Silver Nanoparticles (AgNPs) in Urea Solution in Laboratory Tests and Field Experiments with Crops and Vegetables
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fertiliser Containing AgNPs
2.2. Laboratory Tests
2.3. Field Experiments
2.4. Analysis of Data
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Priestly, B.G. Nanotechnology: A promising new technology—But how safe? Med. J. Aust. 2007, 186, 187–188. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.H.; Al-Whaibi, M.; Mohammad, F. Nanotechnology and Plant Sciences Nanoparticles and Their Impact on Plants; Springer International Publishing: Cham, Switzerland, 2015. [Google Scholar]
- Bayda, S.; Adeel, M.; Tuccinardi, T.; Cordani, M.; Rizzolio, F. The History of Nanoscience and Nanotechnology: From Chemical-Physical Applications to Nanomedicine. Molecules 2019, 25, 112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Payal; Pandey, P. Role of Nanotechnology in Electronics: A Review of Recent Developments and Patents. Recent Pat. Nanotechnol. 2021, 15. [Google Scholar] [CrossRef] [PubMed]
- Baig, N.; Kammakakam, I.; Falath, W. Nanomaterials, a review of synthesis methods, properties, recent progress, and challenges. Mater. Adv. 2021, 2, 1821–1871. [Google Scholar] [CrossRef]
- Kalkidan Mamo, W.; Fufa Abunna, K.; Yonas Tolosa, R. A review on nanotechnology and its application in modern veterinary science. Int. J. Nanomater. Nanotechnol. Nanomed. 2021, 7, 26–31. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Ibrahim, M.N.M.; Rafatullah, M.; Chua, Y.S.; Ahmad, A.; Umar, K. Recent Advances in Anodes for Microbial Fuel Cells: An Overview. Materials 2020, 13, 2078. [Google Scholar] [CrossRef]
- Soares, S.; Sousa, J.; Pais, A.; Vitorino, C. Nanomedicine: Principles, Properties, and Regulatory Issues. Front. Chem. 2018, 6, 360. [Google Scholar] [CrossRef]
- Dahman, Y. Nanotechnology and Functional Materials for Engineers; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Goldmann, E.; Górski, M.; Klemczak, B. Recent Advancements in Carbon Nano-Infused Cementitious Composites. Materials 2021, 14, 5176. [Google Scholar] [CrossRef]
- Dutta, M.M. Nanomaterials for Food and Agriculture. In Applications of Nanomaterials in Agriculture, Food Science, and Medicine; Bhat, M., Wani, I., Ashraf, S., Eds.; IGI Global: Hershey, PA, USA, 2021; pp. 75–97. [Google Scholar]
- Manjunatha, R.L.; Naik, D.; Usharani, K.V. Nanotechnology application in agriculture: A review. J. Pharmacogn. Phytochem. 2019, 8, 1073–1083. [Google Scholar]
- Chhipa, H. Nanofertilizers and nanopesticides for agriculture. Environ. Chem. Lett. 2017, 15, 15–22. [Google Scholar] [CrossRef]
- Badawy, A.A.; Abdelfattah, N.A.H.; Salem, S.S.; Awad, M.F.; Fouda, A. Efficacy Assessment of Biosynthesized Copper Oxide Nanoparticles (CuO-NPs) on Stored Grain Insects and Their Impacts on Morphological and Physiological Traits of Wheat (Triticum aestivum L.) Plant. Biology 2021, 10, 233. [Google Scholar] [CrossRef] [PubMed]
- Salem, S.S.; Fouda, A. Green Synthesis of Metallic Nanoparticles and Their Prospective Biotechnological Applications: An Overview. Biol. Trace Elem. Res. 2021, 199, 344–370. [Google Scholar] [CrossRef] [PubMed]
- León-Silva, S.; Arrieta-Cortes, R.; Fernández-Luqueño, F.; López-Valdez, F. Design and Production of Nanofertilizers. In Agricultural Nanobiotechnology; Springer Science and Business Media LLC: Cham, Switzerland, 2018; pp. 17–31. [Google Scholar]
- Zhao, L.; Lu, L.; Wang, A.; Zhang, H.; Huang, M.; Wu, H.; Xing, B.; Wang, Z.; Ji, R. Nano-Biotechnology in Agriculture: Use of Nanomaterials to Promote Plant Growth and Stress Tolerance. J. Agric. Food Chem. 2020, 68, 1935–1947. [Google Scholar] [CrossRef] [PubMed]
- DeRosa, M.C.; Monreal, C.; Schnitzer, M.; Walsh, R.; Sultan, Y. Nanotechnology in fertilizers. Nat. Nanotechnol. 2010, 5, 91. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.D.; Chirag, G.; Prakash, P.O.; Mohan, M.H.; Prakasha, G. Nano-Fertilizers is a New Way to Increase Nutrients Use Efficiency in Crop Production. Int. J. Agric. Sci. 2017, 9, 3831–3833. [Google Scholar]
- Lin, D.; Xing, B. Phytotoxicity of nanoparticles: Inhibition of seed germination and root growth. Environ. Pollut. 2007, 150, 243–250. [Google Scholar] [CrossRef]
- Liu, R.; Lal, R. Potentials of engineered nanoparticles as fertilizers for increasing agronomic productions. Sci. Total Environ. 2015, 514, 131–139. [Google Scholar] [CrossRef]
- Sanzari, I.; Leone, A.; Ambrosone, A. Nanotechnology in Plant Science: To Make a Long Story Short. Front. Bioeng. Biotechnol. 2019, 7, 120. [Google Scholar] [CrossRef] [Green Version]
- Lansdown, A.B. Silver in health care: Antimicrobial effects and safety in use. Curr. Probl. Dermatol. 2006, 33, 17–34. [Google Scholar]
- Suresh, A.K.; Pelletier, D.A.; Wang, W.; Moon, J.W.; Gu, B.; Mortensen, N.P.; Allison, D.P.; Joy, D.C.; Phelps, T.J.; Doktycz, M.J. Silver nanocrystallites: Biofabrication using Shewanella oneidensis, and an evaluation of their comparative toxicity on gram-negative and gram-positive bacteria. Environ. Sci. Technol. 2010, 44, 5210–5215. [Google Scholar] [CrossRef]
- Nowack, B.; Krug, H.F.; Height, M. Reply to comments on B120 years of nanosilver history: Implications for policy makers. Environ. Sci. Technol. 2011, 45, 7593–7595. [Google Scholar] [CrossRef]
- Kaur, P.; Thakur, R.; Choudhary, A. An in vitro study of the antifungal activity of silver/chitosan nanoformulations against important seed borne pathogens. Int. J. Sci. Technol. Res. 2012, 1, 83–86. [Google Scholar]
- Vinković, T.; Novák, O.; Strnad, M.; Goessler, W.; Jurašin, D.D.; Paraiković, N.; Vrček, I.V. Cytokinin response in pepper plants (Capsicum annuum L.) exposed to silver nanoparticles. Environ. Res. 2017, 156, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Alghuthaymi, M.A.; Almoammar, H.; Rai, M.; Said-Galiev, E.; Abd-Elsalam, K.A. Myconanoparticles: Synthesis and their role in phytopathogens management. Biotechnol. Biotechnol. Equip. 2015, 29, 221–236. [Google Scholar] [CrossRef] [PubMed]
- Barros, C.H.N.; Fulaz, S.; Stanisic, D.; Tasic, L. Biogenic Nanosilver against Multidrug-Resistant Bacteria (MDRB). Antibiotics 2018, 7, 69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehmood, A. Brief overview of the application of silver nanoparticles to improve growth of crop plants. IET Nanobiotechnol. 2018, 12, 701–705. [Google Scholar] [CrossRef]
- Kudrinskiy, A.; Zherebin, P.; Gusev, A.; Shapoval, O.; Pyee, J.; Lisichkin, G.; Krutyakov, Y. New Relevant Descriptor of Linear QNAR Models for Toxicity Assessment of Silver Nanoparticles. Nanomaterials 2020, 10, 1459. [Google Scholar] [CrossRef]
- Wagner, A.J.; Bleckmann, C.A.; Murdock, R.C.; Schrand, A.M.; Schlager, J.J.; Hussain, S.M. Cellular Interaction of Different Forms of Aluminum Nanoparticles in Rat Alveolar Macrophages. J. Phys. Chem. B 2007, 111, 7353–7359. [Google Scholar] [CrossRef]
- Hristozov, D.; Malsch, I. Hazards and Risks of Engineered Nanoparticles for the Environment and Human Health. Sustainability 2009, 1, 1161–1194. [Google Scholar] [CrossRef] [Green Version]
- Mittal, D.; Kaur, G.; Singh, P.; Yadav, K.; Ali, S.A. Nanoparticle-Based Sustainable Agriculture and Food Science: Recent Advances and Future Outlook. Front. Bioeng. Biotechnol. 2020, 2, 9954. [Google Scholar] [CrossRef]
- ISTA International Rules for Seed Testing; International Seed Testing Association: Bassersdorf, Switzerland, 2018.
