Trans. Biomed. Eng. 1985, BME-32, 230–236. [Google Scholar] [CrossRef]
- Barrett, P.M.; Komatireddy, R.; Haaser, S.; Topol, S.; Sheard, J.; Encinas, J.; Fought, A.J.; Topol, E.J. Comparison of 24-hour holter monitoring with 14-day novel adhesive patch electrocardiographic monitoring. Am. J. Med. 2014, 127, 95.e11–95.e17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, W.; Cheng, X.; Xiong, T.; Wang, X. Stretchable bio-potential electrode with self-similar serpentine structure for continuous, long-term, stable ECG recordings. Biomed. Microdevices 2019, 21, 6. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.A.; Yeo, W.H.; Su, Y.; Hattori, Y.; Lee, W.; Jung, S.Y.; Zhang, Y.; Liu, Z.; Cheng, H.; Falgout, L.; et al. Fractal design concepts for stretchable electronics. Nat. Commun. 2014, 5, 3266. [Google Scholar] [CrossRef] [Green Version]
- Morikawa, Y.; Yamagiwa, S.; Sawahata, H.; Numano, R.; Koida, K.; Ishida, M.; Kawano, T.; Morikawa, Y.; Yamagiwa, S.; Sawahata, H.; et al. Ultrastretchable kirigami bioprobes. Adv. Healthc. Mater. 2018, 7, 1701100. [Google Scholar] [CrossRef]
- Xu, B.; Akhtar, A.; Liu, Y.; Chen, H.; Yeo, W.-H.; Park, S.I.I.; Boyce, B.; Kim, H.; Yu, J.; Lai, H.-Y.; et al. An epidermal stimulation and sensing platform for sensorimotor prosthetic control, management of lower back exertion, and electrical muscle activation. Adv. Mater. 2016, 28, 4462–4471. [Google Scholar] [CrossRef]
- Chlaihawi, A.A.; Narakathu, B.B.; Emamian, S.; Bazuin, B.J.; Atashbar, M.Z. Development of printed and flexible dry ECG electrodes. Sens. Bio-Sens. Res. 2018, 20, 9–15. [Google Scholar] [CrossRef]
- Jung, H.; Moon, J.; Baek, D.; Lee, J.; Choi, Y.; Hong, J.; Lee, S. CNT/PDMS composite flexible dry electrodesfor long-term ECG monitoring. IEEE Trans. Biomed. Eng. 2012, 59, 1472–1479. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; He, P.; Luo, M.; Xu, X.; Dai, G.; Yang, J. Highly stretchable polymer/silver nanowires composite sensor for human health monitoring. Nano Res. 2020, 13, 919–926. [Google Scholar] [CrossRef]
- Pani, D.; Dessì, A.; Saenz-Cogollo, J.F.; Barabino, G.; Fraboni, B.; Bonfiglio, A. Fully textile, PEDOT:PSS based electrodes for wearable ECG monitoring systems. IEEE Trans. Biomed. Eng. 2016, 63, 540–549. [Google Scholar] [CrossRef] [PubMed]
- Satti, A.T.; Park, J.; Park, J.; Kim, H.; Cho, S. Fabrication of parylene-coated microneedle array electrode for wearable ECG device. Sensors 2020, 20, 5183. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Li, Z.; Wang, Z.; Yu, H. Miura-ori structured flexible microneedle array electrode for biosignal recording. Microsyst. Nanoeng. 2021, 7, 53. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Thangavel, G.; Li, Y.; Xiong, J.; Gao, D.; Ciou, J.; Tan, M.W.M.; Aziz, I.; Chen, S.; Chen, J.; et al. Printable elastomeric electrodes with sweat-enhanced conductivity for wearables. Sci. Adv. 2021, 7, eabg8433. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Li, Y.; Li, Y.; Ding, M.; Xie, J.; Hu, B. Solution-processed submicron free-standing, conformal, transparent, breathable epidermal electrodes. ACS Appl. Mater. Interfaces 2020, 12, 23689–23696. [Google Scholar] [CrossRef]
