Effects of the PbBr2:PbI2 Molar Ratio on the Formation of Lead Halide Thin Films, and the Ratio’s Application for High Performance and Wide Bandgap Solar Cells
Abstract
:1. Introduction
2. Experimental Setup and Methods
3. Results and Discussion
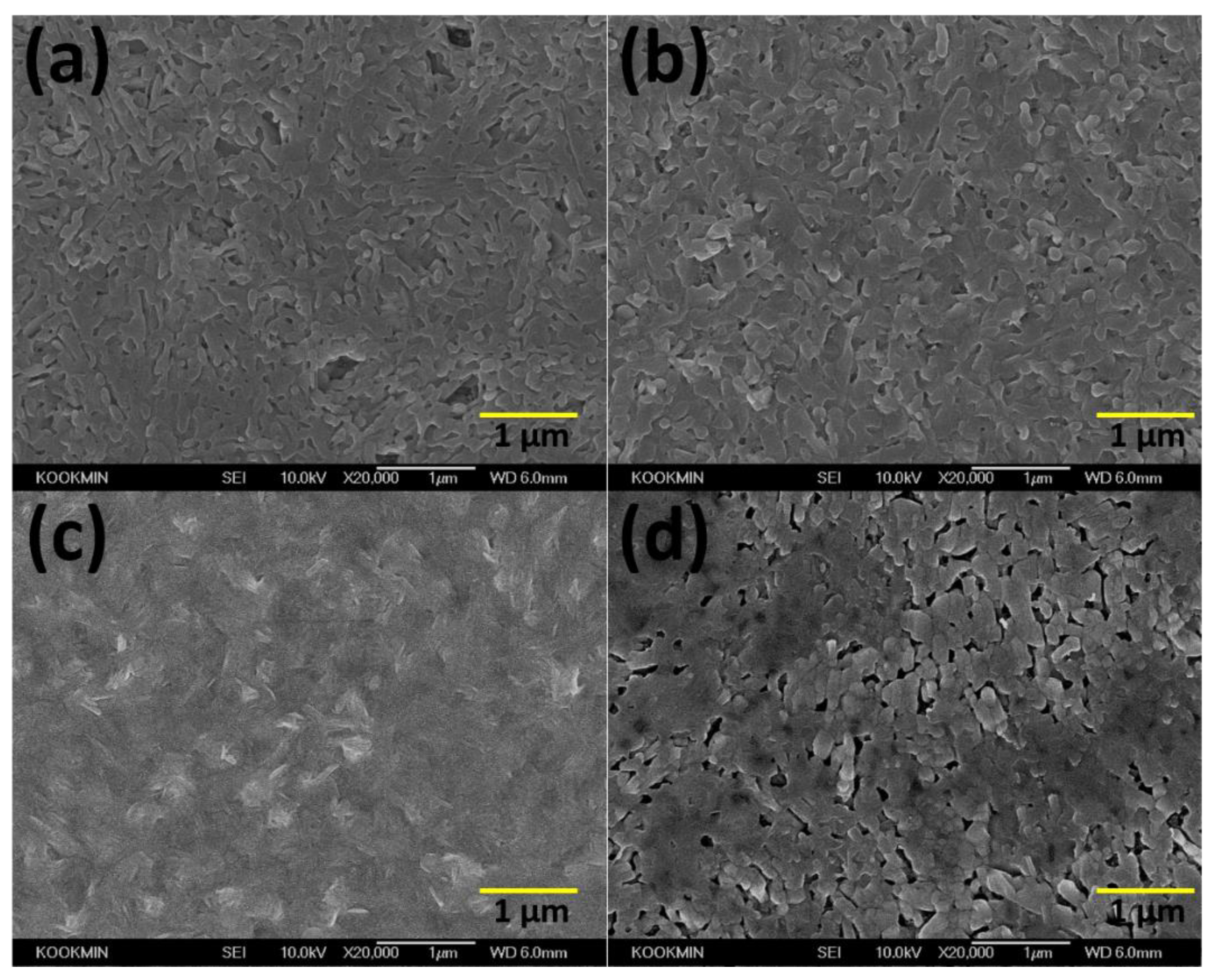
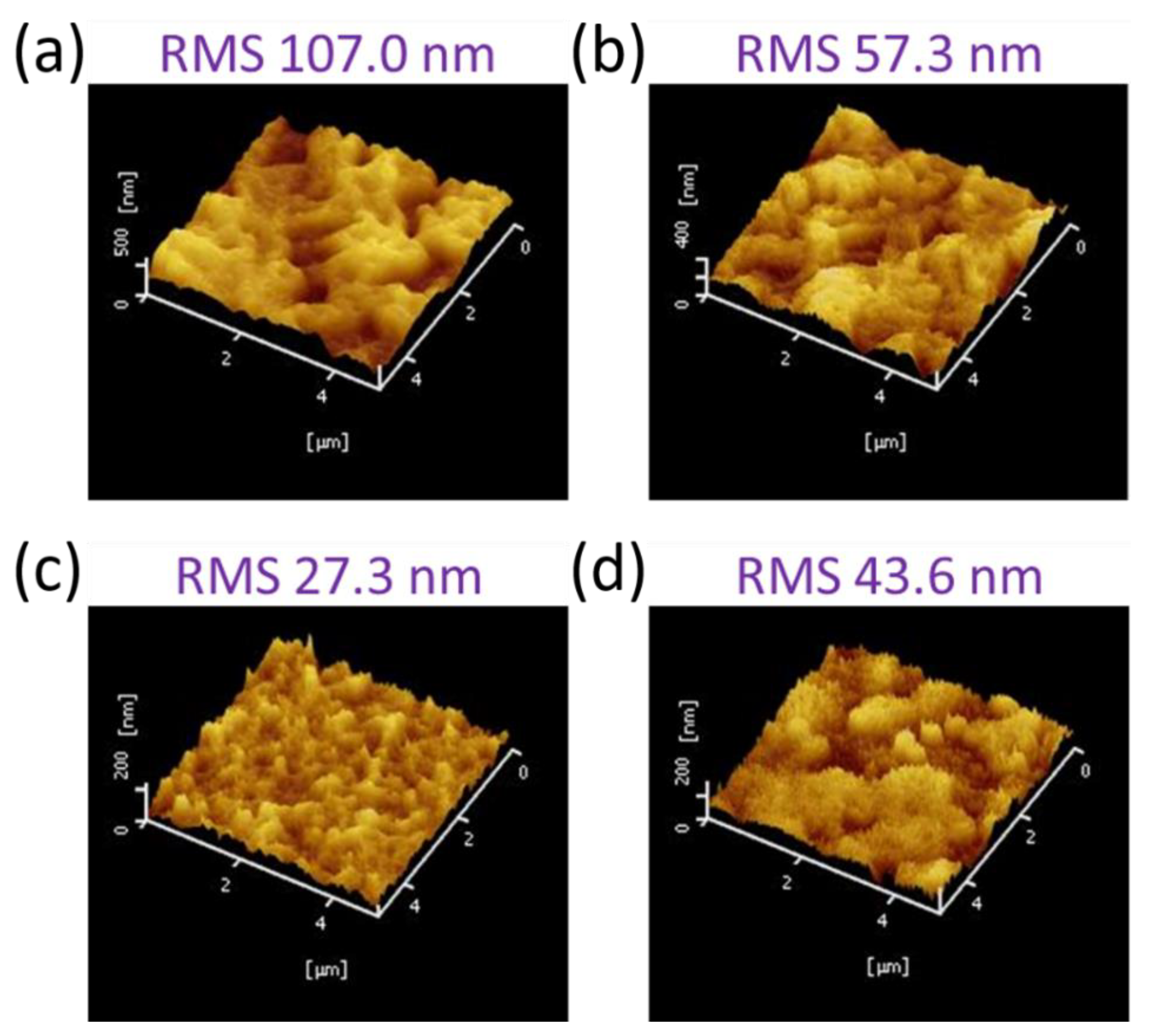
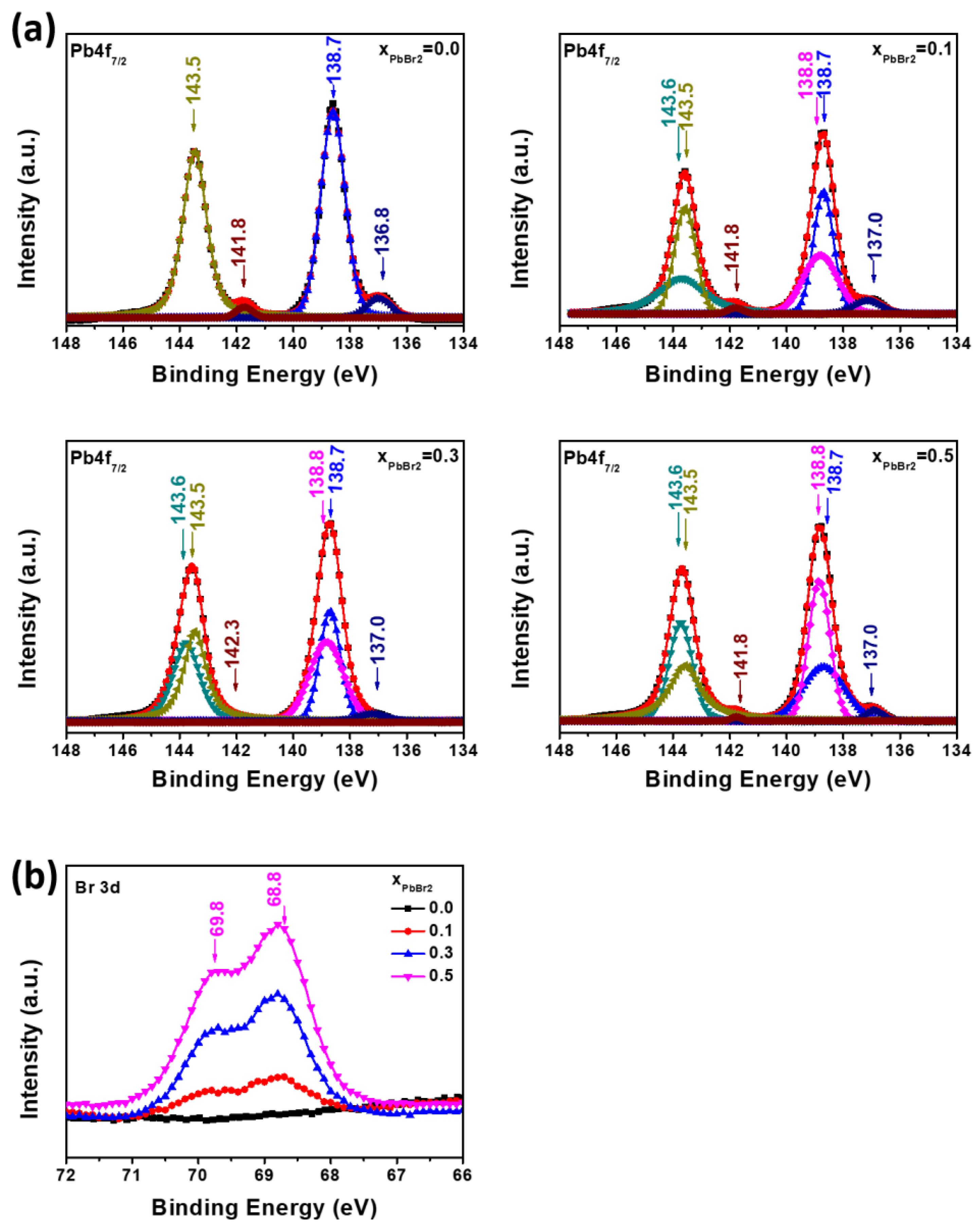
3.1. Formation of Mixed Lead Halides PbI2(1−x)Br2x at Various Molar Ratio(x) of PbBr2
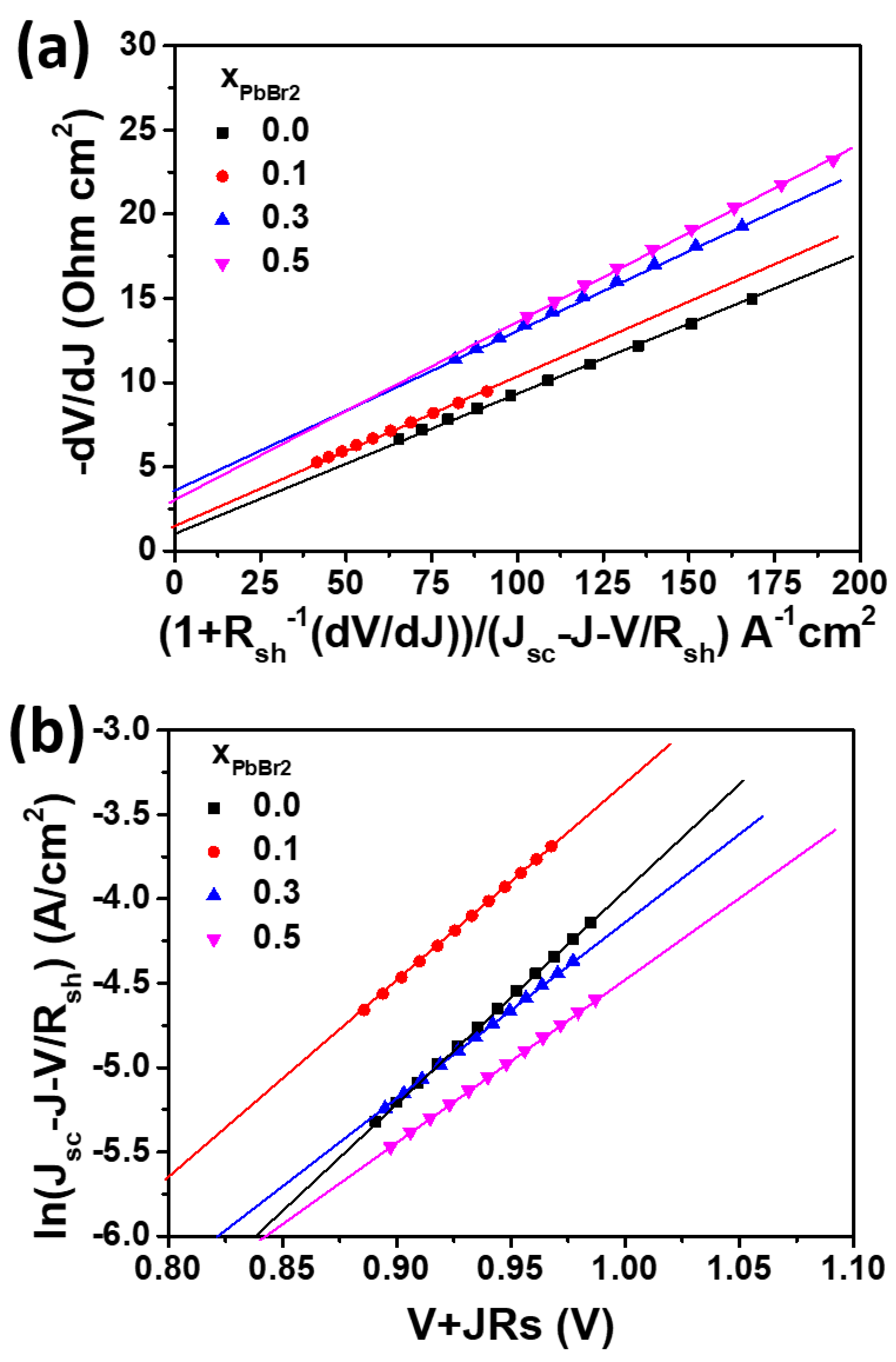
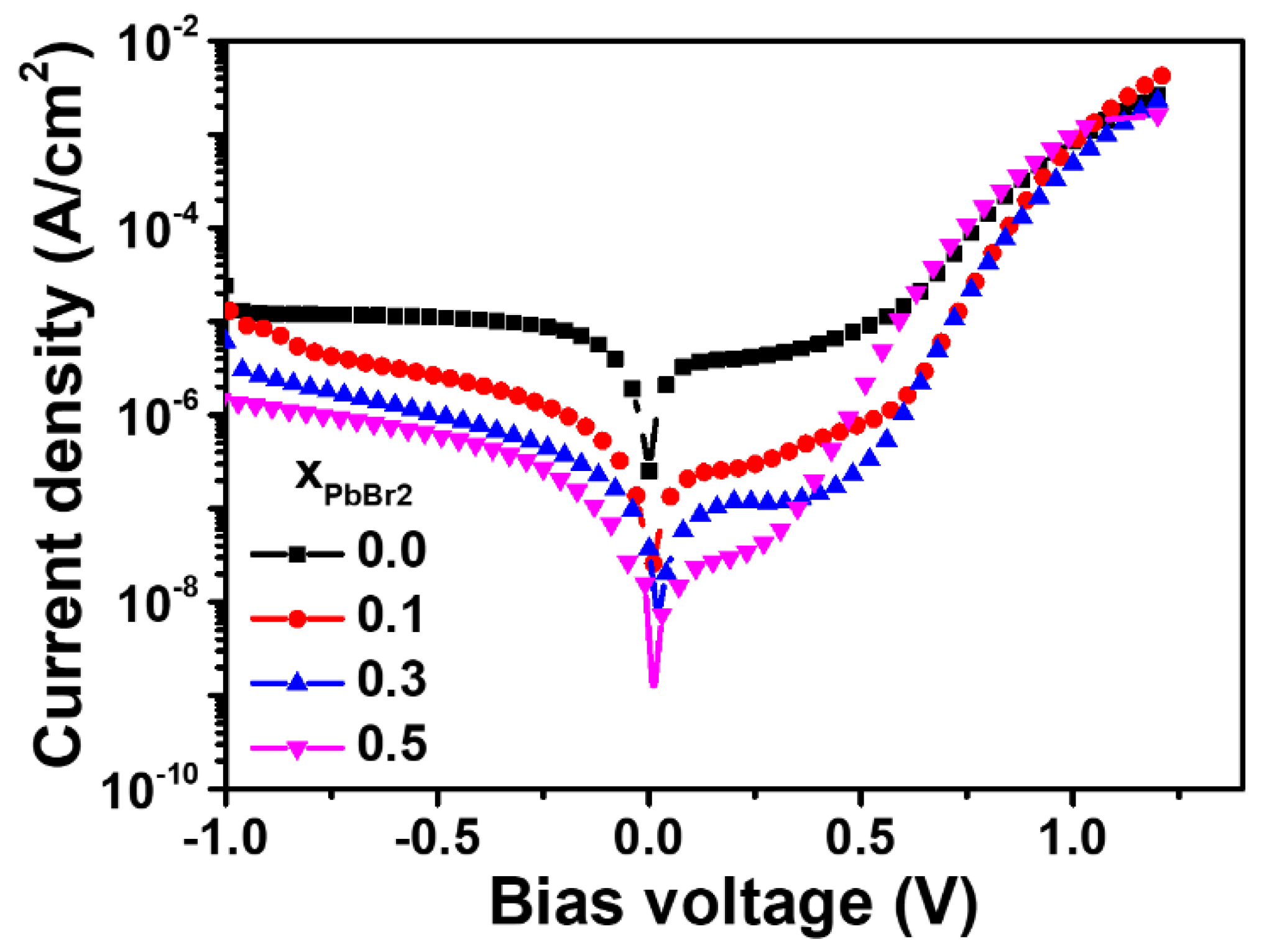
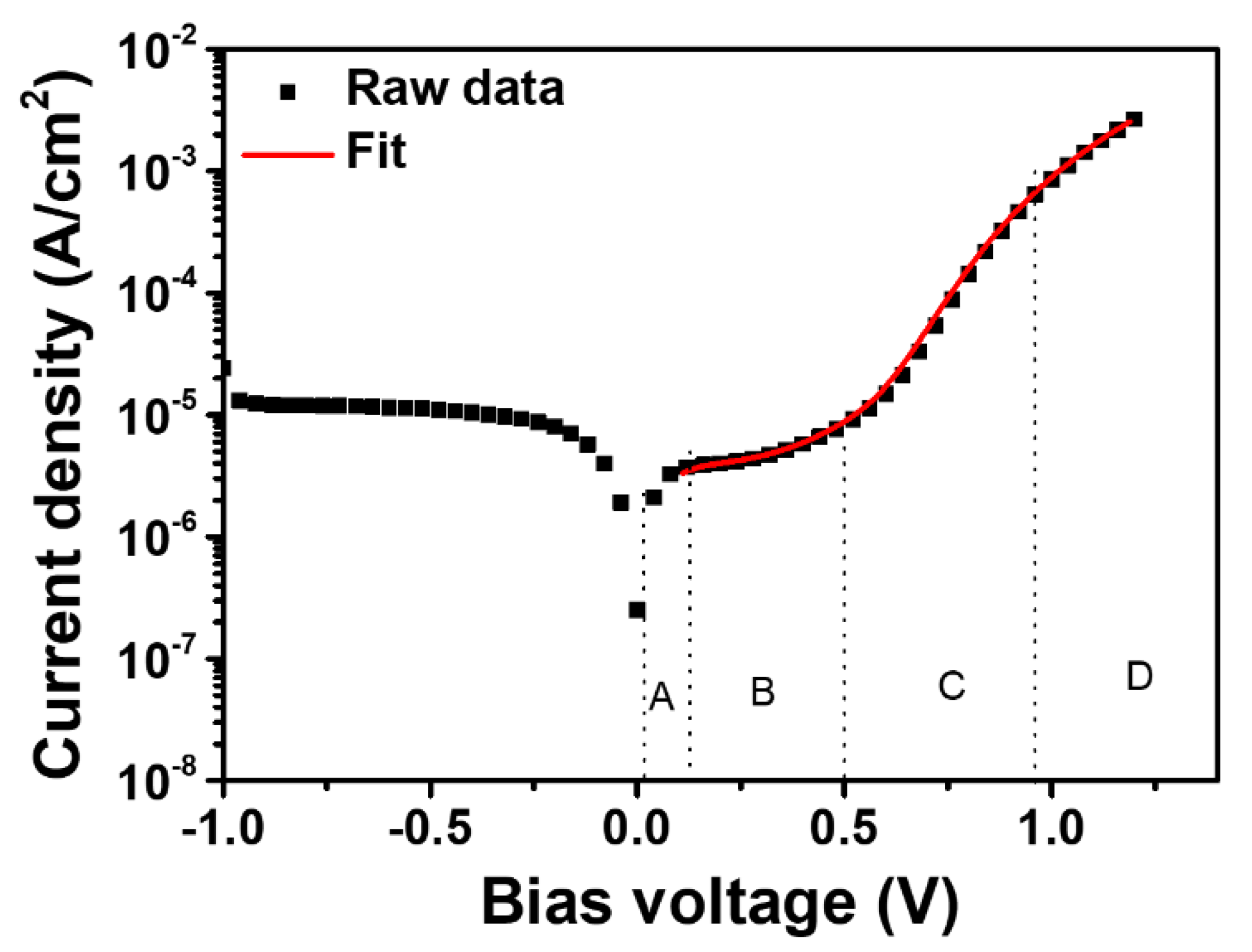
3.2. Photovoltaic Performance and Long-Term Stability of Lead Halide Solar Cell
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kong, W.; Li, G.; Liang, Q.; Ji, X.; Li, G.; Ji, T.; Che, T.; Hao, Y.; Cui, Y. Controllable deposition of regular lead iodide nanoplatelets and their photoluminescence at room temperature. Phys. E Low-Dimens. Syst. Nanostruct. 2018, 97, 130–135. [Google Scholar] [CrossRef]
- Bennett, P.R.; Shah, K.S.; Dmitriev, Y.; Klugerman, M.; Gupta, T.; Squillante, M.; Street, R.; Partain, L.; Zentai, G.; Pavyluchova, R. Polycrystalline lead iodide films for digital X-ray sensors. Nucl. Instrum. Methods Phys. Res. A 2003, 505, 269–272. [Google Scholar] [CrossRef]
- Mousa, A.M. Optical and photoconduction in PbI2 polycrystalline deposited from solution. Int. J. Mod. Phys. B 2007, 21, 3745–3753. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, Y.-Y.; Zhang, S.; Lu, T.-M.; Shi, J. Band gap engineering of a soft inorganic compound PbI2 by incommensurate van der Waals epitaxy. Appl. Phys. Lett. 2016, 108, 013105. [Google Scholar] [CrossRef] [Green Version]
- Malevu, T.D.; Ocaya, R.O.; Tshabalala, K.G. Phase transformations of high-purity PbI2 nanoparticles synthesized from lead-acid accumulator anodes. Phys. B 2016, 496, 69–73. [Google Scholar] [CrossRef]