- World Reference Base for Soil Resources (WRB). International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; IUSS Working Group WRB; World Soil Resources Reports; FAO: Rome, Italy, 2014; Volume 106. [Google Scholar]
- Peel, M.C.; Finlayson, B.L.; McMahon, T.A. Updated world map of the Köppen-Geiger climate classification. Hydrol. Earth Syst. Sci. 2007, 11, 1633–1644. [Google Scholar] [CrossRef] [Green Version]
- Statistica. Data Analysis Software System, Version 12; TIBCO Software INC: Palo Alto, CA, USA, 2017; Available online: http://statistica.io (accessed on 15 January 2019).
- Steinitz, B.; Bilavendran, A.D. Thiosulfate stimulates growth and alleviates silver and copper toxicity in tomato root cultures. Plant Cell Tissue Organ Cult. 2011, 107, 355–363. [Google Scholar] [CrossRef]
- Monica, R.C.; Cremonini, R. Nanoparticles and higher plants. Caryologia 2009, 62, 161–165. [Google Scholar] [CrossRef] [Green Version]
- Yan, A.; Chen, Z. Impacts of Silver Nanoparticles on Plants: A Focus on the Phytotoxicity and Underlying Mechanism. Int. J. Mol. Sci. 2019, 20, 1003. [Google Scholar] [CrossRef]
- Musante, C.; White, J.C. Toxicity of silver and copper to Cucurbita pepo: Differential effects of nano and bulk-size particles. Environ. Toxicol. 2012, 27, 510–517. [Google Scholar] [CrossRef]