- Haddad, P.A.; Servati, A.; Soltanian, S.; Ko, F.; Servati, P. Breathable dry silver/silver chloride electronic textile electrodes for electrodermal activity monitoring. Biosensors 2018, 8, 79. [Google Scholar] [CrossRef] [Green Version]
- Rodeheaver, N.; Herbert, R.; Kim, Y.-S.; Mahmood, M.; Kim, H.; Jeong, J.-W.; Yeo, W.-H. Strain-isolating materials and interfacial physics for soft wearable bioelectronics and wireless, motion artifact-controlled health monitoring. Adv. Funct. Mater. 2021, 31, 2104070. [Google Scholar] [CrossRef]
- Liu, C.; Kim, J.-T.; Kwak, S.S.; Hourlier-Fargette, A.; Avila, R.; Vogl, J.; Tzavelis, A.; Chung, H.U.; Lee, J.Y.; Kim, D.H.; et al. Wireless, skin-interfaced devices for pediatric critical care: Application to continuous, noninvasive blood pressure monitoring. Adv. Healthc. Mater. 2021, 10, 2100383. [Google Scholar] [CrossRef]
- Chung, H.U.; Rwei, A.Y.; Hourlier-Fargette, A.; Xu, S.; Lee, K.; Dunne, E.C.; Xie, Z.; Liu, C.; Carlini, A.; Kim, D.H.; et al. Skin-interfaced biosensors for advanced wireless physiological monitoring in neonatal and pediatric intensive-care units. Nat. Med. 2020, 26, 418–429. [Google Scholar] [CrossRef]
- Kim, Y.-S.; Mahmood, M.; Lee, Y.; Kim, N.K.; Kwon, S.; Herbert, R.; Kim, D.; Cho, H.C.; Yeo, W.-H. All-in-one, wireless, stretchable hybrid electronics for smart, connected, and ambulatory physiological monitoring. Adv. Sci. 2019, 6, 1900939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zulqarnain, M.; Stanzione, S.; Rathinavel, G.; Smout, S.; Willegems, M.; Myny, K.; Cantatore, E. A flexible ECG patch compatible with NFC RF communication. npj Flex. Electron. 2020, 4, 13. [Google Scholar] [CrossRef]
- Elgendi, M.; Fletcher, R.; Liang, Y.; Howard, N.; Lovell, N.H.; Abbott, D.; Lim, K.; Ward, R. The use of photoplethysmography for assessing hypertension. npj Digit. Med. 2019, 2, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamura, T.; Maeda, Y.; Sekine, M.; Yoshida, M. Wearable photoplethysmographic sensors—Past and present. Electronics 2014, 3, 282–302. [Google Scholar] [CrossRef]
- Harfiya, L.N.; Chang, C.-C.; Li, Y.-H. Continuous blood pressure estimation using exclusively photopletysmography by LSTM-based signal-to-signal translation. Sensors 2021, 21, 2952. [Google Scholar] [CrossRef]
- Lee, I.; Park, N.; Lee, H.; Hwang, C.; Kim, J.H.; Park, S. Systematic review on human skin-compatible wearable photoplethysmography sensors. Appl. Sci. 2021, 11, 2313. [Google Scholar] [CrossRef]
- Askarian, B.; Jung, K.; Chong, J.W. Monitoring of heart rate from photoplethysmographic signals using a samsung galaxy note8 in underwater environments. Sensors 2019, 19, 2846. [Google Scholar] [CrossRef] [Green Version]
- Mohan, P.M.; Nisha, A.A.; Nagarajan, V.; Jothi, E.S.J. Measurement of arterial oxygen saturation (SpO2) using PPG optical sensor. In Proceedings of the 2016 International Conference on Communication and Signal Processing (ICCSP), Melmaruvathur, India, 6–8 April 2016; pp. 1136–1140. [Google Scholar]
- L’Her, E.; N’Guyen, Q.-T.; Pateau, V.; Bodenes, L.; Lellouche, F. Photoplethysmographic determination of the respiratory rate in acutely ill patients: Validation of a new algorithm and implementation into a biomedical device. Ann. Intensive Care 2019, 9, 11. [Google Scholar] [CrossRef] [Green Version]
