Compositional Tailoring of Mg–2Zn–1Ca Alloy Using Manganese to Enhance Compression Response and In-Vitro Degradation
Abstract
:1. Introduction
- Mg–2Zn–1Ca/XMn (X = 0.3, 0.5, and 0.7) alloys were developed using disintegrated melt deposition followed by hot extrusion.
- The corrosion resistance of Mg–2Zn–1Ca alloys was improved with the addition of 0.5 wt.% Mn element.
- The enhanced corrosion resistance is due to improved corrosion product film.
- Post-corrosion compressive strength of Mg–2Zn–1Ca/0.3Mn and Mg-2Zn1Ca/0.5Mn stabilized after day 21.
2. Materials and Methods
2.1. Materials
2.2. Processing
2.2.1. Primary Processing Method
2.2.2. Secondary Processing Method
2.3. Characterization
3. Results
3.1. Immersion Studies—Corrosion Rate and pH
3.2. Microstructure Characterisation—Grain Size
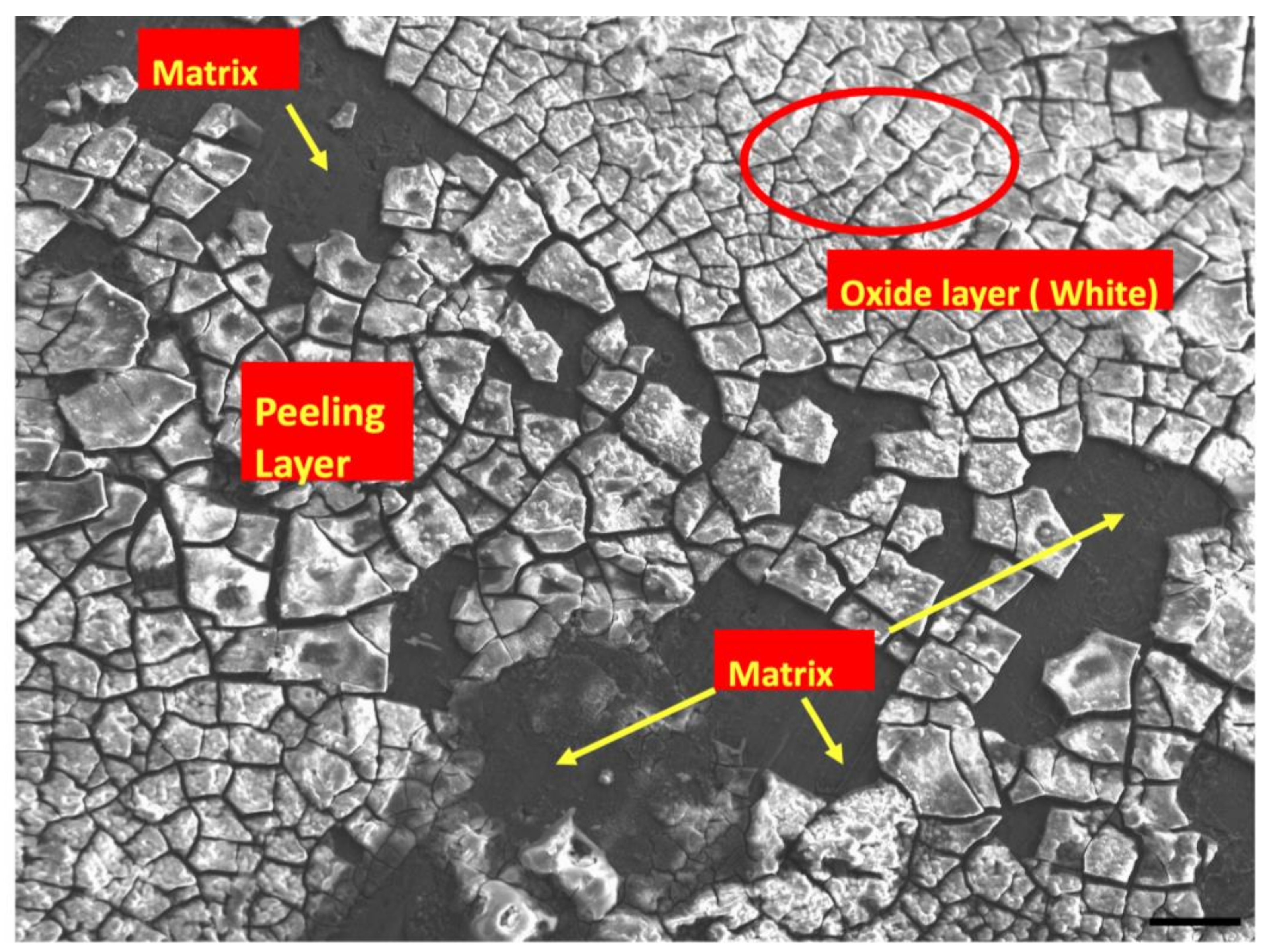
3.3. Post-Corrosion Scanning Electron Microscopy (SEM)
4. Discussion
5. Conclusions
- The effect of manganese addition on the bio-corrosion behavior and post corrosion strength retention was successfully established for Mg–2Zn–1Ca alloy.