- Mazumdar, H.; Ahmed, G.U. Phytotoxicity effect of silver nanoparticles on Oryza sativa. Int. J. Chem. Tech. Res. 2011, 3, 1494–1500. [Google Scholar]
- Dietz, K.-J.; Herth, S. Plant nanotoxicology. Trends Plant Sci. 2011, 16, 582–589. [Google Scholar] [CrossRef]
- Aslani, F.; Bagheri, S.; Muhd Julkapli, N.; Juraimi, A.S.; Hashemi, F.S.G.; Baghdadi, A. Effects of engineered nanomaterials on plants growth: An overview. Sci. World J. 2014, 641759. [Google Scholar] [CrossRef]
- Tripathi, D.K.; Tripathi, A.; Singh, S.; Singh, Y.; Vishwakarma, K.; Yadav, G.; Sharma, S.; Singh, V.K.; Mishra, R.K.; Upadhyay, R.G.; et al. Uptake, accumulation and toxicity of silver nanoparticle in autotrophic plants, and heterotrophic microbes: A concentric review. Front. Microbiol. 2017, 8, 7. [Google Scholar] [CrossRef]
- Sukhanova, A.; Bozrova, S.; Sokolov, P.; Berestovoy, M.; Karaulov, A.; Nabiev, I. Dependence of Nanoparticle Toxicity on Their Physical and Chemical Properties. Nanoscale Res. Lett. 2018, 13, 44. [Google Scholar] [CrossRef] [Green Version]
