Sorption Studies of Tetracycline Antibiotics on Hydroxyapatite (001) Surface—A First-Principles Insight
Abstract
1. Introduction
2. Theoretical Methods
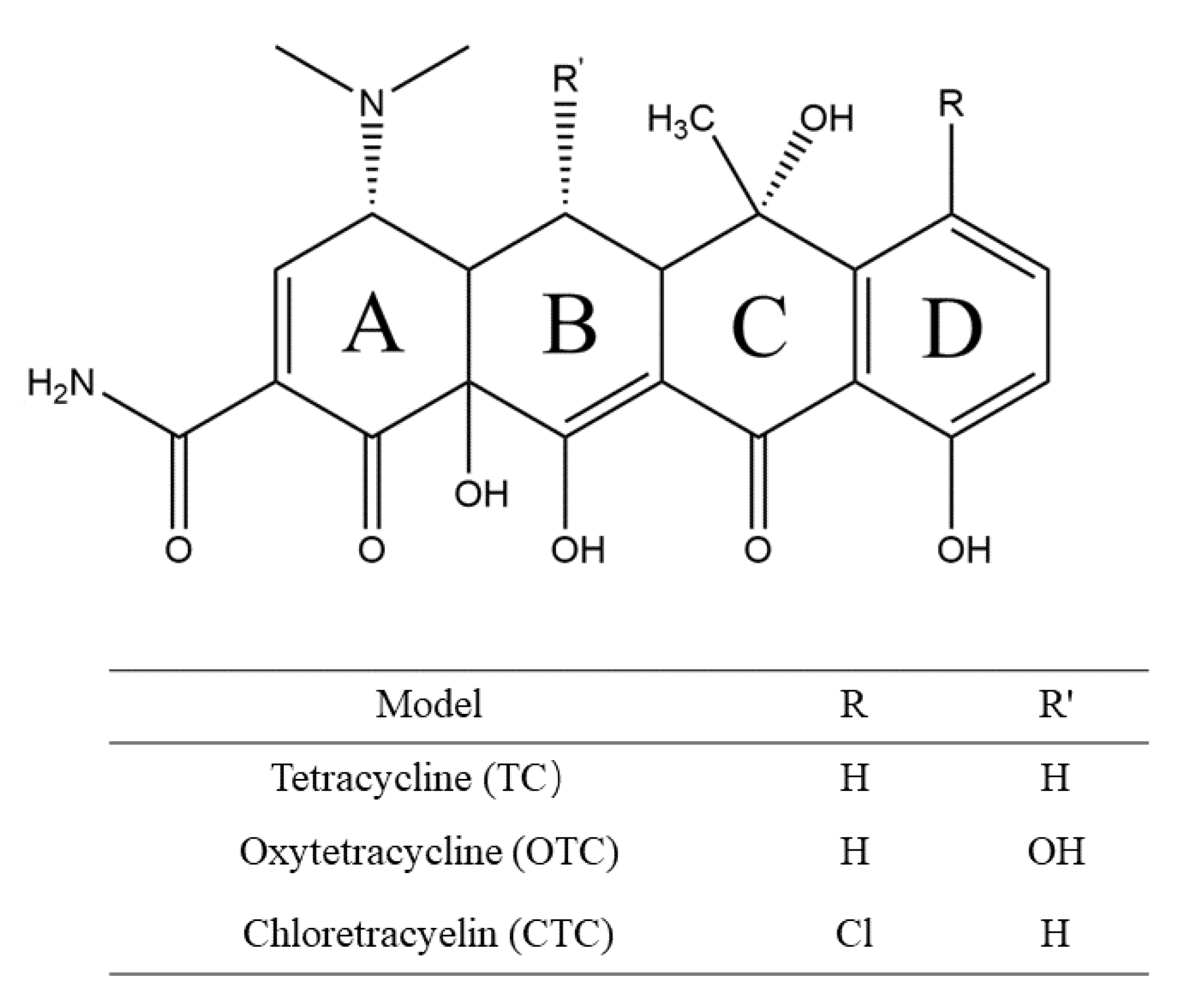
2.1. Model Development
2.2. DFT Calculation
3. Results and Discussion
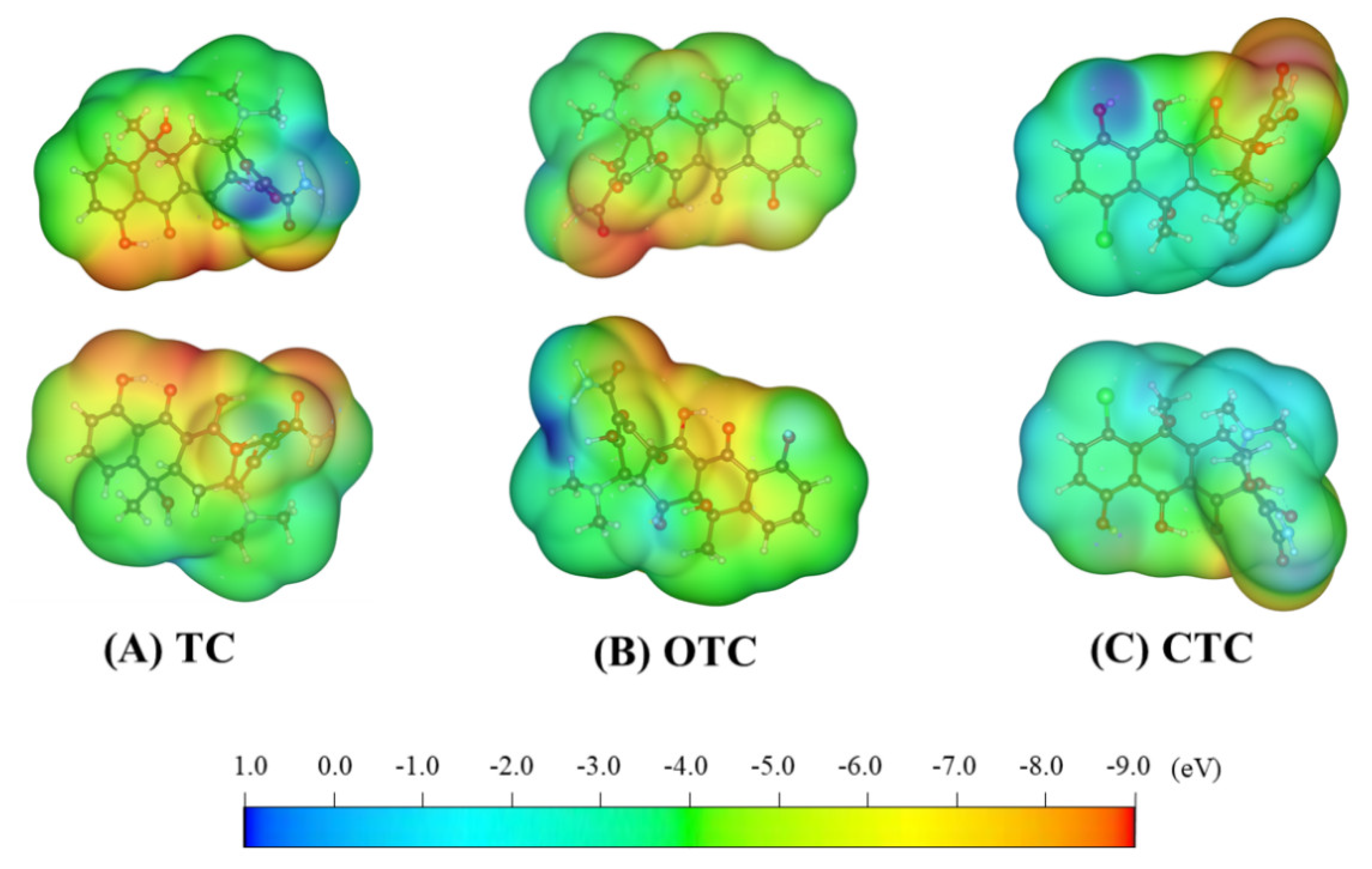