- Chong, J.W.; Cho, C.H.; Tabei, F.; Le-Anh, D.; Esa, N.; Mcmanus, D.D.; Chon, K.H. Motion and noise artifact-resilient atrial fibrillation detection using a smartphone. IEEE J. Emerg. Sel. Top. Circuits Syst. 2018, 8, 230–239. [Google Scholar] [CrossRef]
- Kim, J.; Salvatore, G.A.; Araki, H.; Chiarelli, A.M.; Xie, Z.; Banks, A.; Sheng, X.; Liu, Y.; Lee, J.W.; Jang, K.-I.; et al. Battery-free, stretchable optoelectronic systems for wireless optical characterization of the skin. Sci. Adv. 2022, 2, e1600418. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Xu, Y.; Li, X.; Chen, Y.; Jiang, Y.; Zhang, C.; Lu, B.; Wang, J.; Ma, Y.; Chen, Y.; et al. Epidermal inorganic optoelectronics for blood oxygen measurement. Adv. Healthc. Mater. 2017, 6, 1601013. [Google Scholar] [CrossRef] [PubMed]
- Hong, N.; Chu, R.J.; Kang, S.S.; Ryu, G.; Han, J.-H.; Yu, K.J.; Jung, D.; Choi, W.J. Flexible GaAs photodetector arrays hetero-epitaxially grown on GaP/Si for a low-cost III-V wearable photonics platform. Opt. Express 2020, 28, 36559–36567. [Google Scholar] [CrossRef] [PubMed]
- Yokota, T.; Zalar, P.; Kaltenbrunner, M.; Jinno, H.; Matsuhisa, N.; Kitanosako, H.; Tachibana, Y.; Yukita, W.; Koizumi, M.; Someya, T. Ultraflexible organic photonic skin. Sci. Adv. 2016, 2, e1501856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polat, E.O.; Mercier, G.; Nikitskiy, I.; Puma, E.; Galan, T.; Gupta, S.; Montagut, M.; Piqueras, J.J.; Bouwens, M.; Durduran, T.; et al. Flexible graphene photodetectors for wearable fitness monitoring. Sci. Adv. 2021, 5, eaaw7846. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Kang, T.-H.; Ahn, J.; Han, H.; Park, S.; Kim, S.J.; Park, M.-C.; Paik, S.; Hwang, D.K.; Yi, H.; et al. Spirally wrapped Carbon nanotube microelectrodes for fiber optoelectronic devices beyond geometrical limitations toward smart wearable e-textile applications. ACS Nano 2020, 14, 17213–17223. [Google Scholar] [CrossRef] [PubMed]
- Pribadi, E.F.; Pandey, R.K.; Chao, P.C.P. Optimizing a novel PPG sensor patch via optical simulations towards accurate heart rates. Microsyst. Technol. 2020, 26, 3409–3420. [Google Scholar] [CrossRef]
- Khan, Y.; Han, D.; Ting, J.; Ahmed, M.; Nagisetty, R.; Arias, A.C. Organic multi-channel optoelectronic sensors for wearable health monitoring. IEEE Access 2019, 7, 128114–128124. [Google Scholar] [CrossRef]
- La, T.-G.; Le, L.H. Flexible and wearable ultrasound device for medical applications: A review on materials, structural designs, and current challenges. Adv. Mater. Technol. 2021, 2100798. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, S.; Zhang, Y.; Geng, X.; Zhang, J.; Zhang, H. A novel flexible pressure sensor array for depth information of radial artery. Sens. Actuators A Phys. 2018, 272, 92–101. [Google Scholar] [CrossRef]
- Lee, S.-M.; Lee, T.; Kim, H.; Jo, Y.; Kim, M.-G.; Kim, S.; Bae, H.-M.; Lee, H.J. Calcium-modified silk patch as a next-generation ultrasound coupling medium. ACS Appl. Mater. Interfaces 2021, 13, 55827–55839. [Google Scholar] [CrossRef]