- Egbuna, C.; Parmar, V.K.; Jeevanandam, J.; Ezzat, S.M.; Patrick-Iwuanyanwu, K.C.; Adetunji, C.O.; Khan, J.; Onyeike, E.N.; Uche, C.Z.; Akram, M.; et al. Toxicity of Nanoparticles in Biomedical Application: Nanotoxicology. J. Toxicol. 2021, 2021, 9954443. [Google Scholar] [CrossRef] [PubMed]
- Franci, G.; Falanga, A.; Galdiero, S.; Palomba, L.; Rai, M.; Morelli, G.; Galdiero, M. Silver nanoparticles as potential antibacterial agents. Molecules 2015, 20, 8856–8874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salomoni, R.; Léo, P.; Montemor, A.F.; Rinaldi, B.G.; Rodrigues, M. Antibacterial effect of silver nanoparticles in Pseudomonas aeruginosa. Nanotechnol. Sci. Appl. 2017, 10, 115–121. [Google Scholar] [CrossRef] [Green Version]
- Elumalai, E.K.; Vinothkumar, P. Role of Silver Nanoparticle Against Plant Pathogens. Nano Biomed. Eng. 2013, 5, 90–93. [Google Scholar] [CrossRef]
- Khan, M.; Khan, A.U.; Bogdanchikova, N.; Garibo, D. Antibacterial and Antifungal Studies of Biosynthesized Silver Nanoparticles against Plant Parasitic Nematode Meloidogyne incognita, Plant Pathogens Ralstonia solanacearum and Fusarium oxysporum. Molecules 2021, 26, 2462. [Google Scholar] [CrossRef] [PubMed]
- Wolny-Kładka, K.; Malina, D.; Sobczak-Kupiec, A.; Wzorek, Z. Synthesis and physicochemical characteristics of the silver nanoparticles and evaluation of their toxicity to fungi of the species Fusarium culmorum isolated from winter wheat. Pol. J. Agron. 2013, 15, 69–74. [Google Scholar]