- Dmitriev, Y.; Bennett, P.R.; Cirignano, L.J.; Klugerman, M.; Shah, K.S. Physical modeling of the electrical properties of PbI2 films. Nucl. Instrum. Methods Phys. Res. A 2008, 592, 334–345. [Google Scholar] [CrossRef]
- Shi, Z.; Jayatissa, A.H. Perovskites-Based Solar Cells: A Review of Recent Progress. Mater. Process. Methods Mater. 2018, 11, 729. [Google Scholar]
- Zhang, J.; Huang, Y.; Tan, Z.; Li, T.; Zhang, Y.; Jia, K.; Lin, L.; Sun, L.; Chen, L.Z.; Tan, C.; et al. Low-temperature heteroepitaxy of 2D PbI2/graphene for large-area flexible photodetectors. Adv. Mater. 2018, 30, 1803194. [Google Scholar] [CrossRef]
- Ahmad, S.; Prakash, G.V. Two-step fabrication of R-PbI4(1−y)Br4y type light emitting inorganic-organic hybrid photonic structures. Opt. Mater. Express 2014, 4, 101–110. [Google Scholar] [CrossRef]
- Zhong, M.; Huang, L.; Deng, H.-X.; Wang, X.; Li, B.; Wei, Z.; Li, J. Flexible photodetectors based on phase dependent PbI2 single crystals. J. Mater. Chem. C 2016, 4, 6492–6499. [Google Scholar] [CrossRef]
- Zhang, J.; Song, T.; Zhang, Z.; Ding, K.; Huang, F.; Sun, B. Layered ultrathin PbI2 single crystals for high sensitivity flexible photodetectors. J. Mater. Chem. C 2015, 3, 4402–4406. [Google Scholar] [CrossRef]
- Sun, H.; Zhao, B.; Yang, D.; Wangyang, P.; Gao, X.; Zhu, X. Flexible X-ray detector based on sliced lead iodide crystal. Phys. Status Solidi RRL 2017, 11, 1600397. [Google Scholar] [CrossRef]
- Liu, X.; Ha, S.T.; Zhang, Q.; de Mata, M.L.; Magen, C.; Arbiol, J.; Sum, T.C.; Xiong, Q. Whispering gallery mode lasing from hexagonal shaped layered lead iodide crystals. ACS Nano 2015, 9, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Kinpara, M.; Wang, J.F.; Mimura, K.; Issiki, M. Growth of PbI2 single crystals from stoichiometric and Pb excess melts. J. Cryst. Growth 2008, 310, 47–50. [Google Scholar] [CrossRef]