3.1. ESP
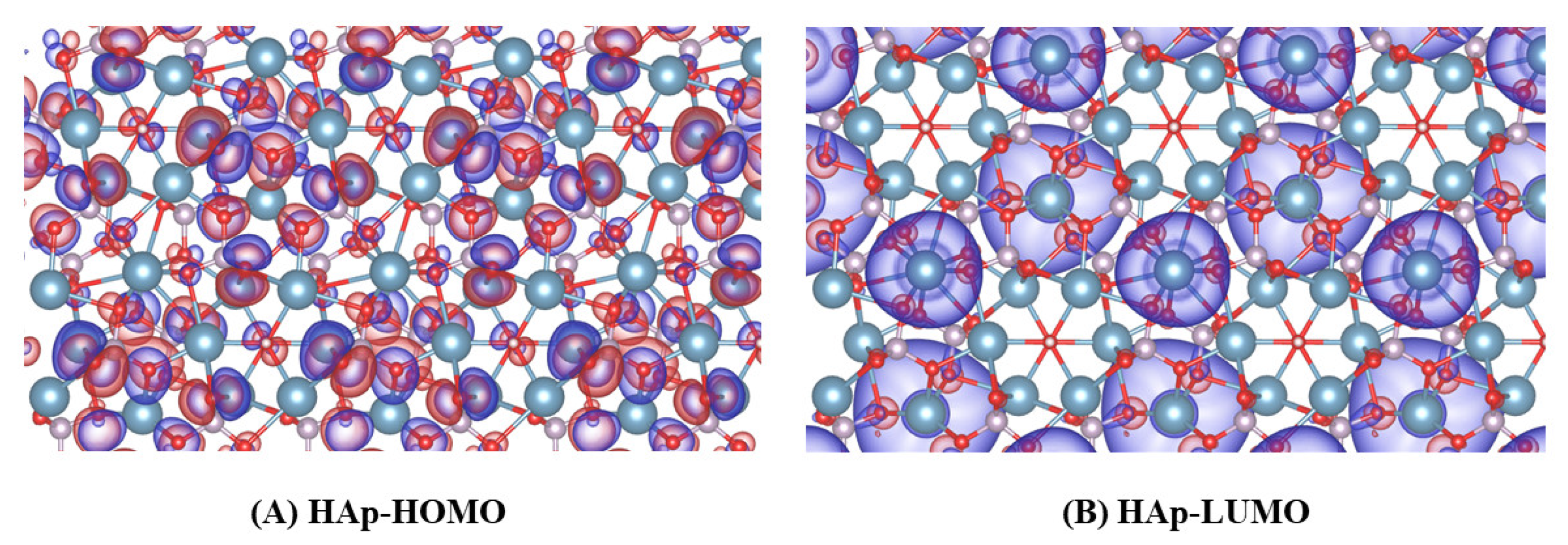
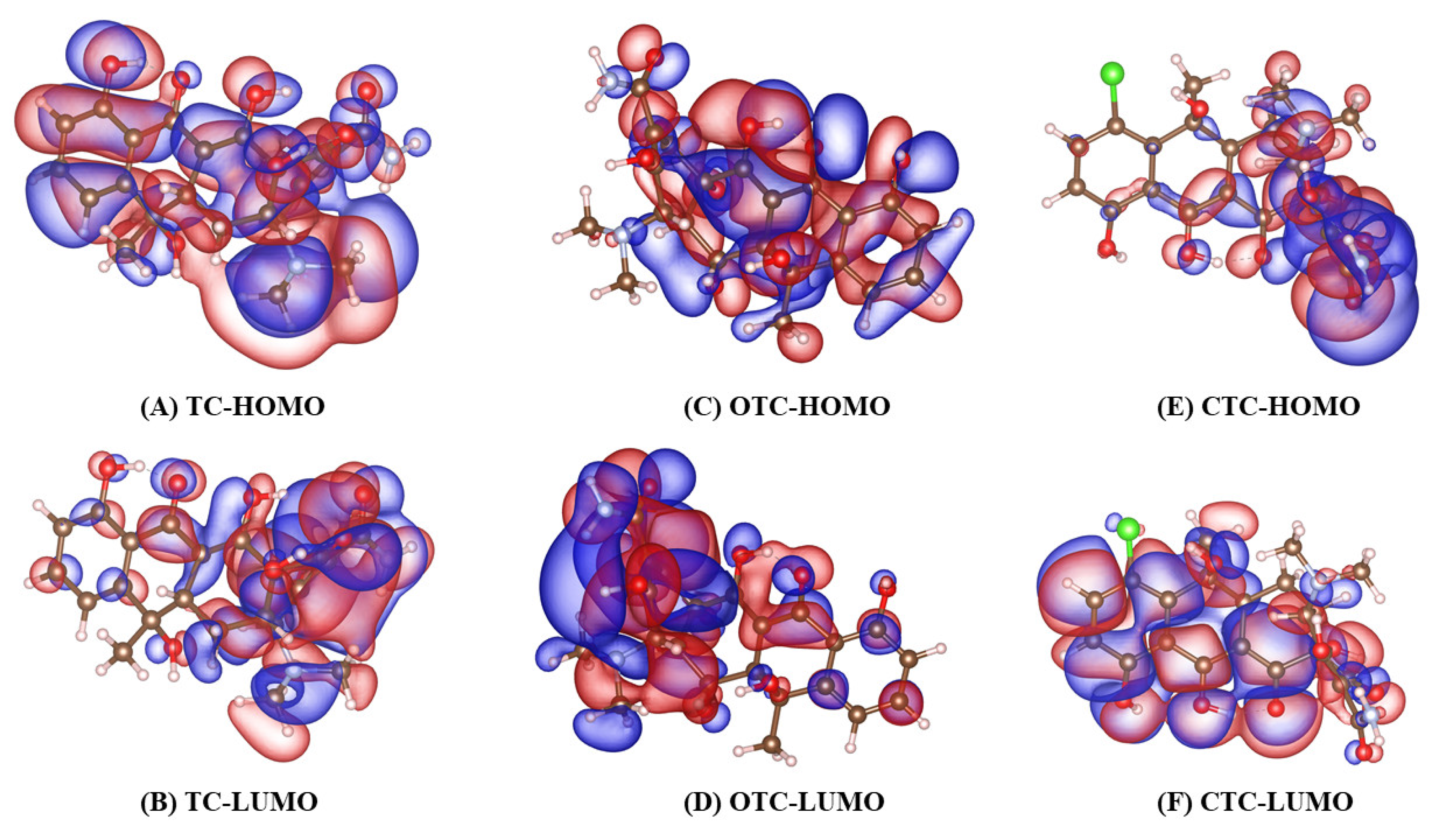
3.2. Molecular Orbital
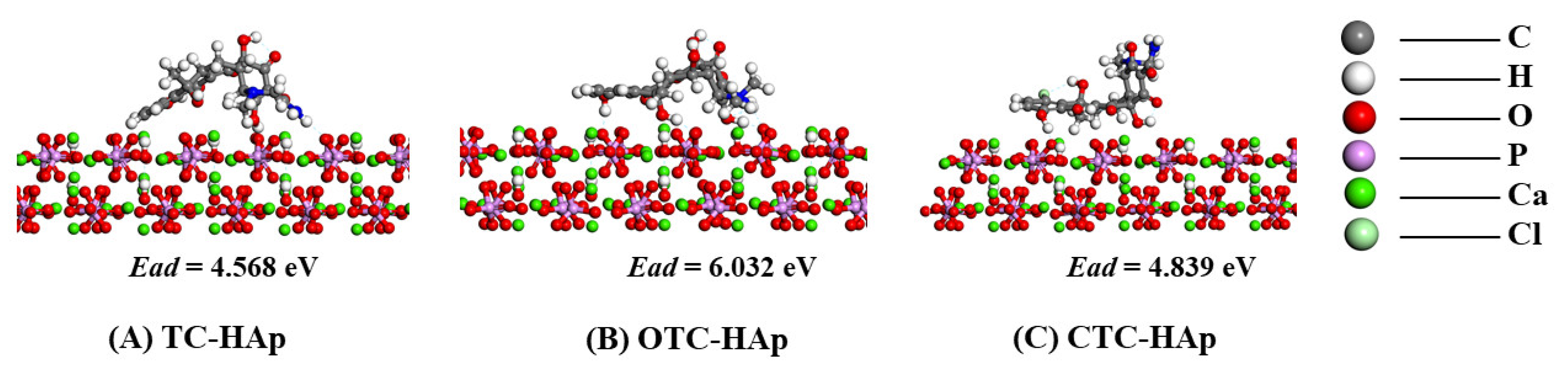