- The results showed the lowest corrosion rate for Mg–2Zn–1Ca/0.5Mn alloy. This can be attributed to the optimized presence of secondary phases (near absence of clusters) and reduced grain size. Corrosion resistance of the alloys can be represented as: Mg–2Zn–1Ca/0.5Mn > Mg–2Zn–1Ca > Mg–2Zn–1Ca/0.3Mn > Mg–2Zn–1Ca/0.7Mn.
- The post-corrosion compression properties displayed superior strength retention for all the samples except Mg–2Zn–1Ca/0.7Mn. The relative order of post-corrosion strength retention of the alloys can be represented as: Mg–2Zn–1Ca/0.5Mn > Mg–2Zn–1Ca > Mg–2Zn–1Ca/0.3Mn > Mg-2Zn1-Ca/0.7Mn.
- The presence of a controlled amount of Mn addition to the Mg2Zn1Ca alloy up to 0.5 wt.% can be attributed to the ability of the material to develop a uniform passivating layer as a result of the formation of the hydroxide and phosphate compounds. Addition of Mn above 0.5 wt.% has resulted in the decrement in the corrosion resistance of Mg–2Zn–1Ca owing to the non-uniform segregation of Mn along the grain boundaries of the alloy.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shahin, M.; Munir, K.; Wen, C.; Li, Y. Magnesium matrix nanocomposites for orthopedic applications: A review from mechanical, corrosion, and biological perspectives. Acta Biomater. 2019, 96, 1–19. [Google Scholar] [CrossRef]
- Parande, G.; Manakari, V.; Gupta, H.; Gupta, M. Magnesium-β-Tricalcium Phosphate Composites as a Potential Orthopedic Implant: A Mechanical/Damping/Immersion Perspective. Metals 2018, 8, 343. [Google Scholar] [CrossRef] [Green Version]
- Walker, J.; Shadanbaz, S.; Woodfield, T.B.F.; Staiger, M.P.; Dias, G.J. Magnesium biomaterials for orthopedic application: A review from a biological perspective. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014, 102, 1316–1331. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Hussein, M.; Al-Aqeeli, N. Magnesium-based composites and alloys for medical applications: A review of mechanical and corrosion properties. J. Alloy. Compd. 2019, 792, 1162–1190. [Google Scholar] [CrossRef]
- Zheng, Y.F.; Gu, X.N.; Witte, F. Biodegradable metals. Mater. Sci. Eng. R Rep. 2014, 77, 1–34. [Google Scholar] [CrossRef]
- Mozafari, M. Handbook of Biomaterials Biocompatibility; Woodhead Publishing: Sawston, UK, 2020. [Google Scholar]
- Liu, C.; Ren, Z.; Xu, Y.; Pang, S.; Zhao, X.; Zhao, Y. Biodegradable Magnesium Alloys Developed as Bone Repair Materials: A Review. Scanning 2018, 2018, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Zhang, M.; Li, Y.; Zhao, J.; Qin, L.; Lai, Y. Corrosion and biocompatibility improvement of magnesium-based alloys as bone implant materials: A review. Regen. Biomater. 2017, 4, 129–137. [Google Scholar] [CrossRef] [Green Version]
- Hou, X.; Cao, Z.; Zhao, L.; Wang, L.; Wu, Y.; Wang, L. Microstructure, texture and mechanical properties of a hot rolled Mg–6.5 Gd–1.3 Nd–0.7 Y–0.3 Zn alloy. Mater. Des. 2012, 34, 776–781. [Google Scholar] [CrossRef]
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti based biomaterials, the ultimate choice for orthopaedic implants–a review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Paital, S.R.; Dahotre, N.B. Calcium phosphate coatings for bio-implant applications: Materials, performance factors, and methodologies. Mater. Sci. Eng. R Rep. 2009, 66, 1–70. [Google Scholar] [CrossRef]