- Taebi, A.; Solar, B.E.; Bomar, A.J.; Sandler, R.H.; Mansy, H.A. Recent advances in seismocardiography. Vibration 2019, 2, 64–86. [Google Scholar] [CrossRef] [Green Version]
- Salernod, M.; Zanetti, J. Seismocardiography: A new technique for recording cardiac vibrations. Concept, method, and initial observations. J. Cardiovasc. Technol. (N. Y.) 1990, 9, 111–118. [Google Scholar]
- Liu, Y.; Norton, J.J.; Qazi, R.; Zou, Z.; Ammann, K.R.; Liu, H.; Yan, L.; Tran, P.L.; Jang, K.I.; Lee, J.W.; et al. Epidermal mechano-acoustic sensing electronics for cardiovascular diagnostics and human-machine interfaces. Sci. Adv. 2021, 2, e1601185. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.; Ni, X.; Lee, J.Y.; Arafa, H.; Pe, D.J.; Xu, S.; Avila, R.; Irie, M.; Lee, J.H.; Easterlin, R.L.; et al. Mechano-acoustic sensing of physiological processes and body motions via a soft wireless device placed at the suprasternal notch. Nat. Biomed. Eng. 2020, 4, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Kwak, Y.H.; Kim, W.; Park, K.B.; Kim, K.; Seo, S. Flexible heartbeat sensor for wearable device. Biosens. Bioelectron. 2017, 94, 250–255. [Google Scholar] [CrossRef]
- Lee, H.; Hong, Y.J.; Baik, S.; Hyeon, T.; Kim, D.-H. Enzyme-based glucose sensor: From invasive to wearable device. Adv. Healthc. Mater. 2018, 7, 1701150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bandodkar, A.J.; Jia, W.; Yardımcı, C.; Wang, X.; Ramirez, J.; Wang, J. Tattoo-based noninvasive glucose monitoring: A proof-of-concept study. Anal. Chem. 2015, 87, 394–398. [Google Scholar] [CrossRef] [PubMed]
- Sempionatto, J.R.; Lin, M.; Yin, L.; De la Paz, E.; Pei, K.; Sonsa-Ard, T.; de Loyola Silva, A.N.; Khorshed, A.A.; Zhang, F.; Tostado, N.; et al. An epidermal patch for the simultaneous monitoring of haemodynamic and metabolic biomarkers. Nat. Biomed. Eng. 2021, 5, 737–748. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, Y.; Fujita, H.; Oh, S.L.; Tan, J.H.; Tan, R.S.; Ciaccio, E.J.; Acharya, U.R. Computer-aided diagnosis of atrial fibrillation based on ECG Signals: A review. Inf. Sci. 2018, 467, 99–114. [Google Scholar] [CrossRef]
- Martis, R.J.; Acharya, U.R.; Adeli, H. Current methods in electrocardiogram characterization. Comput. Biol. Med. 2014, 48, 133–149. [Google Scholar] [CrossRef] [PubMed]
- Addison, P.S. Wavelet transforms and the ECG: A review. Physiol. Meas. 2005, 26, R155–R199. [Google Scholar] [CrossRef] [Green Version]
- Raghuram, M.; Madhav, K.V.; Krishna, E.H.; Komalla, N.R.; Sivani, K.; Reddy, K.A. Dual-tree complex wavelet transform for motion-artifact reduction of PPG signals. In Proceedings of the 2012 IEEE International Symposium on Medical Measurements and Applications Proceedings, Budapest, Hungary, 18–19 May 2012; pp. 1–4. [Google Scholar]
- Gupta, V.; Mittal, M. Arrhythmia detection in ECG signal using fractional wavelet transform with principal component analysis. J. Inst. Eng. Ser. B 2020, 101, 451–461. [Google Scholar] [CrossRef]
- Liu, S.; Shao, J.; Kong, T.; Malekian, R. ECG arrhythmia classification using high order spectrum and 2D graph fourier transform. Appl. Sci. 2020, 10, 4741. [Google Scholar] [CrossRef]