3.3. Adsorption Energy and Geometry
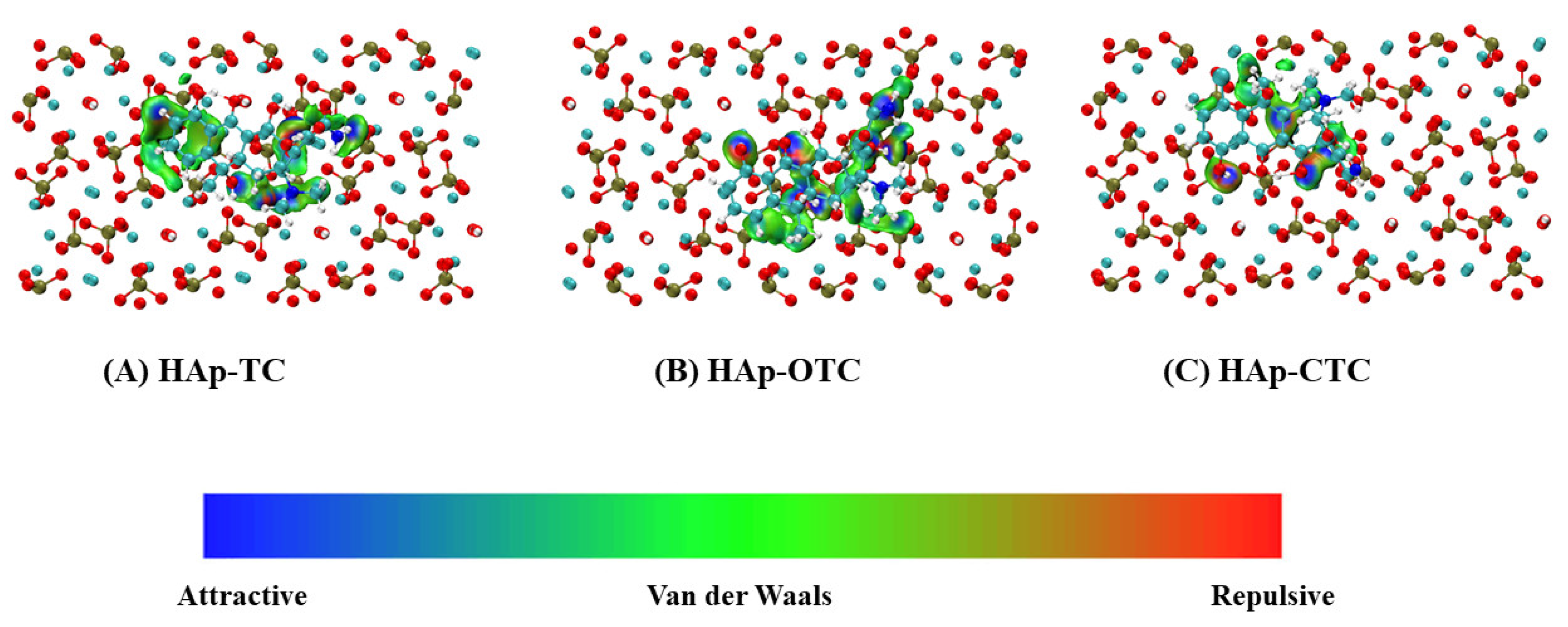
3.4. IGM Analysis
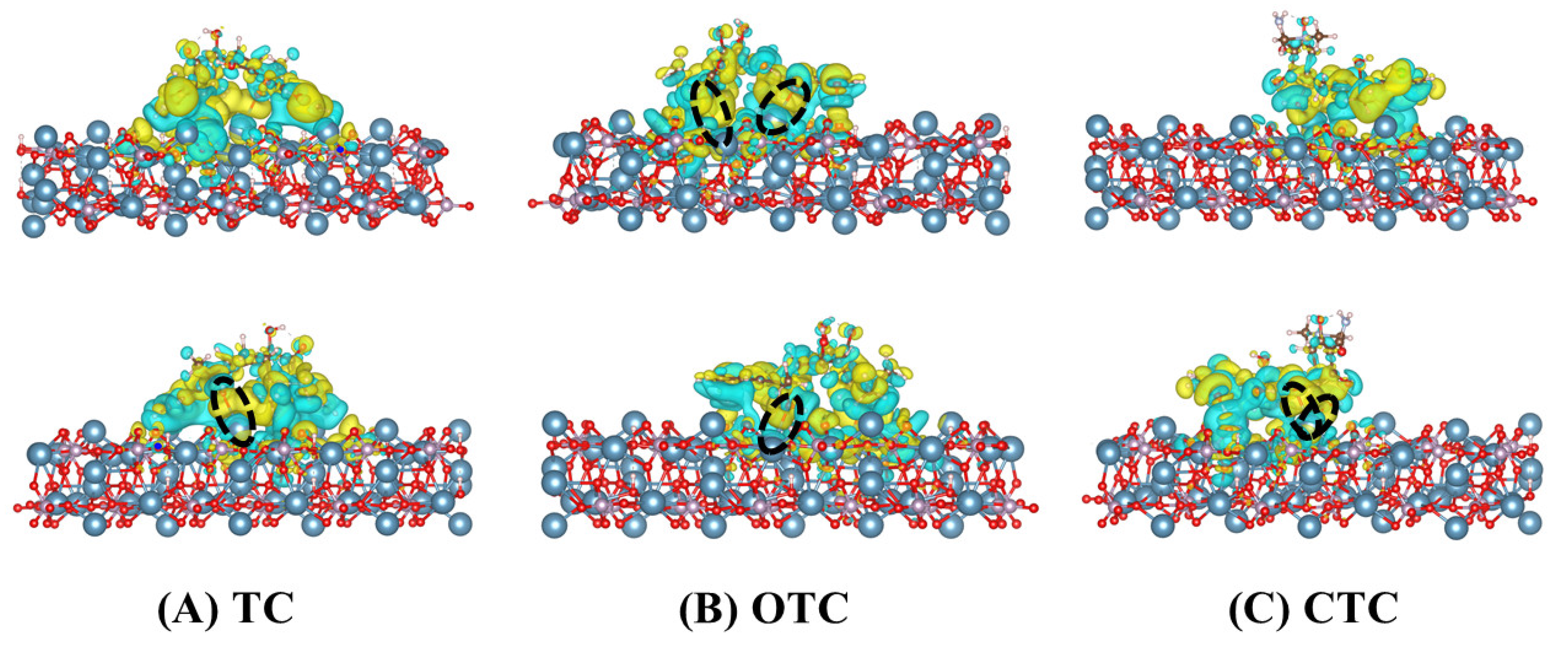
3.5. Analysis of the Charge Density Difference
4. Conclusions
- The order of affinity of the three TCs on the surface of HAp was OTC-HAp > CTC-HAp > TC-HAp.