- Selvaraj, D.; Raja, J.; Prasath, S. Interdisciplinary approach for bilateral maxillary canine: First premolar transposition with complex problems in an adult patient. J. Pharm. Bioallied Sci. 2013, 5, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, H.; Moghaddam, N.; Elahinia, M. Mechanical and In Vitro Corrosion Properties of a Heat-Treated Mg-Zn-Ca-Mn Alloy as a Potential Bioresorbable Material. Sci. Pages Metall. Mater. Eng 2017, 1, 1–7. [Google Scholar]
- Prakash, C.; Singh, S.; Pabla, B.S.; Sidhu, S.S.; Uddin, M. Bio-inspired low elastic biodegradable Mg-Zn-Mn-Si-HA alloy fabricated by spark plasma sintering. Mater. Manuf. Process. 2018, 34, 357–368. [Google Scholar] [CrossRef]
- Wong, P.-C.; Tsai, P.-H.; Li, T.-H.; Cheng, C.-K.; Jang, J.; Huang, J. Degradation behavior and mechanical strength of Mg-Zn-Ca bulk metallic glass composites with Ti particles as biodegradable materials. J. Alloy. Compd. 2017, 699, 914–920. [Google Scholar] [CrossRef]
- Witte, F. The history of biodegradable magnesium implants: A review. Acta Biomater. 2010, 6, 1680–1692. [Google Scholar] [CrossRef]
- Witte, F.; Fischer, J.; Nellesen, J.; Crostack, H.-A.; Kaese, V.; Pisch, A.; Beckmann, F.; Windhagen, H. In vitro and in vivo corrosion measurements of magnesium alloys. Biomaterials 2006, 27, 1013–1018. [Google Scholar] [CrossRef]
- Witte, F.; Kaese, V.; Haferkamp, H.; Switzer, E.; Meyer-Lindenberg, A.; Wirth, C.; Windhagen, H. In vivo corrosion of four magnesium alloys and the associated bone response. Biomaterials 2005, 26, 3557–3563. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Gu, X.; Xi, Y.; Chai, D. In vitro degradation and cytotoxicity of Mg/Ca composites produced by powder metallurgy. Acta Biomater. 2010, 6, 1783–1791. [Google Scholar] [CrossRef]
- Parande, G.; Manakari, V.; Prasadh, S.; Chauhan, D.; Rahate, S.; Wong, R.; Gupta, M. Strength retention, corrosion control and biocompatibility of Mg–Zn–Si/HA nanocomposites. J. Mech. Behav. Biomed. Mater. 2020, 103, 103584. [Google Scholar] [CrossRef]
- Johnston, S.; Shi, Z.; Atrens, A. The influence of pH on the corrosion rate of high-purity Mg, AZ91 and ZE41 in bicarbonate buffered Hanks’ solution. Corros. Sci. 2015, 101, 182–192. [Google Scholar] [CrossRef] [Green Version]
- Radha, R.; Sreekanth, D. Insight of magnesium alloys and composites for orthopedic implant applications–a review. J. Magnes. Alloy. 2017, 5, 286–312. [Google Scholar] [CrossRef]
- Waizy, H.; Seitz, J.-M.; Reifenrath, J.; Weizbauer, A.; Bach, F.-W.; Meyer-Lindenberg, A.; Denkena, B.; Windhagen, H. Biodegradable magnesium implants for orthopedic applications. J. Mater. Sci. 2013, 48, 39–50. [Google Scholar] [CrossRef]
- Vatsal, A.; Prasadh, S.; Deepamala, S.; Patil, A.; Sulochana, K.; Shruthi, D. Comparative evaluation of dimensional changes of elastomeric impression materials after disinfection with glutaraldehyde and microwave irradiation. J. Int. Oral Health 2015, 7, 44–46. [Google Scholar]
- Witte, F.; Hort, N.; Vogt, C.; Cohen, S.; Kainer, K.U.; Willumeit, R.; Feyerabend, F. Degradable biomaterials based on magnesium corrosion. Curr. Opin. Solid State Mater. Sci. 2008, 12, 63–72. [Google Scholar] [CrossRef] [Green Version]