- Shan, S.; Tang, S.; Huang, P.; Lin, Y.; Huang, W.; Lai, D.; Wu, A.A. Reliable PPG-based algorithm in atrial fibrillation detection. In Proceedings of the 2016 IEEE Biomedical Circuits and Systems Conference (BioCAS), Shanghai, China, 17–19 October 2016; pp. 340–343. [Google Scholar]
- Kanawade, R.; Tewary, S.; Sardana, H.K. Photoplethysmography based arrhythmia detection and classification. In Proceedings of the 2019 6th International Conference on Signal Processing and Integrated Networks (SPIN), Noida, India, 7–8 March 2019; pp. 944–948. [Google Scholar]
- Homaeinezhad, M.R.; Atyabi, S.A.; Tavakkoli, E.; Toosi, H.N.; Ghaffari, A.; Ebrahimpour, R. ECG arrhythmia recognition via a neuro-SVM–KNN hybrid classifier with virtual QRS image-based geometrical features. Expert Syst. Appl. 2012, 39, 2047–2058. [Google Scholar] [CrossRef]
- Savalia, S.; Emamian, V. Cardiac arrhythmia classification by multi-layer perceptron and convolution neural networks. Bioengineering 2018, 5, 35. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Pandey, S.K.; Pawar, U.; Janghel, R.R. Classification of ECG arrhythmia using recurrent neural networks. Procedia Comput. Sci. 2018, 132, 1290–1297. [Google Scholar] [CrossRef]
- Ebrahimi, Z.; Loni, M.; Daneshtalab, M.; Gharehbaghi, A. A review on deep learning methods for ECG arrhythmia classification. Expert Syst. Appl. X 2020, 7, 100033. [Google Scholar] [CrossRef]
- Liu, M.; Kim, Y. Classification of heart diseases based on ecg signals using long short-term memory. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18–21 July 2018; pp. 2707–2710. [Google Scholar]
- Sayantan, G.; Kien, P.T.; Kadambari, K.V. Classification of ECG beats using deep belief network and active learning. Med. Biol. Eng. Comput. 2018, 56, 1887–1898. [Google Scholar] [CrossRef]
- Kaisti, M.; Panula, T.; Leppänen, J.; Punkkinen, R.; Jafari Tadi, M.; Vasankari, T.; Jaakkola, S.; Kiviniemi, T.; Airaksinen, J.; Kostiainen, P.; et al. Clinical assessment of a non-invasive wearable MEMS pressure sensor array for monitoring of arterial pulse waveform, heart rate and detection of atrial fibrillation. npj Digit. Med. 2019, 2, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, L.; Zhang, B.; Xin, Y.; Liu, Y.; Xing, R.; Yang, Y.; Wang, X.; Que, W.; Liu, W.; Geng, L. Fully integrated flexible long-term electrocardiogram recording patch with gel-less adhesive electrodes for arrhythmia detection. Sens. Actuators A Phys. 2021, 332, 113063. [Google Scholar] [CrossRef]
- Tison, G.; Sanchez, J.M.; Ballinger, B.; Singh, A.; Olgin, J.E.; Pletcher, M.J.; Vittinghoff, E.; Lee, E.S.; Fan, S.M.; Gladstone, R.A.; et al. Passive detection of atrial fibrillation using a commercially available smartwatch. JAMA Cardiol. 2018, 3, 409–416. [Google Scholar] [CrossRef] [Green Version]
- Petmezas, G.; Haris, K.; Stefanopoulos, L.; Kilintzis, V.; Tzavelis, A.; Rogers, J.A.; Katsaggelos, A.K.; Maglaveras, N. Automated atrial fibrillation detection using a hybrid CNN-LSTM network on imbalanced ECG datasets. Biomed. Signal Process. Control 2021, 63, 102194. [Google Scholar] [CrossRef]