- Kasprowicz, M.; Gorczyca, A.; Koziol, M. The effect of Silver Nanoparticles on phytopathogenic spores of Fusarium culmorum. Can. J. Microbiol. 2010, 56, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.S.; Jung, J.H.; Lamsal, K.; Kim, Y.S.; Min, S.J.; Lee, Y.S. Antifungal Effects of Silver Nanoparticles (AgNPs) against Various Plant Pathogenic Fungi. Mycobiology 2012, 40, 53–58. [Google Scholar] [CrossRef] [Green Version]
- Bystrzejewska-Piotrowska, G.; Golimowski, J.; Urban, P.L. Nanoparticles: Their potential toxicity, waste and environmental management. Waste Manag. 2009, 29, 2587–2595. [Google Scholar] [CrossRef]
- Sharma, V.K.; Siskova, K.M.; Zboril, R.; Gardea-Torresdey, J.L. Organic-coated silver nanoparticles in biological and environmental conditions: Fate, stability and toxicity. Adv. Colloid Interface Sci. 2014, 204, 15–34. [Google Scholar] [CrossRef]
- Chichiriccó, G.; Poma, A. Penetration and toxicity of nanomaterials in higher plants. Nanomaterials 2015, 5, 851–873. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, M.V.J.; Sharma, P.K. Effect of copper oxide nanoparticles on growth, morphology, photosynthesis, and antioxidant response in Oryza sativa. Photosynthetica 2016, 54, 110–119. [Google Scholar] [CrossRef]