- Bazhenov, V.; Li, A.; Komissarov, A.; Koltygin, A.; Tavolzhanskii, S.; Bautin, V.; Voropaeva, O.; Mukhametshina, A.; Tokar, A. Microstructure and mechanical and corrosion properties of hot-extruded Mg–Zn–Ca–(Mn) biodegradable alloys. J. Magnes. Alloy. 2020, 9, 1428–1442. [Google Scholar] [CrossRef]
- Cho, D.H.; Lee, B.W.; Park, J.Y.; Cho, K.M.; Park, I.M. Effect of Mn addition on corrosion properties of biodegradable Mg-4Zn-0.5Ca-xMn alloys. J. Alloy. Compd. 2017, 695, 1166–1174. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Idris, M.H.; Abdul-Kadir, M.R.; Ourdjini, A.; Medraj, M.; Daroonparvar, M.; Hamzah, E. Mechanical and bio-corrosion properties of quaternary Mg–Ca–Mn–Zn alloys compared with binary Mg–Ca alloys. Mater. Des. 2014, 53, 283–292. [Google Scholar] [CrossRef]
- Kujur, M.S.; Manakari, V.; Parande, G.; Prasadh, S.; Wong, R.; Mallick, A.; Gupta, M. Effect of samarium oxide nanoparticles on degradation and invitro biocompatibility of magnesium. Mater. Today Commun. 2021, 26, 102171. [Google Scholar] [CrossRef]
- Kujur, M.S.; Manakari, V.; Parande, G.; Prasadh, S.; Wong, R.; Mallick, A.; Gupta, M. Development of rare-earth oxide reinforced magnesium nanocomposites for orthopaedic applications: A mechanical/immersion/biocompatibility perspective. J. Mech. Behav. Biomed. Mater. 2020, 114, 104162. [Google Scholar] [CrossRef]
- Song, G. Control of biodegradation of biocompatable magnesium alloys. Corros. Sci. 2007, 49, 1696–1701. [Google Scholar] [CrossRef]
- Tong, L.; Chu, J.; Sun, W.; Jiang, Z.; Zou, D.; Wang, K.; Kamado, S.; Zheng, M. Development of high-performance Mg–Zn–Ca–Mn alloy via an extrusion process at relatively low temperature. J. Alloy. Compd. 2020, 825, 153942. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Hamzah, E.; Farahany, S.; Staiger, M.P. The Mechanical Properties and Corrosion Behavior of Quaternary Mg-6Zn-0.8 Mn-x Ca Alloys. J. Mater. Eng. Perf. 2015, 24, 598–608. [Google Scholar] [CrossRef]
- Cai, S.; Lei, T.; Li, N.; Feng, F. Effects of Zn on microstructure, mechanical properties and corrosion behavior of Mg–Zn alloys. Mater. Sci. Eng. C 2012, 32, 2570–2577. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, X.; Zhao, C.; Li, J.; Song, Y.; Xie, C.; Tao, H.; Zhang, Y.; He, Y.; Jiang, Y.; et al. Research on an Mg–Zn alloy as a degradable biomaterial. Acta Biomater. 2010, 6, 626–640. [Google Scholar] [CrossRef]
- Yan, Y.; Cao, H.; Kang, Y.; Yu, K.; Xiao, T.; Luo, J.; Deng, Y.; Fang, H.; Xiong, H.; Dai, Y. Effects of Zn concentration and heat treatment on the microstructure, mechanical properties and corrosion behavior of as-extruded Mg-Zn alloys produced by powder metallurgy. J. Alloy. Compd. 2017, 693, 1277–1289. [Google Scholar] [CrossRef]
- Pogosova, M.A.; Kasin, P.E.; Tretyakov, Y.D.; Jansen, M. Synthesis, structural features, and color of calcium-yttrium hydroxyapatite with copper ions in hexagonal channels. Russ. J. Inorg. Chem. 2013, 58, 381–386. [Google Scholar] [CrossRef]
- Li, Y.C.; Li, M.H.; Hu, W.Y.; Hodgson, P.D.; Wen, C.E. Biodegradable Mg-Ca and Mg-Ca-Y Alloys for Regenerative Medicine. Mater. Sci. Forum 2010, 654-656, 2192–2195. [Google Scholar] [CrossRef]