- In terms of the adsorption configurations of TC-PCL, TC, OTC, and CTC were adsorbed on HAp in an arch shape, a broken-line shape, and a twisted shape, respectively.
- Both OTC and CTC formed more hydrogen bonds and coordination bonds with HAp. TC exhibited van der Waals interactions and hydrogen bonds but formed only weak coordination bonds.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wong, S.K.; Mohamad, N.-V.; Giaze, T.R.; Chin, K.-Y.; Mohamed, N.; Ima-Nirwana, S. Prostate Cancer and Bone Metastases: The Underlying Mechanisms. Int. J. Mol. Sci. 2019, 20, 2587. [Google Scholar] [CrossRef]
- Cleeland, C.; Von Moos, R.; Walker, M.S.; Wang, Y.; Gao, J.; Chavez-MacGregor, M.; Liede, A.; Arellano, J.; Balakumaran, A.; Qian, Y. Burden of symptoms associated with development of metastatic bone disease in patients with breast cancer. Support. Care Cancer 2016, 24, 3557–3565. [Google Scholar] [CrossRef]
- Rotman, S.; Grijpma, D.; Richards, R.; Moriarty, F.; Eglin, D.; Guillaume, O. Drug delivery systems functionalized with bone mineral seeking agents for bone targeted therapeutics. J. Control. Release 2018, 269, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Yu, X.; Carbone, E.J.; Nelson, C.; Kan, H.M.; Lo, K.W.-H. Poly aspartic acid peptide-linked PLGA based nanoscale particles: Potential for bone-targeting drug delivery applications. Int. J. Pharm. 2014, 475, 547–557. [Google Scholar] [CrossRef]
- Gu, W.; Wu, C.; Chen, J.; Xiao, Y. Nanotechnology in the targeted drug delivery for bone diseases and bone regeneration. Int. J. Nanomed. 2013, 8, 2305–2317. [Google Scholar] [CrossRef] [PubMed]
- de Miguel, L.; Noiray, M.; Surpateanu, G.; Iorga, B.I.; Ponchel, G. Poly(γ-benzyl-l-glutamate)-PEG-alendronate multivalent nanoparticles for bone targeting. Int. J. Pharm. 2014, 460, 73–82. [Google Scholar] [CrossRef]
- Pierce, W.M.; Waite, L.C. Bone-Targeted Carbonic Anhydrase Inhibitors: Effect of a Proinhibitor on Bone Resorption In Vitro. Exp. Biol. Med. 1987, 186, 96–102. [Google Scholar] [CrossRef]
- Cawthray, J.; Wasan, E.; Wasan, K. Bone-seeking agents for the treatment of bone disorders. Drug Deliv. Transl. Res. 2017, 7, 466–481. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, M.; Sawamoto, K.; Alméciga-Díaz, C.J.; MacKenzie, W.G.; Mason, R.W.; Orii, T.; Tomatsu, S. Development of Bone Targeting Drugs. Int. J. Mol. Sci. 2017, 18, 1345. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Tan, X.; Huang, J.; Huang, H.; Zou, P.; Hu, J. Atorvastatin-loaded micelles with bone-targeted ligand for the treatment of osteoporosis. Drug Deliv. 2017, 24, 1067–1076. [Google Scholar] [CrossRef][Green Version]
- Xie, Y.; Liu, C.; Huang, H.; Huang, J.; Deng, A.; Zou, P.; Tan, X. Bone-targeted delivery of simvastatin-loaded PEG-PLGA micelles conjugated with tetracycline for osteoporosis treatment. Drug Deliv. Transl. Res. 2018, 8, 1090–1102. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Wang, H.; Liu, J.; Tao, S.; Chai, G.; Wang, J.; Hu, F. Tetracycline-grafted PLGA nanoparticles as bone-targeting drug delivery system. Int. J. Nanomed. 2015, 10, 5671–5685. [Google Scholar] [CrossRef]
- Neale, J.R.; Richter, N.B.; Merten, K.E.; Taylor, K.G.; Singh, S.; Waite, L.C.; Emery, N.K.; Smith, N.B.; Cai, J.; Pierce, W.M. Bone selective effect of an estradiol conjugate with a novel tetracycline-derived bone-targeting agent. Bioorg. Med. Chem. Lett. 2009, 19, 680–683. [Google Scholar] [CrossRef] [PubMed]
- E Shea, J.; Miller, S.C. Skeletal function and structure: Implications for tissue-targeted therapeutics. Adv. Drug Deliv. Rev. 2005, 57, 945–957. [Google Scholar] [CrossRef]
- Lammi, M.J.; Häyrinen, J.; Mahonen, A. Proteomic analysis of cartilage- and bone-associated samples. Electrophoresis 2006, 27, 2687–2701. [Google Scholar] [CrossRef]