- Jiang, H.S.; Yin, L.Y.; Ren, N.N.; Zhao, S.T.; Li, Z.; Zhi, Y.; Shao, H.; Li, W.; Gontero, B. Silver nanoparticles induced reactive oxygen species via photosynthetic energy transport imbalance in an aquatic plant. Nanotoxicology 2017, 11, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Ahammed, G.J.; Li, C.; Bao, X.; Yu, J.; Huang, C.; Yin, H.; Zhou, J. Brassinosteroid ameliorates zinc oxide nanoparticles-induced oxidative stress by improving antioxidant potential and redox homeostasis in tomato seedling. Front. Plant Sci. 2016, 7, 615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saha, N.; Gupta, S.D. A Glimpse on Silver Nanoparticles Genotoxicity in Higher Plants Global. Nanomed. J. 2017, 2, 1–2. [Google Scholar]
- Kong, I.C.; Ko, K.S.; Koh, D.C. Evaluation of the Effects of Particle Sizes of Silver Nanoparticles on Various Biological Systems. Int. J. Mol. Sci. 2020, 21, 8465. [Google Scholar] [CrossRef]
- Szőllősi, R.; Molnár, Á.; Kondak, S.; Kolbert, Z. Dual Effect of Nanomaterials on Germination and Seedling Growth: Stimulation vs. Phytotoxicity. Plants 2020, 9, 1745. [Google Scholar] [CrossRef]