- Li, Z.; Gu, X.; Lou, S.; Zheng, Y. The development of binary Mg–Ca alloys for use as biodegradable materials within bone. Biomaterials 2008, 29, 1329–1344. [Google Scholar] [CrossRef]
- Mohamed, A.; El-Aziz, A.M.; Breitinger, H.-G. Study of the degradation behavior and the biocompatibility of Mg–0.8Ca alloy for orthopedic implant applications. J. Magnes. Alloy. 2019, 7, 249–257. [Google Scholar] [CrossRef]
- Liu, Y.-C.; Liu, D.-B.; Zhao, Y.; Chen, M.-F. Corrosion degradation behavior of Mg–Ca alloy with high Ca content in SBF. Trans. Nonferrous Met. Soc. China 2015, 25, 3339–3347. [Google Scholar] [CrossRef]
- Erikson, K.M.; Syversen, T.; Aschner, J.L.; Aschner, M. Interactions between excessive manganese exposures and dietary iron-deficiency in neurodegeneration. Environ. Toxicol. Pharmacol. 2005, 19, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Woolf, A.; Wright, R.; Amarasiriwardena, C.; Bellinger, D. A child with chronic manganese exposure from drinking water. Environ. Health Perspect. 2002, 110, 613–616. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, H.; Esfahani, S.N.; Poorganji, B.; Dean, D.; Elahinia, M. Resorbable bone fixation alloys, forming, and post-fabrication treatments. Mater. Sci. Eng. C 2017, 70, 870–888. [Google Scholar] [CrossRef] [Green Version]
- Gu, X.; Li, S.; Li, X.; Fan, Y. Frontiers of Mater. Sci. China 2010, 4, 111–115. [Google Scholar]
- Gu, X.; Zheng, Y.; Cheng, Y.; Zhong, S.; Xi, T. In vitro corrosion and biocompatibility of binary magnesium alloys. Biomaterials 2009, 30, 484–498. [Google Scholar] [CrossRef]
- Gupta, M.; Wong, W. Magnesium-based nanocomposites: Lightweight materials of the future. Mater. Charact. 2015, 105, 30–46. [Google Scholar] [CrossRef]
- Sripathy, A.P.; Handjaja, C.; Manakari, V.; Parande, G.; Gupta, M. Development of Lightweight Magnesium/Glass Micro Balloon Syntactic Foams Using Microwave Approach with Superior Thermal and Mechanical Properties. Metals 2021, 11, 827. [Google Scholar] [CrossRef]
- Prasadh, S.; Manakari, V.; Parande, G.; Wong, R.C.W.; Gupta, M. Hollow silica reinforced magnesium nanocomposites with enhanced mechanical and biological properties with computational modeling analysis for mandibular reconstruction. Int. J. Oral Sci. 2020, 12, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Chen, L.; Tang, J.; Li, Z.; Zhao, G.; Zhang, C. Investigation on corrosion behavior and mechanical properties of an extruded Mg-Zn-Al-Sn-Mn alloy. Mater. Charact. 2021, 180, 111439. [Google Scholar] [CrossRef]
- Duley, P.; Bairagi, D.; Bairi, L.; Bandyopadhyay, T.; Mandal, S. Effect of microstructural evolution and texture change on the in-vitro bio-corrosion behaviour of hard-plate hot forged Mg-4Zn-0.5Ca-0.16Mn (wt%) alloy. Corros. Sci. 2021, 192, 109860. [Google Scholar] [CrossRef]
- Prasadh, S.; Manakari, V.; Parande, G.; Srivatsan, T.S.; Wong, R.; Gupta, M. Bioresorbable Nano-Hydroxyapatite Reinforced Magnesium Alloplastic Bone Substitute for Biomedical Applications: A Study. In Nanocomposites VI: Nanoscience and Nanotechnology in Advanced Composites; Springer: New York, NY, USA, 2019; pp. 71–82. [Google Scholar]