- Zhu, X.H.; Wei, Z.R.; Jin, Y.R.; Xiang, A.P. Growth and characterization of a PbI2 single crystal used for gamma ray detectors. Cryst. Res. Technol. 2007, 42, 456–459. [Google Scholar] [CrossRef]
- Shah, K.S.; Olschner, F.; Moy, L.P.; Bennett, P.; Misra, M.; Zhang, J.; Squillante, M.R.; Lund, J.C. Lead iodide X-ray detection systems. Nucl. Instrum. Methods Phys. Res. A 1996, 380, 266–270. [Google Scholar] [CrossRef]
- Matuchova, M.; Zdansky, K.; Zavadil, J.; Danilewsky, A.; Maixner, J.; Alexiev, D. Electrical, optical and structural properties of lead iodide. J. Mater. Sci. Mater. Electron. 2009, 20, 289–294. [Google Scholar] [CrossRef]
- Sun, H.; Zhu, X.; Yang, D.; Yang, J.; Gao, X.; Li, X. Electrical characteristics of lead iodide crystal devices on flexible substrate under mechanical tensile strain. Phys. Status Solidi A 2014, 211, 823–827. [Google Scholar] [CrossRef]
- Tiwary, D.; Fermin, D.J. Textured PbI2 photocathodes obtained by gas phase anion replacement. Electrochim. Acta 2017, 254, 223–229. [Google Scholar] [CrossRef]
- Dmitriev, Y.; Bennett, P.R.; Cirignano, L.J.; Klugerman, M.; Shah, K.S. PbI2 thick films: Growth, properties, and problems. Nucl. Instrum. Methods Phys. Res. A 2008, 584, 165–173. [Google Scholar] [CrossRef]
- Schieber, M.; Zamoshchik, N.; Khakhan, O.; Zuck, A. Structural changes during vapor-phase deposition of polycrystalline-PbI2 films. J. Cryst. Growth 2006, 310, 3168–3173. [Google Scholar] [CrossRef]
- Condeles, J.F.; Lofrano, R.C.Z.; Rosolen, J.M.; Mulato, M. Stoichiometry, surface and structural characterization of lead iodide thin films. Braz. J. Phys. 2006, 36, 320–323. [Google Scholar] [CrossRef]
- Chaudhuri, T.K.; Acharya, H.N. Preparation of lead iodide films by iodination of chemically deposited lead sulphide films. Mat. Res. Bull. 1982, 17, 279–286. [Google Scholar] [CrossRef]