- Poschenrieder, C.; Tolrà, R.; Barceló, J. Can metals defend plants against biotic stress? Trends Plant Sci. 2006, 11, 288–295. [Google Scholar] [CrossRef]
- Hillegass, J.M.; Shukla, A.; Lathrop, S.A.; MacPherson, M.B.; Fukagawa, N.K.; Mossman, B.T. Assessing nanotoxicity in cells in vitro. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2010, 2, 219–231. [Google Scholar] [CrossRef] [Green Version]
- Nel, A.E.; Mädler, L.; Velegol, D.; Xia, T.; Hoek, E.M.V.; Somasundaran, P.; Klaessig, F.; Castranova, V.; Thompson, M. Understanding biophysicochemical interactions at the nano–bio interface. Nat. Mater. 2009, 8, 543–557. [Google Scholar] [CrossRef]
- Chen, M.; Zhou, S.; Zhu, Y.; Sun, Y.; Zeng, G.; Yang, C.; Xu, P.; Yan, M.; Liu, Z.; Zhang, W. Toxicity of carbon nanomaterials to plants, animals and microbes: Recent progress from 2015-present. Chemosphere 2018, 206, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Docter, D.; Westmeier, D.; Markiewicz, M.; Stolte, S.; Knauer, S.K.; Stauber, R.H. The nanoparticle biomolecule corona: Lessons learned-Challenge accepted? Chem. Soc. Rev. 2015, 44, 6094–6121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almutairi, Z.M.; Alharbi, A. Effect of silver nanoparticles on seed germination of crop plants. J. Adv. Agric. 2015, 4, 283–288. [Google Scholar] [CrossRef]