- Prasadh, S.; Ratheesh, V.; Manakari, V.; Parande, G.; Gupta, M.; Wong, R. The Potential of Magnesium Based Materials in Mandibular Reconstruction. Metals 2019, 9, 302. [Google Scholar] [CrossRef] [Green Version]
- Beck, G.R., Jr.; Ha, S.-W.; Camalier, C.E.; Yamaguchi, M.; Li, Y.; Lee, J.-K.; Weitzmann, M.N. Bioactive silica-based nanoparticles stimulate bone-forming osteoblasts, suppress bone-resorbing osteoclasts, and enhance bone mineral density in vivo. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 793–803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, W.F.; Chiu, K.Y.; Cheng, F.T. Effect of pH on the in vitro corrosion rate of magnesium degradable implant material. Mater. Sci. Eng. C 2010, 30, 898–903. [Google Scholar] [CrossRef]
- Agha, N.A.; Willumeit-Römer, R.; Laipple, D.; Luthringer, B.; Feyerabend, F. The Degradation Interface of Magnesium Based Alloys in Direct Contact with Human Primary Osteoblast Cells. PLoS ONE 2016, 11, e0157874. [Google Scholar] [CrossRef]
- Badawy, W.A.; Hilal, N.H.; El-Rabiee, M.; Nady, H. Electrochemical behavior of Mg and some Mg alloys in aqueous solutions of different pH. Electrochim. Acta 2010, 55, 1880–1887. [Google Scholar] [CrossRef]
- Yang, C.; Yuan, G.; Zhang, J.; Tang, Z.; Zhang, X.; Dai, K. Effects of magnesium alloys extracts on adult human bone marrow-derived stromal cell viability and osteogenic differentiation. Biomed. Mater. 2010, 5, 045005. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Atrens, A. Understanding Magnesium Corrosion—A Framework for Improved Alloy Performance. Adv. Eng. Mater. 2003, 5, 837–858. [Google Scholar] [CrossRef]
- Kujur, M.S.; Mallick, A.; Manakari, V.; Parande, G.; Tun, K.S.; Gupta, M. Significantly Enhancing the Ignition/Compression/Damping Response of Monolithic Magnesium by Addition of Sm2O3 Nanoparticles. Metals 2017, 7, 357. [Google Scholar] [CrossRef] [Green Version]
- Kujur, M.S.; Manakari, V.; Parande, G.; Tun, K.S.; Mallick, A.; Gupta, M. Enhancement of thermal, mechanical, ignition and damping response of magnesium using nano-ceria particles. Ceram. Int. 2018, 44, 15035–15043. [Google Scholar] [CrossRef]
- Zhao, T.; Hu, Y.; He, B.; Zhang, C.; Zheng, T.; Pan, F. Effect of manganese on microstructure and properties of Mg-2Gd magnesium alloy. Mater. Sci. Eng. A 2019, 765, 138292. [Google Scholar] [CrossRef]
- Han, Y.Y.; You, C.; Zhao, Y.; Chen, M.F.; Wang, L. Effect of Mn Element Addition on the Microstructure, Mechanical Properties, and Corrosion Properties of Mg-3Zn-0.2Ca Alloy. Front. Mater. 2019, 6. [Google Scholar] [CrossRef]
- Zheng, H.R.; Li, Z.; You, C.; Liu, D.B.; Chen, M.F. Effects of MgO modified β-TCP nanoparticles on the microstructure and properties of β-TCP/Mg-Zn-Zr composites. Bioact. Mater. 2017, 2, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Chen, L.; Li, Z.; Zhao, G.; Zhang, C.; Lin, J.; Zuo, Y. Microstructure characterization and corrosion behavior of hollow ZK60 Mg profile containing longitudinal welds. Corros. Sci. 2021, 193, 109875. [Google Scholar] [CrossRef]
- Dunne, C.F.; Levy, G.K.; Hakimi, O.; Aghion, E.; Twomey, B.; Stanton, K.T. Corrosion behaviour of biodegradable magnesium alloys with hydroxyapatite coatings. Surf. Coat. Technol. 2016, 289, 37–44. [Google Scholar] [CrossRef]