- Ren, F.Z.; Xin, R.L.; Ge, X.; Leng, Y. An Experimental and Computational Study on Zn-Substituted Hydroxyapatite. Adv. Mater. Res. 2008, 47–50, 1379–1382. [Google Scholar] [CrossRef]
- Bystrov, V.S.; Paramonova, E.V.; Costa, M.E.V.; Santos, C.; Almeida, M.; Kopyl, S.; Dekhtyar, Y.; Bystrova, A.V.; Maevsky, E.I.; Pullar, R.C.; et al. A Computational Study of the Properties and Surface Interactions of Hydroxyapatite, Structure and Properties: Modeling and Synchrotron Data Analysis of Modified Hydroxyapatite Structure. Ferroelectrics 2013, 449, 94–101. [Google Scholar] [CrossRef]
- Nielsen, J.J.; Low, S.A. Bone-Targeting Systems to Systemically Deliver Therapeutics to Bone Fractures for Accelerated Healing. Curr. Osteoporos. Rep. 2020, 18, 449–459. [Google Scholar] [CrossRef]
- Low, S.A.; Kopeček, J. Targeting polymer therapeutics to bone. Adv. Drug Deliv. Rev. 2012, 64, 1189–1204. [Google Scholar] [CrossRef]
- Ryu, T.-K.; Kang, R.-H.; Jeong, K.-Y.; Jun, D.-R.; Koh, J.-M.; Kim, D.; Bae, S.K.; Choi, S.-W. Bone-targeted delivery of nanodiamond-based drug carriers conjugated with alendronate for potential osteoporosis treatment. J. Control. Release 2016, 232, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, J.; Mura, S.; Brambilla, D.; Mackiewicz, N.; Couvreur, P. Design, functionalization strategies and biomedical applications of targeted biodegradable/biocompatible polymer-based nanocarriers for drug delivery. Chem. Soc. Rev. 2012, 42, 1147–1235. [Google Scholar] [CrossRef]
- Hans, M.; Lowman, A. Biodegradable nanoparticles for drug delivery and targeting. Curr. Opin. Solid State Mater. Sci. 2002, 6, 319–327. [Google Scholar] [CrossRef]
- Abou-Zeid, A.-Z.A. Tetracyclines. Zbl. Bakt. Abt. II Bo. 1973, 128, 51–60. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, H.; Xiong, P.; Zhu, Q.; Liao, C.; Jiang, G. Occurrence, fate, and risk assessment of typical tetracycline antibiotics in the aquatic environment: A review. Sci. Total Environ. 2020, 753, 141975. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, P.; Gyrd-Hansen, N. Bioavailability of oxytetracycline, tetracycline and chlortetracycline after oral administration to fed and fasted pigs. J. Vet. Pharmacol. Ther. 1996, 19, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Jiang, L.; Liang, J.; Xu, W.; Yu, H.; Zhang, J.; Ye, S.; Xing, W.; Yuan, X. Photocatalytic degradation of tetracycline antibiotics using delafossite silver ferrite-based Z-scheme photocatalyst: Pathways and mechanism insight. Chemosphere 2020, 270, 128651. [Google Scholar] [CrossRef]
- Gong, X.; Li, X.; Qing, T.; Zhang, P.; Feng, B. Amplified colorimetric detection of tetracycline based on an enzyme-linked aptamer assay with multivalent HRP-mimicking DNAzyme. Analyst 2019, 144, 1948–1954. [Google Scholar] [CrossRef] [PubMed]
- Alanazi, F.; Almugbel, R.; Maher, H.M.; Alodaib, F.M.; Alzoman, N.Z. Determination of tetracycline, oxytetracycline and chlortetracycline residues in seafood products of Saudi Arabia using high performance liquid chromatography–Photo diode array detection. Saudi Pharm. J. 2021, 29, 566–575. [Google Scholar] [CrossRef]
- Perrin, D. Binding of Tetracyclines to Bone. Nature 1965, 208, 787–788. [Google Scholar] [CrossRef]
- Zhou, Q.; Wang, H.; Zhu, K.; Yao, C.; Peng, J.; Chen, Y.; Zhou, S.; Zhu, L. Evaluation of Hydroxyapatite as Adsorbent in the Analysis of Trace Tetracyclines in Complex Matrices. Food Anal. Methods 2020, 14, 28–35. [Google Scholar] [CrossRef]
- Harja, M.; Ciobanu, G. Removal of oxytetracycline from aqueous solutions by hydroxyapatite as a low-cost adsorbent. E3S Web Conf. 2017, 22, 62. [Google Scholar] [CrossRef]