- Kwon, S.; Hong, J.; Choi, E.-K.; Lee, E.; Hostallero, D.E.; Kang, W.J.; Lee, B.; Jeong, E.-R.; Koo, B.-K.; Oh, S.; et al. Deep learning approaches to detect atrial fibrillation using photoplethysmographic signals: Algorithms development study. JMIR mHealth uHealth 2019, 7, e12770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dezaki, F.T.; Ginsberg, T.; Luong, C.; Vaseli, H.; Rohling, R.; Gin, K.; Abolmaesumi, P.; Tsang, T. Echo-rhythm net: Semi-supervised learning for automatic detection of atrial fibrillation in echocardiography. In Proceedings of the 2021 IEEE 18th International Symposium on Biomedical Imaging (ISBI), Nice, France, 13–16 April 2021; pp. 110–113. [Google Scholar]
- Lee, W.; Kobayashi, S.; Nagase, M.; Jimbo, Y.; Saito, I.; Inoue, Y.; Yambe, T.; Sekino, M.; Malliaras, G.G.; Yokota, T.; et al. Nonthrombogenic, stretchable, active multielectrode array for electroanatomical mapping. Sci. Adv. 2022, 4, eaau2426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Qiu, Y.; Ameri, S.K.; Jang, H.; Dai, Z.; Huang, Y.; Lu, N. Low-cost, μm-thick, tape-free electronic tattoo sensors with minimized motion and sweat artifacts. npj Flex. Electron. 2018, 2, 6. [Google Scholar] [CrossRef]
- Xu, X.; Luo, M.; He, P.; Yang, J. Washable and flexible screen printed graphene electrode on textiles for wearable healthcare monitoring. J. Phys. D Appl. Phys. 2020, 53, 125402. [Google Scholar] [CrossRef]
- Kwon, Y.-T.; Kim, Y.-S.; Kwon, S.; Mahmood, M.; Lim, H.-R.; Park, S.-W.; Kang, S.-O.; Choi, J.J.; Herbert, R.; Jang, Y.C.; et al. All-printed nanomembrane wireless bioelectronics using a biocompatible solderable graphene for multimodal human-machine interfaces. Nat. Commun. 2020, 11, 3450. [Google Scholar] [CrossRef]
- Zavanelli, N.; Kim, J.; Yeo, W.-H. Recent advances in high-throughput nanomaterial manufacturing for hybrid flexible bioelectronics. Materials 2021, 14, 2973. [Google Scholar] [CrossRef] [PubMed]



| Reference | Measured Signal | Sensor Location | Substrate Material | Sensor Material | Flexibility |
|---|---|---|---|---|---|
| [14] | PPG | Wrist | PEN | PEDOT:PSS | Flexible |
| [17] | ECG | Arm | unknown | Ag/AgCl | Flexible |
| [18] | ECG | Chest | PDMS | Carbon black-PDMS nanocomposite | Stretchable |
| [19] | PPG | Finger | PI | Sb2Se3 | Rigid |
| [20] | ECG | Wrist | Polythiophene | Polyvinyl alcohol/cellulose /PEDOT:PSS | Flexible |
| [21] | ECG | Forearm | unknown | PEDOT:PSS/WPU/D-sorbitol | Flexible |
| [22] | Ultrasound | Neck | PI | 1–3 Piezoelectric composite | Stretchable |
| [16] | SCG, ECG | Chest | Tegaderm | PVDF | Stretchable |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guess, M.; Zavanelli, N.; Yeo, W.-H. Recent Advances in Materials and Flexible Sensors for Arrhythmia Detection. Materials 2022, 15, 724. https://doi.org/10.3390/ma15030724
Guess M, Zavanelli N, Yeo W-H. Recent Advances in Materials and Flexible Sensors for Arrhythmia Detection. Materials. 2022; 15(3):724. https://doi.org/10.3390/ma15030724
Chicago/Turabian StyleGuess, Matthew, Nathan Zavanelli, and Woon-Hong Yeo. 2022. "Recent Advances in Materials and Flexible Sensors for Arrhythmia Detection" Materials 15, no. 3: 724. https://doi.org/10.3390/ma15030724
APA StyleGuess, M., Zavanelli, N., & Yeo, W.-H. (2022). Recent Advances in Materials and Flexible Sensors for Arrhythmia Detection. Materials, 15(3), 724. https://doi.org/10.3390/ma15030724