- Seitz, J.-M.; Durisin, M.; Goldman, J.; Drelich, J.W. Recent Advances in Biodegradable Metals for Medical Sutures: A Critical Review. Adv. Heal. Mater. 2015, 4, 1915–1936. [Google Scholar] [CrossRef] [PubMed]
- Torbati-Sarraf, H.; Torbati-Sarraf, S.A.; Poursaee, A.; Langdon, T.G. Electrochemical behavior of a magnesium ZK60 alloy processed by high-pressure torsion. Corros. Sci. 2019, 154, 90–100. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Yang, H.; Li, X.; Zheng, Y. In Vitro Evaluation of the Feasibility of Commercial Zn Alloys as Biodegradable Metals. J. Mater. Sci. Technol. 2016, 32, 909–918. [Google Scholar] [CrossRef]
- Kubasek, J.; Vojtěch, D. Zn-based alloys as an alternative biodegradable materials. Proc. Metal 2012, 5, 23–25. [Google Scholar]
- Kubásek, J.; Vojtěch, D.; Jablonská, E.; Pospíšilová, I.; Lipov, J.; Ruml, T. Structure, mechanical characteristics and in vitro degradation, cytotoxicity, genotoxicity and mutagenicity of novel biodegradable Zn–Mg alloys. Mater. Sci. Eng. C 2016, 58, 24–35. [Google Scholar] [CrossRef]
- Törne, K.; Larsson, M.; Norlin, A.; Weissenrieder, J. Degradation of zinc in saline solutions, plasma, and whole blood. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 104, 1141–1151. [Google Scholar] [CrossRef]
- Bolz, A.; Popp, T. Implantable, Bioresorbable Vessel Wall Support, in Particular Coronary Stent. U.S. Patent 6,287,332, 11 September 2001. [Google Scholar]
- Plum, L.M.; Rink, L.; Haase, H. The Essential Toxin: Impact of Zinc on Human Health. Int. J. Environ. Res. Public Health 2010, 7, 1342–1365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, G.; Gongqi, Q.; Gong, H. Kind of Absorbable High Strength and Toughness Corrosion-Resistant Zinc Alloy Implant Material for Human Body. U.S. Patent App. 15/102,355, 2 February 2017. [Google Scholar]
- Bornapour, M.; Muja, N.; Shum-Tim, D.; Cerruti, M.; Pekguleryuz, M. Biocompatibility and biodegradability of Mg–Sr alloys: The formation of Sr-substituted hydroxyapatite. Acta Biomater. 2013, 9, 5319–5330. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Ling, S.N.M. Magnesium, Magnesium Alloys, and Magnesium Composites; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Prasadh, S.; Suresh, S.; Ratheesh, V.; Wong, R.; Gupta, M. Biocompatibility of Metal Matrix Composites Used for Biomedical Applications. Encycl. Mater. Compos. 2020, 1, 474–501. [Google Scholar]
- Anvari-Yazdi, A.F.; Tahermanesh, K.; Hadavi, S.M.M.; Talaei-Khozani, T.; Razmkhah, M.; Abed, S.M.; Mohtasebi, M.S. Cytotoxicity assessment of adipose-derived mesenchymal stem cells on synthesized biodegradable Mg-Zn-Ca alloys. Mater. Sci. Eng. C 2016, 69, 584–597. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Yan, Z.; Bi, S.; Ma, Z.; Qian, J. Investigation of heat treatment and subsequently surface modification by nano-TiO2 on Mg–Zn–Ca–Mn bio-magnesium alloy. Mater. Express 2019, 9, 931–939. [Google Scholar] [CrossRef]







| Raw Material | Size (Morphology) Purity | Supplier |
|---|---|---|
| Mg | Turnings | ACROS Organics, USA |
| >99.9% | ||
| ~149 µm | ||
| Zn | (Spherical) | Alfa Aesar, USA |
| 99.9% | ||
| Ca | granules | |
| 99.9% | Alfa Aesar, USA | |
| ~100 µm | ||
| Mn | (Spherical) | Alfa Aesar, USA |
| 99.9% |
| Material | Mg | Zn | Ca | Mn |
|---|---|---|---|---|