- Jsa, B.; Kva, A.; Pvn, A. Tetracyclines in the environment: An overview on the occurrence, fate, toxicity, detection, removal methods, and sludge management. Sci. Total Environ. 2021, 771, 145291. [Google Scholar] [CrossRef]
- Harja, M.; Ciobanu, G. Studies on adsorption of oxytetracycline from aqueous solutions onto hydroxyapatite. Sci. Total Environ. 2018, 628–629, 36–43. [Google Scholar] [CrossRef]
- Zhou, X.; Cornel, E.J.; He, S.; Du, J. Recent advances in bone-targeting nanoparticles for biomedical applications. Mater. Chem. Front. 2021, 5, 6735–6759. [Google Scholar] [CrossRef]
- Rogalski, W. Chemical Modification of the Tetracyclines. In The Tetracyclines; Springer: Berlin/Heidelberg, Germany, 1985; pp. 179–316. [Google Scholar]
- Albert, A.; Rees, C.W. Avidity of the tetracyclines for the cations of metals. Nature 1956, 177, 433–434. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hu, J.; Zhang, S. Studies on the sorption of tetracycline onto clays and marine sediment from seawater. J. Colloid Interface Sci. 2010, 349, 578–582. [Google Scholar] [CrossRef]
- Kühne, T.D.; Iannuzzi, M.; Del Ben, M.; Rybkin, V.V.; Seewald, P.; Stein, F.; Laino, T.; Khaliullin, R.Z.; Schütt, O.; Schiffmann, F.; et al. CP2K: An electronic structure and molecular dynamics software package—Quickstep: Efficient and accurate electronic structure calculations. J. Chem. Phys. 2020, 152, 194103. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Bayly, C.I.; Cieplak, P.; Cornell, W.; Kollman, P.A. A well-behaved electrostatic potential based method using charge restraints for deriving atomic charges: The RESP model. J. Phys. Chem. 1993, 97, 10269–10280. [Google Scholar] [CrossRef]
- Sjoberg, P.; Politzer, P. Use of the electrostatic potential at the molecular surface to interpret and predict nucleophilic processes. J. Phys. Chem. 1990, 94, 3959–3961. [Google Scholar] [CrossRef]
- Ai, Y.; Liu, Y.; Huo, Y.; Zhao, C.; Sun, L.; Han, B.; Cao, X.; Wang, X. Insights into the adsorption mechanism and dynamic behavior of tetracycline antibiotics on reduced graphene oxide (RGO) and graphene oxide (GO) materials. Environ. Sci. Nano 2019, 6, 3336–3348. [Google Scholar] [CrossRef]
- Lefebvre, C.; Rubez, G.; Khartabil, H.; Boisson, J.-C.; Contreras-García, J.; Hénon, E. Accurately extracting the signature of intermolecular interactions present in the NCI plot of the reduced density gradient versus electron density. Phys. Chem. Chem. Phys. 2017, 19, 17928–17936. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]

| Models | ||||
|---|---|---|---|---|
| TC-HAp | −141,794.223 | −7763.023 | −134,026.542 | 4.658 |
| OTC-HAp | −142,227.494 | −8194.920 | −134,026.542 | 6.032 |
| CTC-HAp | −142,182.725 | −8151.344 | −134,026.542 | 4.839 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, J.; Cui, N.; Mao, X.; Huang, Q.; Lee, E.-S.; Jiang, H. Sorption Studies of Tetracycline Antibiotics on Hydroxyapatite (001) Surface—A First-Principles Insight. Materials 2022, 15, 797. https://doi.org/10.3390/ma15030797
Song J, Cui N, Mao X, Huang Q, Lee E-S, Jiang H. Sorption Studies of Tetracycline Antibiotics on Hydroxyapatite (001) Surface—A First-Principles Insight. Materials. 2022; 15(3):797. https://doi.org/10.3390/ma15030797
Chicago/Turabian StyleSong, Jiaming, Naiyu Cui, Xuran Mao, Qixuan Huang, Eui-Seok Lee, and Hengbo Jiang. 2022. "Sorption Studies of Tetracycline Antibiotics on Hydroxyapatite (001) Surface—A First-Principles Insight" Materials 15, no. 3: 797. https://doi.org/10.3390/ma15030797
APA StyleSong, J., Cui, N., Mao, X., Huang, Q., Lee, E.-S., & Jiang, H. (2022). Sorption Studies of Tetracycline Antibiotics on Hydroxyapatite (001) Surface—A First-Principles Insight. Materials, 15(3), 797. https://doi.org/10.3390/ma15030797