- Condeles, J.F.; Ando, R.A.; Mulato, M. Optical and structural properties of PbI2 thin films. J. Mater. Sci. 2008, 43, 525–529. [Google Scholar] [CrossRef]
- Sheikh, M.A.K.; Abdur, R.; Singh, S.; Kim, J.-H.; Min, K.-S.; Kim, J.; Lee, J. Effects of chlorine contents on perovskite solar cell structure formed on CdS electron transport layer probed by Rutherford Backscattering. Electron. Mater. Lett. 2018, 14, 700–711. [Google Scholar] [CrossRef]
- Gil-Edcrig, L.; Dreessen, C.; Palazon, F.; Hawash, Z.; Moons, E.; Albrecht, S.; Sessolo, M.; Bolink, H.J. Efficient wide-bandgap mixed-cation and mixed-halide perovskite solar cells by vacuum deposition. ACS Energy Lett. 2021, 6, 827–836. [Google Scholar] [CrossRef]
- Choe, H.; Jeon, D.; Lee, S.J.; Cho, J. Mixed or segregated: Toward efficient and stable mixed halide perovskite—Based devices. ACS Omega 2021, 6, 24304–24315. [Google Scholar] [CrossRef]
- Hamed, M.S.G.; Mola, G.T. Mixed halide perovskite solar cells: Progress and challenges. Crit. Rev. Solid State Mater. Sci. 2020, 45, 85–112. [Google Scholar] [CrossRef]
- Karlsson, M.; Yi, Z.; Reichert, S.; Luo, X.; Lin, W.; Zhang, Z.; Bao, C.; Zhang, R.; Bai, S.; Zheng, G.; et al. Mixed halide perovskites for spectrally stable and high-efficiency blue light-emitting diodes. Nat. Commun. 2021, 12, 361. [Google Scholar] [CrossRef]
- Palosz, B. Reasons for polytypism of crystals of the type MX2 II. Classification of faults and structural series of polytypes; conditions of polytypic growth of CdI2, PbI2, CdBr2, SnS2, SnSe2 and Ti1.2S2 2. Phys. Stat. Sol. A 1983, 80, 11–41. [Google Scholar] [CrossRef]
- Chaudhary, S.K.; Kaur, H. Impurity induced structural phase transformations in melt grown single crystals of lead iodide. Cryst. Res. Technol. 2011, 46, 1235–1240. [Google Scholar] [CrossRef]