| Mg–2Zn–1Ca | Bal. | 1.89 | 0.63 | 0 |
| Mg–2Zn–1Ca/0.3Mn | Bal. | 1.88 | 0.83 | 0.15 |
| Mg–2Zn–1Ca/0.5Mn | Bal. | 1.98 | 0.94 | 0.49 |
| Mg–2Zn–1Ca/0.7Mn | Bal. | 1.92 | 0.99 | 0.66 |
| Immersion Time (Days) | Corrosion Rate (mm. Year−1) | |||
|---|---|---|---|---|
| Mg–2Zn–1Ca | Mg–2Zn–1Ca/0.3Mn | Mg–2Zn–1Ca/0.5Mn | Mg–2Zn–1Ca/0.7Mn | |
| Day 1 | 0.19 | 0.43 | 0.07 | 0.21 |
| Day 2 | 0.26 | 0.32 | 0.16 | 0.26 |
| Day 3 | 0.27 | 0.36 | 0.13 | 0.31 |
| Day 7 | 0.33 | 0.42 | 0.09 | 0.50 |
| Day 14 | 0.32 | 1.10 | 0.19 | 1.56 |
| Day 21 | 0.89 | 1.58 | 0.22 | 1.79 |
| Day 28 | 0.87 | 1.82 | 0.25 | 2.15 |
| Materials | Mg–2Zn–1Ca | Mg–2Zn–1Ca /0.3Mn | Mg–2Zn–1Ca /0.5Mn | Mg–2Zn–1Ca /0.7Mn |
| Grain Size (µm) | 23 ± 7 | 18 ± 8 (↓ 22%) | 16 ± 4 (↓ 30%) | 18 ± 7 (↓ 22%) |
| Material | 0.2CYS (MPa) | UCS (MPa) | Fracture Strain (%) |
|---|---|---|---|
| Mg–2Zn–1Ca | 148 | 481 | 18.8 |
| Mg–2Zn–1Ca | 111± 11 | 302 | 10 ± 2 |
| (Day 14) | (↓ 25%) | (↓ 37%) | (↓ 44%) |
| Mg–2Zn–1Ca/0.3Mn | 226 | 568 | 11.3 |
| Mg–2Zn–1Ca/0.3Mn | 155 ± 10 | 301 ± 14 | 13 ± 1 |
| (Day 14) | (↓ 31%) | (↓ 47%) | (↑ 15%) |
| Mg–2Zn–1Ca-/0.5Mn | 214 | 645 | 13.4 |
| Mg–2Zn–1Ca/0.5Mn | 211 ± 16 | 352 ± 12 | 11 ± 3 |
| (Day 14) | (↓ 1%) | (↓ 45%) | (↓ 15%) |
| Mg–2Zn–1Ca/0.7Mn | 249 | 565 | 13.2 |
| Mg–2Zn–1Ca/0.7Mn | 85 ± 12 | 144 ± 9 | 9 ± 1 |
| (Day 14) | (↓ 66%) | (↓ 74%) | (↓ 30%) |
| Natural bone | 130–180 | - | - |
| Cortical bone | - | 131–224 | 2–12 |
| Stainless steel | - | 170–310 | - |
| Titanium | - | 758–1117 | 29–49 |
| alloy | |||
| Co-Cr alloy | - | 450–1000 | - |
| AZ91D | 130 | 300 | 12.4 |
| AM50 | 110 | 312 | 11.5 |
| WE43 | 261 | 420 | 16.3 |
| Alloys | Solution | Corrosion Rate (mm/y) |
|---|---|---|
| Mg–2Zn–1Ca/0.5Mn (This work) | Hank’s | 0.25 |
| Mg-4Zn-0.5Ca-0.75Mn | Hank’s | 0.12 |
| ZK60 | Hank’s | 0.32 |
| MAO-coated ZK60 | Hank’s | 0.003 |
| Mg-2Zn-Ca-0.5Mn | Hank’s | 1.58 |
| Mg-2Zn-Ca-0.5Mn-1.3Ce | Hank’s | 1.34 |
| Mg–2Ca–0.5Mn–2Zn | SBF | 1.78 |
| Mg–2Ca–0.5Mn–4Zn | SBF | 2.21 |
| Mg–2Ca–0.5Mn–7Zn | SBF | 3.98 |
| Mg-Zn-1.5-Ca-1.1Mn | Hank’s | 1.40 |
| 6h-coated Mg-2Zn-Ca-0.5Mn-1.3Ce | Hank’s | 1.29 |
| Mg-Zn-Ca BMGC | SBF | 0.26 |
| Annealed Mg-Zn-Ca | SBF | 1.20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prasadh, S.; Parande, G.; Gupta, M.; Wong, R. Compositional Tailoring of Mg–2Zn–1Ca Alloy Using Manganese to Enhance Compression Response and In-Vitro Degradation. Materials 2022, 15, 810. https://doi.org/10.3390/ma15030810
Prasadh S, Parande G, Gupta M, Wong R. Compositional Tailoring of Mg–2Zn–1Ca Alloy Using Manganese to Enhance Compression Response and In-Vitro Degradation. Materials. 2022; 15(3):810. https://doi.org/10.3390/ma15030810
Chicago/Turabian StylePrasadh, Somasundaram, Gururaj Parande, Manoj Gupta, and Raymond Wong. 2022. "Compositional Tailoring of Mg–2Zn–1Ca Alloy Using Manganese to Enhance Compression Response and In-Vitro Degradation" Materials 15, no. 3: 810. https://doi.org/10.3390/ma15030810
APA StylePrasadh, S., Parande, G., Gupta, M., & Wong, R. (2022). Compositional Tailoring of Mg–2Zn–1Ca Alloy Using Manganese to Enhance Compression Response and In-Vitro Degradation. Materials, 15(3), 810. https://doi.org/10.3390/ma15030810