- Levkovets, S.I.; Khyzhun, O.Y.; Myronchuk, G.L.; Fochuk, P.M.; Piasecki, M.; Kityk, I.V.; Fedorchuk, A.O.; Levkovets, V.I.; Parasyuk, L.V. Synthesis, electronic structure and optical properties of PbBr1.2I0.8. J. Electron Spectrosc. Relat. Phenom. 2017, 218, 13–20. [Google Scholar] [CrossRef]
- Moulder, J.; Stickle, W.; Sobol, P.; Bomben, K. Handbook of X-ray Photoelectron Spectroscopy; Physical Electronics Division: Eden Prairie, MN, USA, 1995; pp. 1–261. [Google Scholar]
- Roy, A.; Sarma, D.D.; Sood, A.K. Spectroscopic studies on quantum dots of PbI2. Spectrochim. Acta Part A 1992, 48, 1779–1787. [Google Scholar]
- Mayerhöfer, T.G.; Pipa, A.V.; Popp, J. Beer’s law-why integrated absorbance depends linearly on concentration. ChemPhysChem 2019, 20, 2748. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhu, H.; Wei, J.; Wang, K.; Xu, E.; Li, Z.; Wu, D. Determination of band gaps of self-assembled carbon nanotube films using Tauc/Davis–Mott model. Appl. Phys. A 2009, 97, 341–344. [Google Scholar] [CrossRef]
- Salje, E.; Palosz, B.; Wruck, B. In situ observation of the polytypic phase transition 2H-12R in PbI2: Investigations of the thermodynamic, structural and dielectric properties. J. Phys. C Solid State Phys. 1987, 20, 4077–4096. [Google Scholar] [CrossRef]
- Chaudhary, S.K. Lead iodide crystals as input material for radiation detectors. Cryst. Struct. Theory Appl. 2012, 1, 21–24. [Google Scholar] [CrossRef] [Green Version]
- Ichikawa, R.; Ikeda, T. Effects of cooling rate and supercooling degree on solidified structures of Al-Mn, Al-Cr and Al-Zr alloys in rapid solidification. Trans. Jap. Inst. Met. 1971, 12, 280–284. [Google Scholar] [CrossRef] [Green Version]
- Kim, B.J.; Kim, D.H.; Kwon, S.L.; Park, S.Y.; Li, Z.; Zhu, K.; Jung, H.S. Selective dissolution of halide perovskites as a step towards recycling solar cells. Nat. Commun. 2016, 7, 11735. [Google Scholar] [CrossRef]
- Wakamiya, A.; Endo, M.; Sasamori, T.; Tokitoh, N.; Ogomi, Y.; Hayase, S.; Murata, Y. Reproducible fabrication of efficient perovskite-based solar cells: X-ray crystallographic studies on the formation of CH3NH3PbI3 layers. Chem. Lett. 2014, 43, 711–713. [Google Scholar] [CrossRef] [Green Version]
- Arain, Z.; Liu, C.; Yang, Y.; Mateen, M.; Ren, Y.; Ding, Y.; Liu, X.; Ali, Z.; Kumar, M.; Dai, S. Elucidating the dynamics of solvent engineering for perovskite solar cells. Sci. China Mater. 2019, 62, 161–172. [Google Scholar] [CrossRef] [Green Version]
- Petrov, A.A.; Sokolova, I.P.; Belich, N.A.; Peters, G.S.; Dorovatovskii, P.V.; Zubavichus, Y.V.; Khrustalev, V.N.; Petrov, A.V.; Grätzel, M.; Goodilin, E.A.; et al. Crystal structure of dmf-intermediate phases uncovers the link between CH3NH3PbI3 morphology and precursor stoichiometry. J. Phys. Chem. C 2017, 121, 20739–20743. [Google Scholar] [CrossRef]
- Kelso, M.V.; Mahenderkar, N.K.; Chen, Q.; Tubbesing, J.Z.; Switzer, J.A. Spin coating epitaxial films. Science 2019, 364, 166–169. [Google Scholar] [CrossRef]
- Valizadeh, A.R.; Rashid, A.R.K.; Gharavol, M.H.S.; Karimi, E.Z. The Influence of Cooling Rate on the Microstructure and Microsegregation in Al–30Sn Binary Alloy. Metallogr. Microstruct. Anal. 2013, 2, 107–112. [Google Scholar] [CrossRef] [Green Version]
- Moffatt, W.G.; Pearsall, G.W.; Wulff, J. The Structure and Properties of Materials; John Wiley & Sons, Inc.: New York, NY, USA; London, UK; Sydney, Australia, 1964. [Google Scholar]
- Gordon, P. Principles of Phase Diagrams in Materials Systems; McGraw Hill: New York, NY, USA, 1968. [Google Scholar]
- Cao, D.H.; Stoumpos, C.C.; Malliakas, C.D.; Katz, M.J.; Farha, O.K.; Hupp, J.T.; Kanatzidis, M.G. Remnant PbI2, an unforeseen necessity in high-efficiency hybrid perovskite-based solar cells. APL Mater. 2014, 2, 091101. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Bu, S.; Zhang, D.; Peng, R.; Wei, Q.; Ge, Z.; Zhang, J. Schottky/p-n cascade heterojunction constructed by intentional n-Type doping perovskite toward efficient electron layer-free perovskite solar cells. Sol. RRL 2019, 3, 1800274. [Google Scholar] [CrossRef]
- Sites, J.R.; Mauk, P.H. Diode quality factor determination for thin-film solar cells. Sol. Cells 1989, 27, 411–417. [Google Scholar] [CrossRef]
- Shkir, M.; AlFaify, S. Tailoring the structural, morphological, optical and dielectric properties of lead iodide through Nd3+ doping. Sci. Rep. 2017, 7, 16091. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, J.; Guerrero, A.; Rahimnejad, S.; Almora, O.; Zarazua, I.; Marza, E.M.; Bisquert, J.; Belmonte, G.G. Ionic reactivity at contacts and aging of methylammonium lead triiodide perovskite solar cells. Adv. Energy Mater. 2016, 6, 1502246. [Google Scholar] [CrossRef]
- Chen, B.; Yang, M.; Zheng, X.; Wu, C.; Li, W.; Yan, Y.; Bisquert, J.; Belmonte, G.G.; Zhu, K.; Priya, S. Impact of capacitive effect and ion migration on the hysteretic behavior of perovskite solar cells. J. Phys. Chem. Lett. 2015, 6, 4693–4700. [Google Scholar] [CrossRef]
- Almora, O.; Zarazua, I.; Marza, E.M.; Sero, I.M.; Bisquert, J.; Belmonte, G.G. Capacitive dark currents, hysteresis, and electrode polarization in lead halide perovskite solar cells. J. Phys. Chem. Lett. 2015, 6, 1645–1652. [Google Scholar] [CrossRef] [PubMed]
- Almora, O.; Guerrero, A.; Belmonte, G.G. Ionic charging by local imbalance at interfaces in hybrid lead halide perovskites. APL 2016, 108, 043903. [Google Scholar] [CrossRef] [Green Version]
- Mitoff, S.P.; Charles, R.J. Electrode polarization of ionic conductors. J. Appl. Phys. 1972, 43, 927–934. [Google Scholar] [CrossRef]


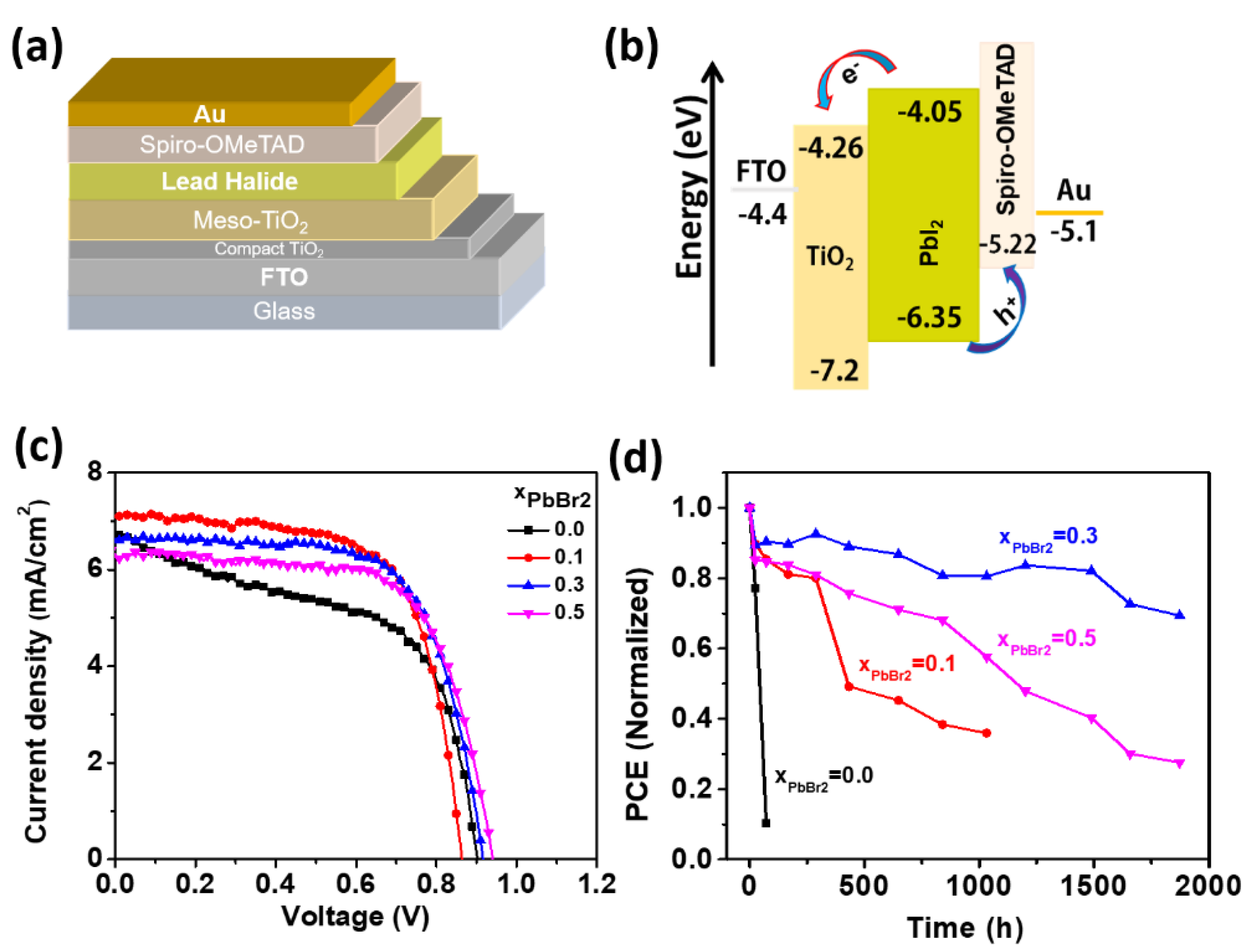

| x | Jsc (mA/cm2) | Voc (V) | FF | PCE (%) |
|---|---|---|---|---|
| 0.0 | 6.74 | 0.90 | 0.55 | 3.36 |
| 0.1 | 7.09 | 0.86 | 0.68 | 4.15 |
| 0.3 | 6.63 | 0.92 | 0.67 | 4.11 |
| 0.5 | 6.31 | 0.94 | 0.67 | 3.97 |
| x = 0.0 | x = 0.1 | x = 0.3 | x = 0.5 | |
|---|---|---|---|---|
| J0 | 7.1 × 10−8 | 2.6 × 10−7 | 4.1 × 10−7 | 6.9 × 10−7 |
| m | 3.1 | 3.2 | 3.6 | 3.9 |
| Rsh | 675 | 4.74 × 104 | 2.75 × 104 | 2.04 × 106 |
| Rs | 1.42 | 1.79 | 3.76 | 3.47 |
| x = 0.0 | x = 0.1 | x = 0.3 | x = 0.5 | |
|---|---|---|---|---|
| J0 (dark) (A/cm2) | 1.9 × 10−9 | 1.0 × 10−11 | 1.0 × 10−11 | 3.7 × 10−10 |
| Jcap (A/cm2) | 2 × 10−6 | 1 × 10−7 | 8 × 10−8 | 2 × 10−8 |
| C (μF/cm2) | 100 | 5 | 4 | 1 |
| Rsh (dark) (Ω cm2) | 2 × 105 | 9 × 105 | 4 × 106 | 5 × 106 |
| Jsh (=V/Rsh) at 0.1 V | 5 × 10−7 | 1.1 × 10−7 | 2.5 × 10−8 | 2 × 10−8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheikh, M.A.K.; Singh, S.; Abdur, R.; Lee, S.-M.; Kim, J.-H.; Nam, H.-S.; Lee, H.; Lee, J. Effects of the PbBr2:PbI2 Molar Ratio on the Formation of Lead Halide Thin Films, and the Ratio’s Application for High Performance and Wide Bandgap Solar Cells. Materials 2022, 15, 837. https://doi.org/10.3390/ma15030837
Sheikh MAK, Singh S, Abdur R, Lee S-M, Kim J-H, Nam H-S, Lee H, Lee J. Effects of the PbBr2:PbI2 Molar Ratio on the Formation of Lead Halide Thin Films, and the Ratio’s Application for High Performance and Wide Bandgap Solar Cells. Materials. 2022; 15(3):837. https://doi.org/10.3390/ma15030837
Chicago/Turabian StyleSheikh, Md. Abdul Kuddus, Son Singh, Rahim Abdur, Sung-Min Lee, Jae-Hun Kim, Ho-Seok Nam, Hyunseung Lee, and Jaegab Lee. 2022. "Effects of the PbBr2:PbI2 Molar Ratio on the Formation of Lead Halide Thin Films, and the Ratio’s Application for High Performance and Wide Bandgap Solar Cells" Materials 15, no. 3: 837. https://doi.org/10.3390/ma15030837