- Barrena, R.; Casals, E.; Colón, J.; Font, X.; Sánchez, A.; Puntes, V. Evaluation of the ecotoxicity of model nanoparticles. Chemosphere 2009, 75, 850–857. [Google Scholar] [CrossRef] [Green Version]
- Prażak, R.; Święciło, A.; Krzepiłko, A.; Michałek, S.; Arczewska, M. Impact of Ag Nanoparticles on Seed Germination and Seedling Growth of Green Beans in Normal and Chill Temperatures. Agriculture 2020, 10, 312. [Google Scholar] [CrossRef]
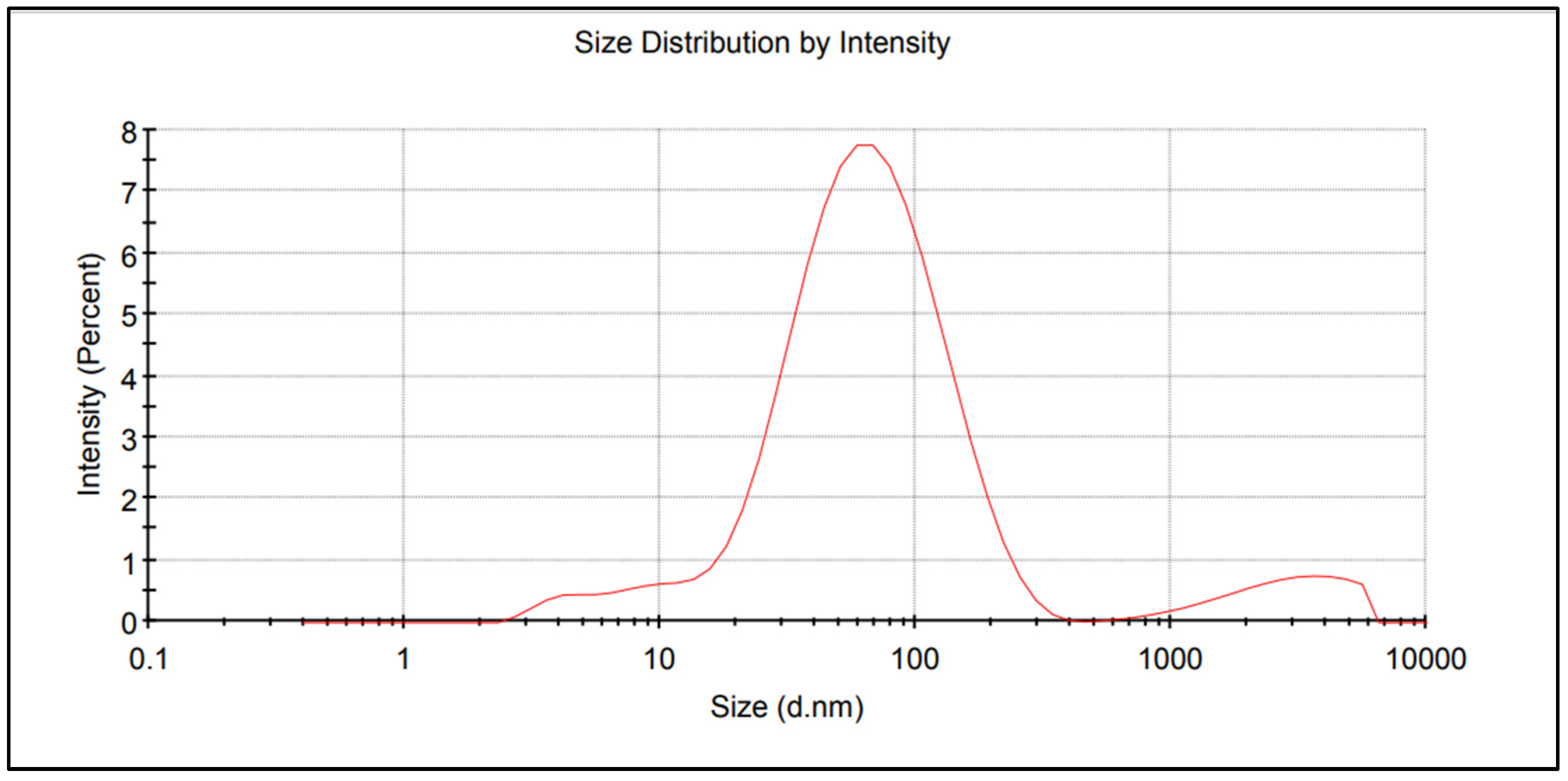
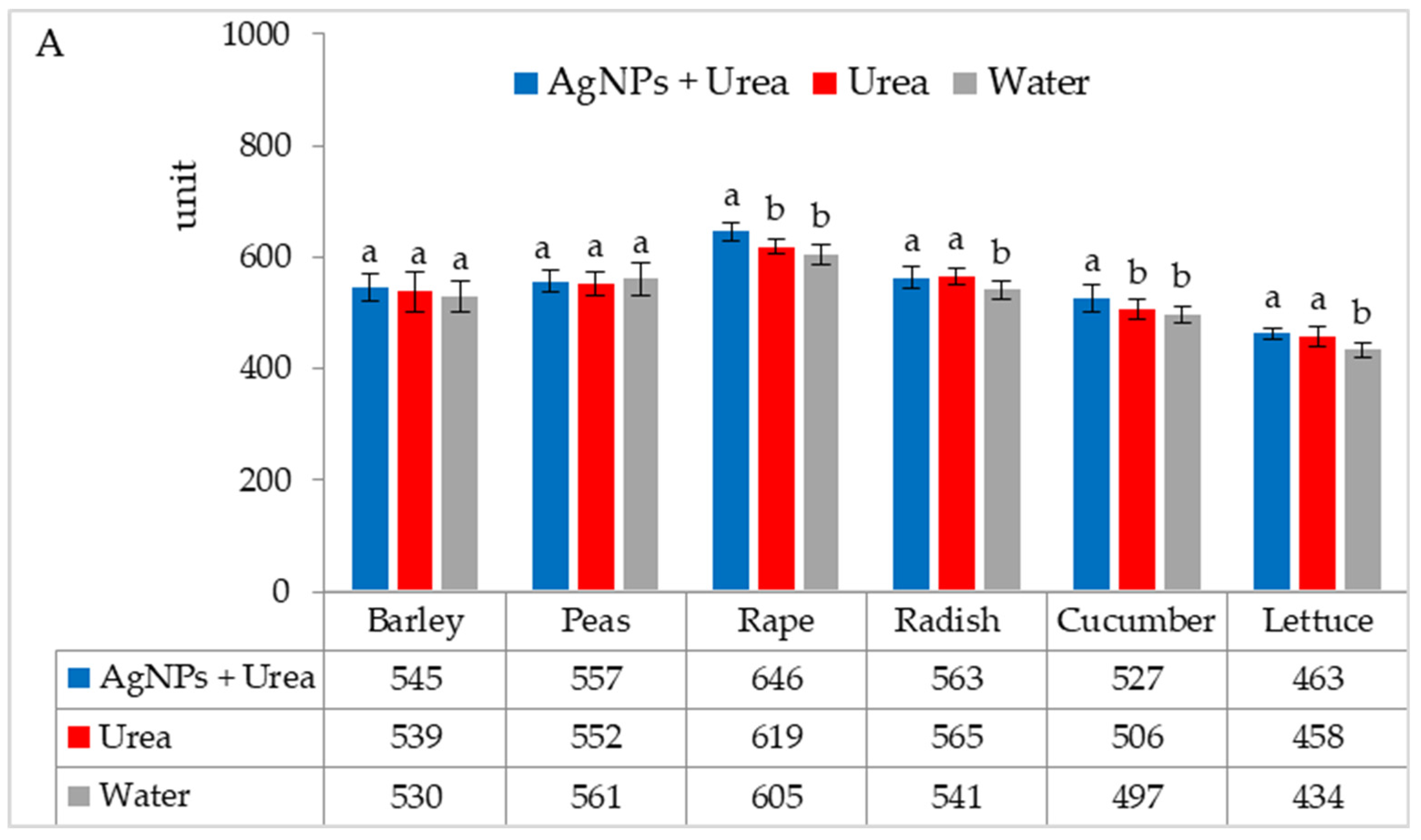
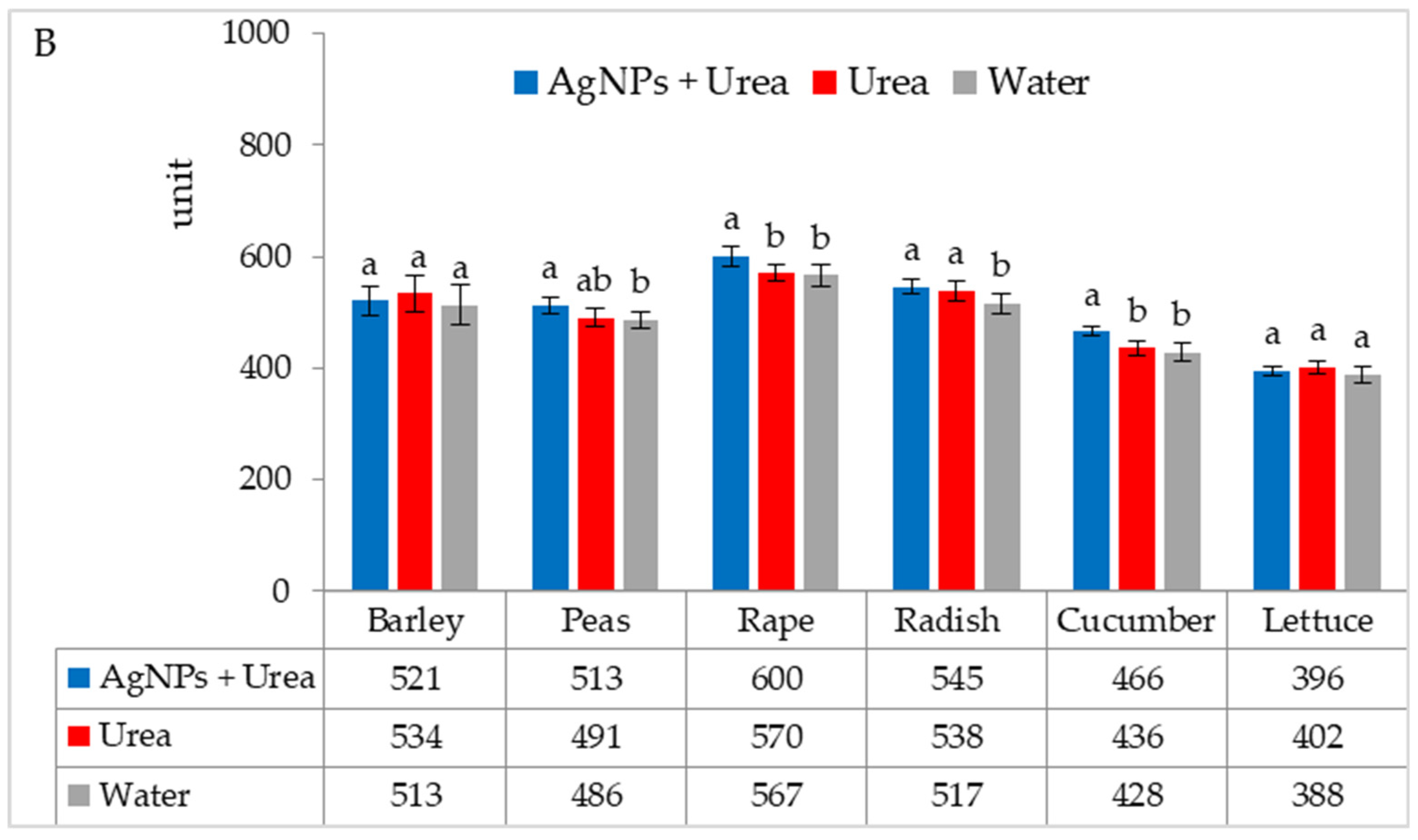

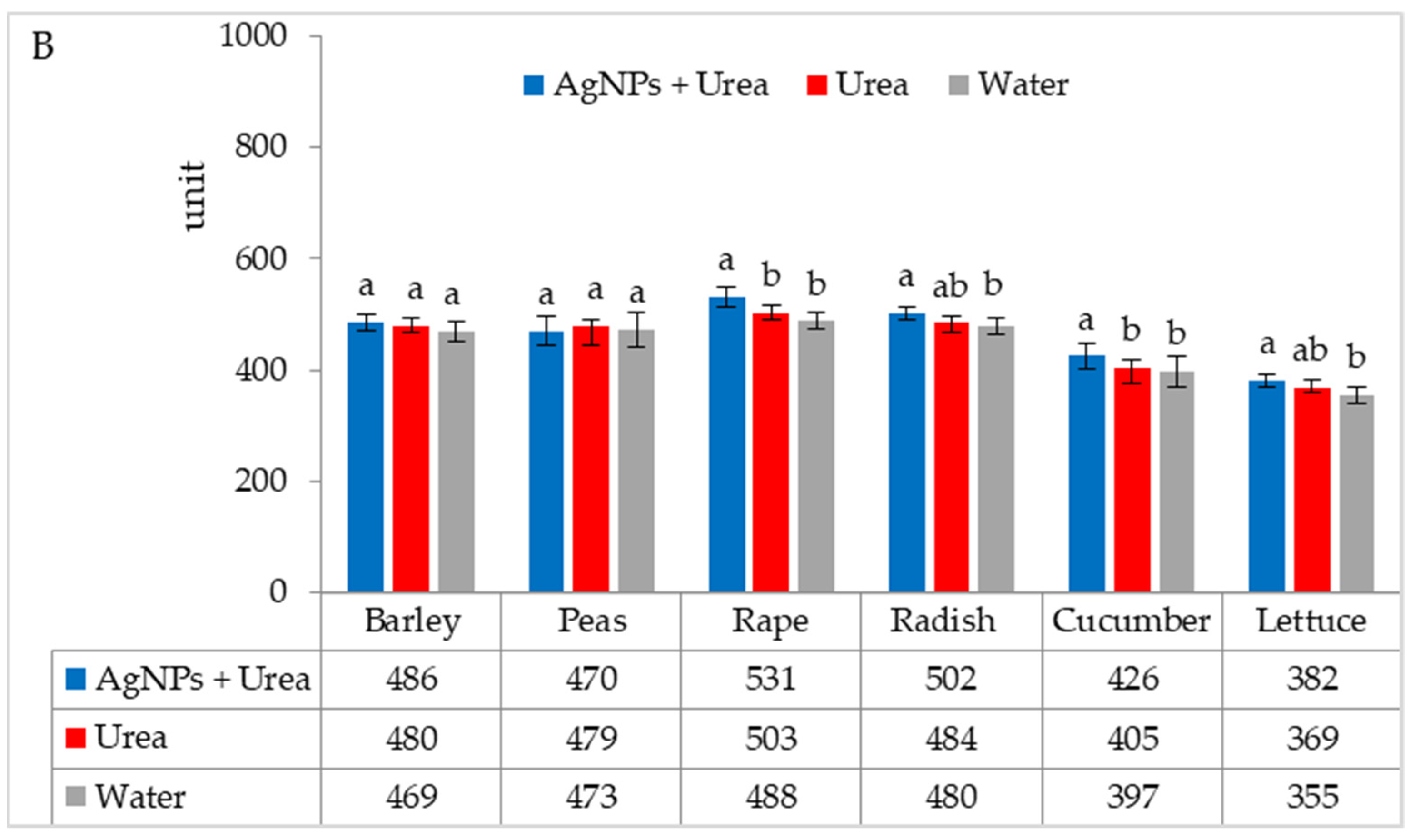
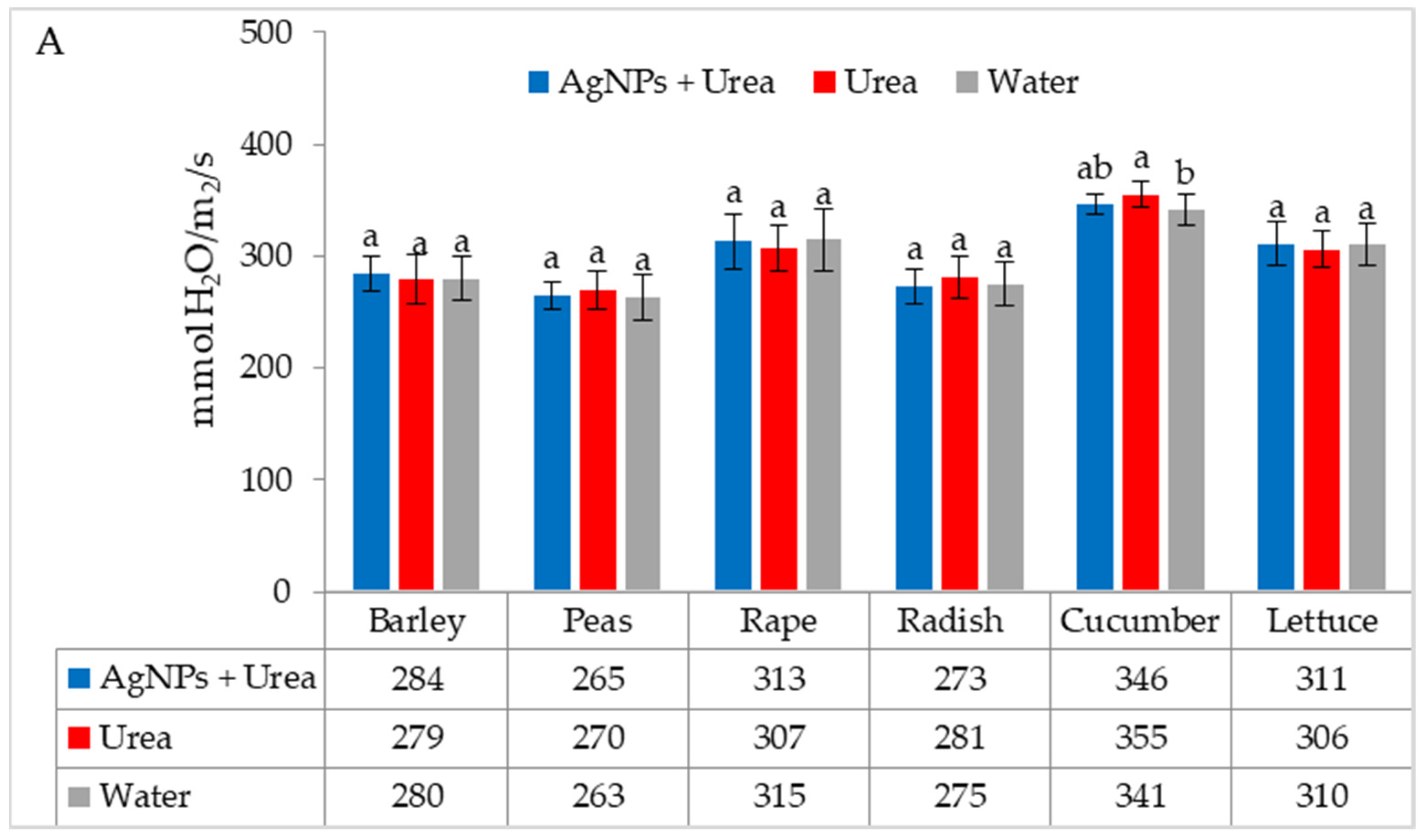
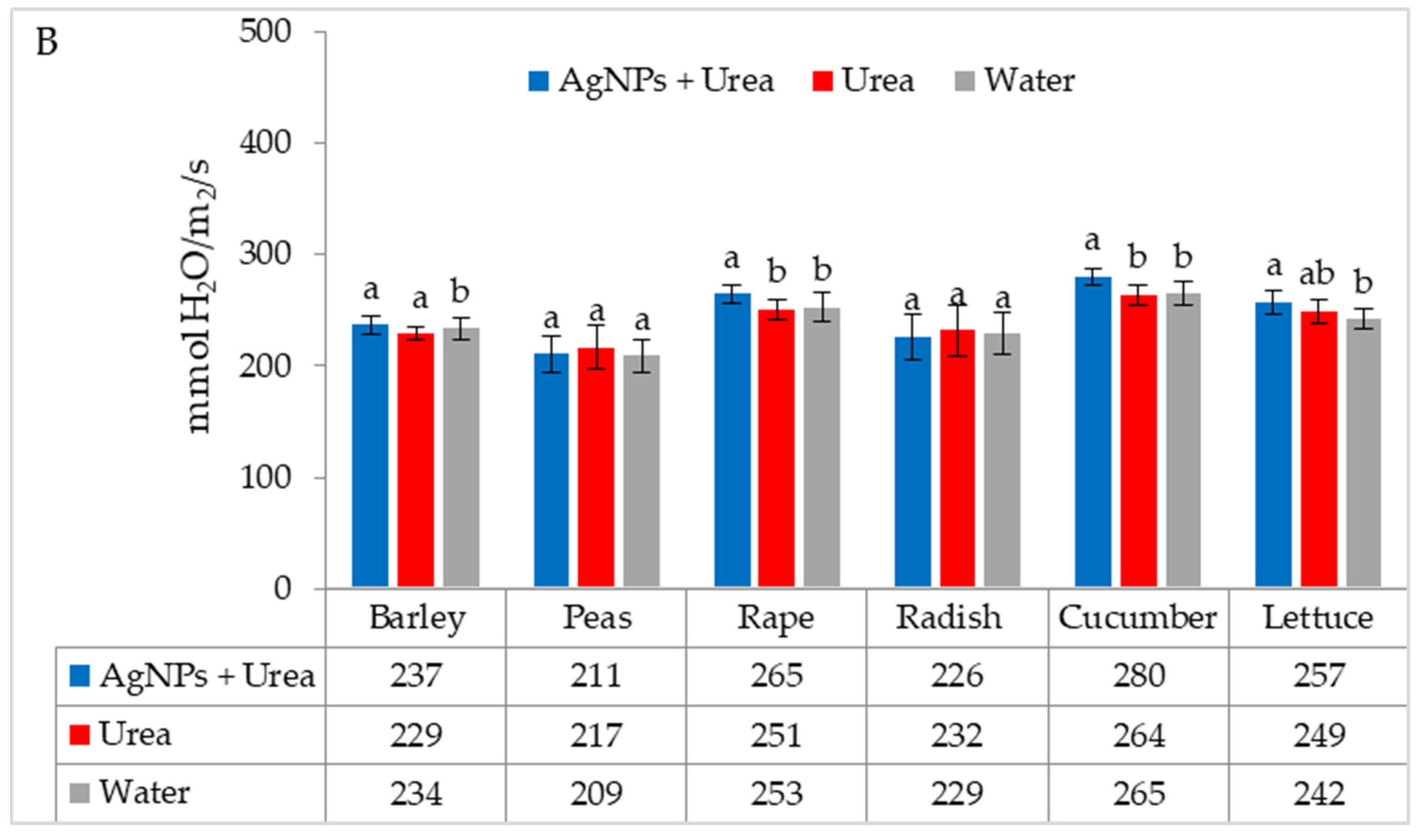
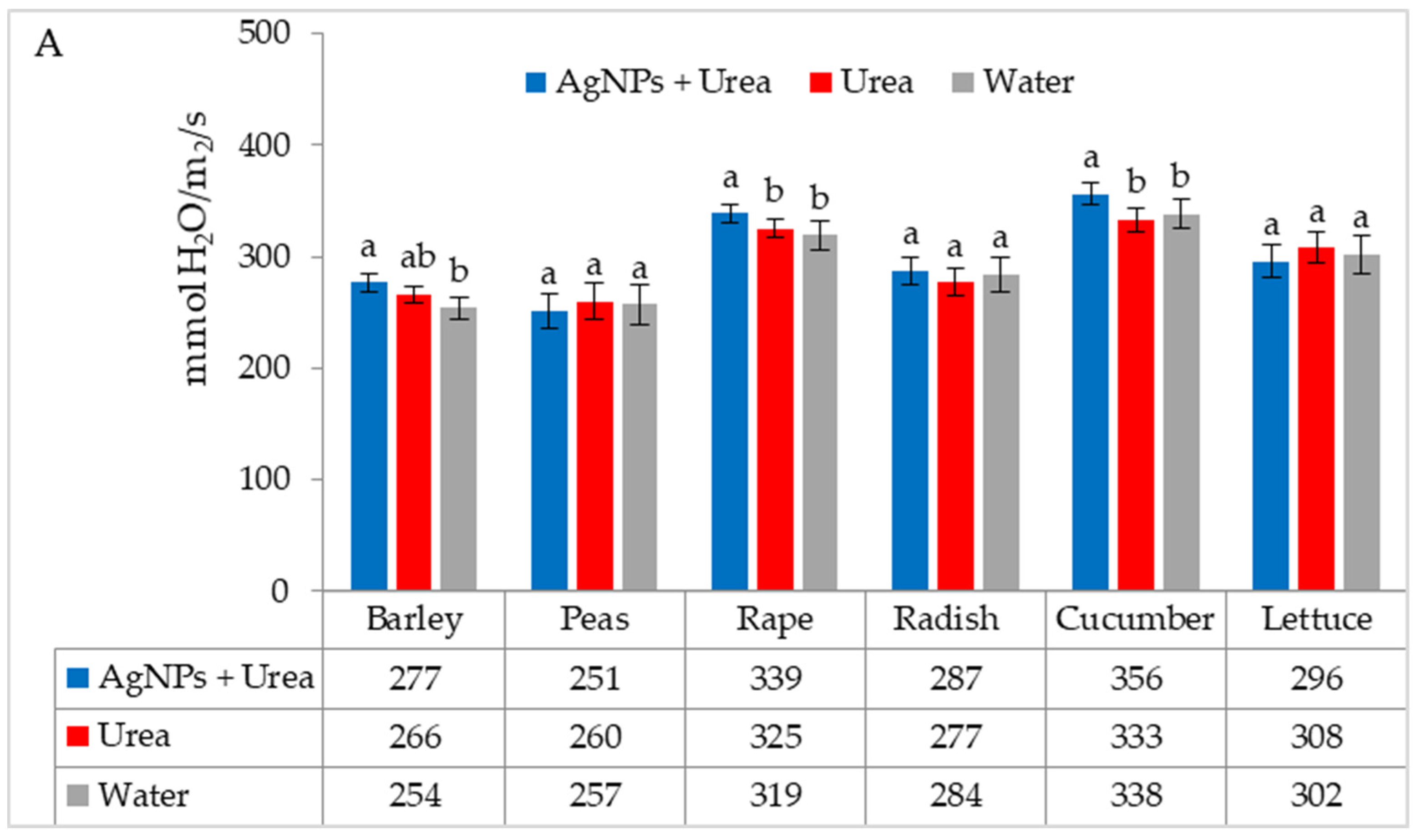


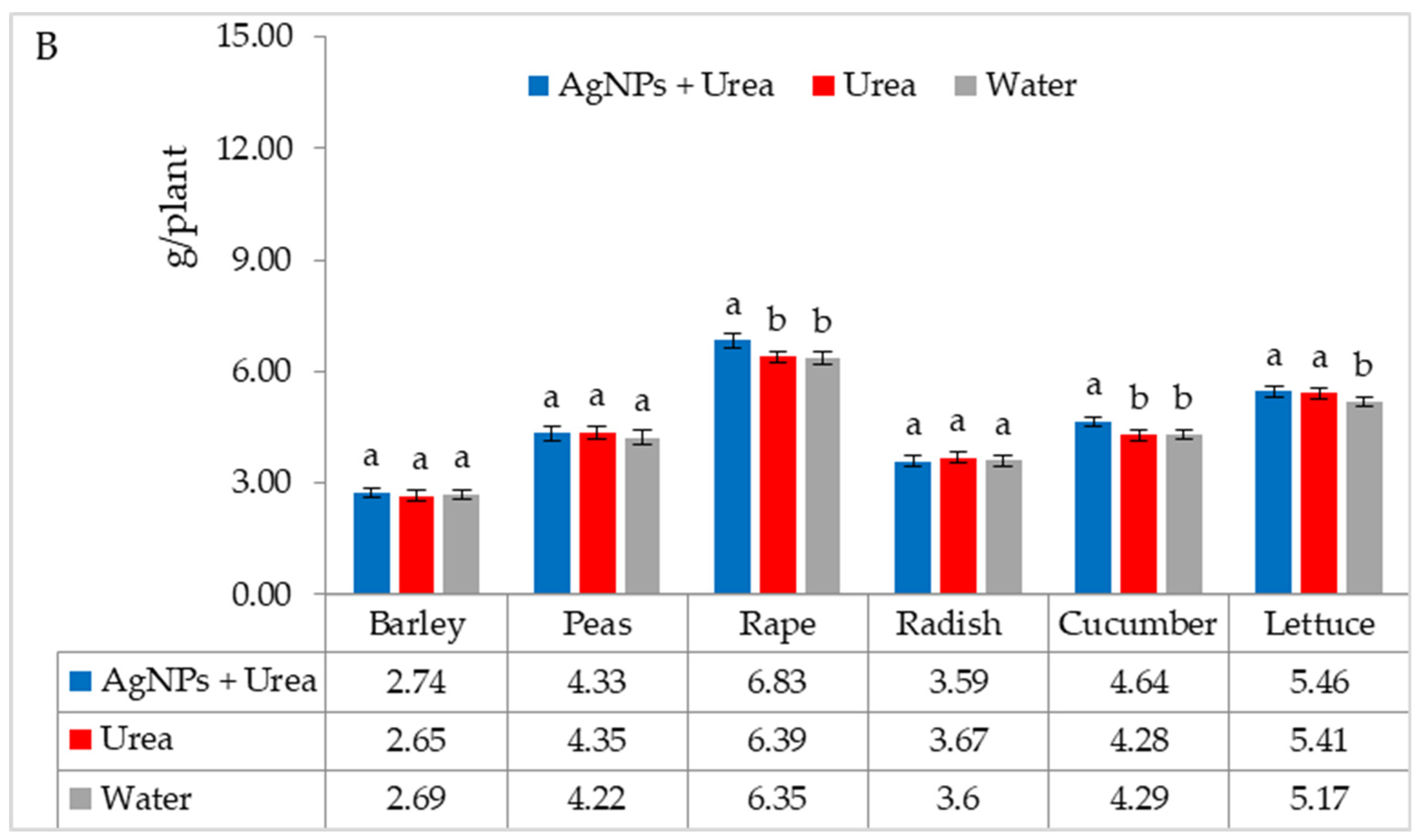

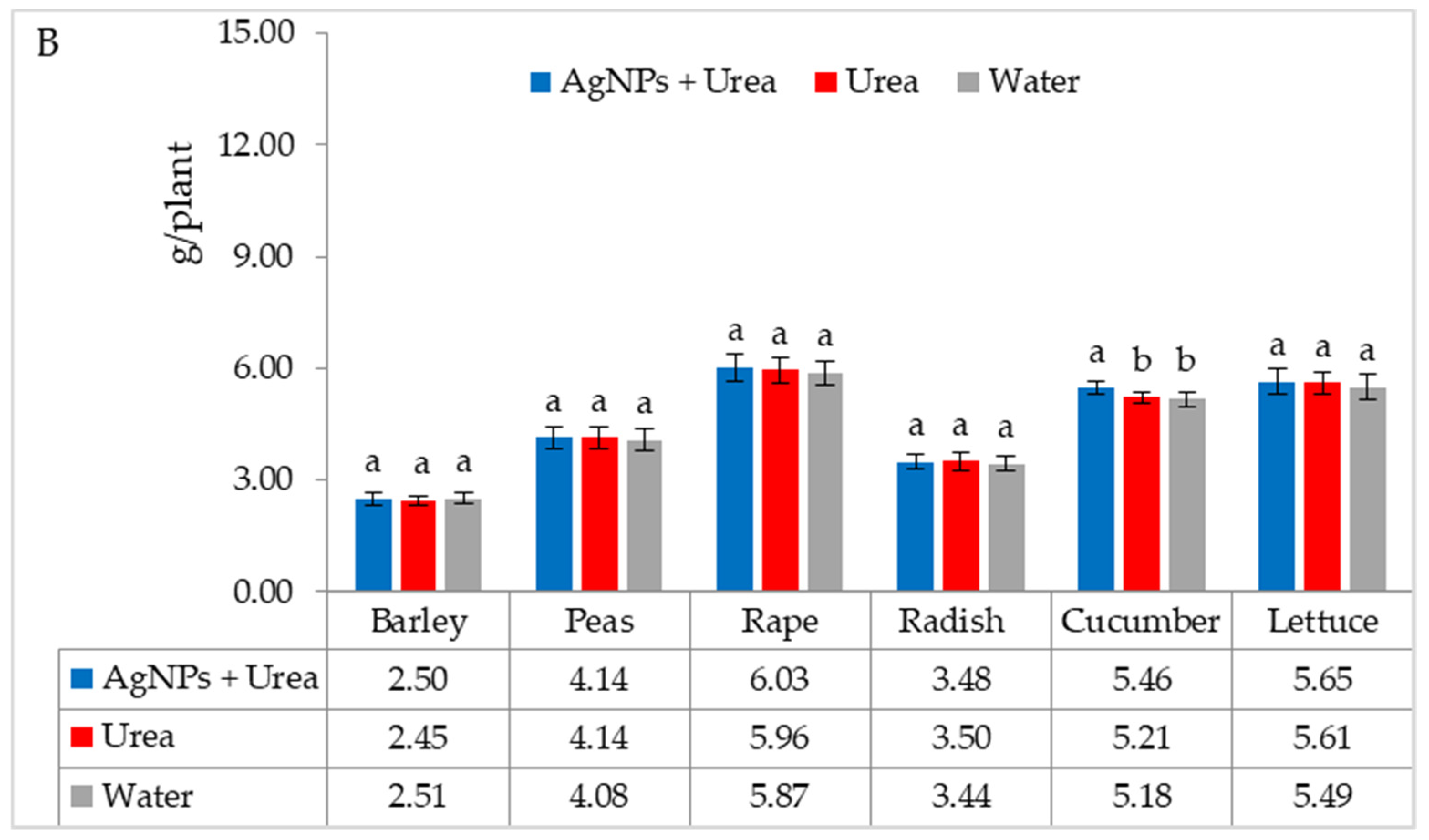
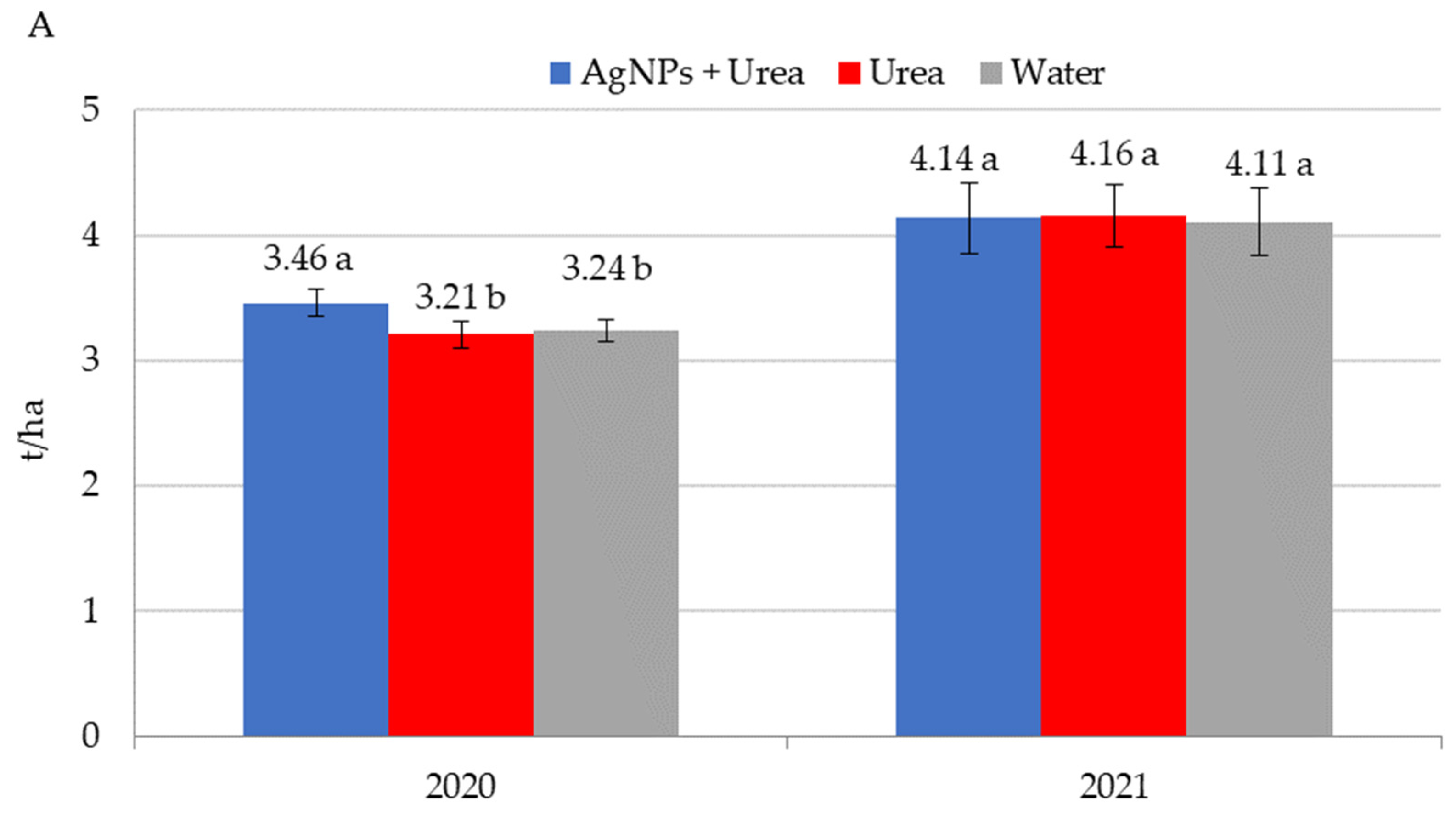

| Property | 2019 | 2020 | 2021 |
|---|---|---|---|
| Rape | |||
| Texture (%) | |||
| Sand (2–0.05 mm) | 49.5 | 53.6 | - |
| Silt (0.05–0.002 mm) | 45.2 | 44.1 | - |
| Clay (<0.002 mm) | 5.3 | 2.3 | - |
| pHKCl | 6.52 | 5.93 | - |
| Organic carbon (g C kg−1 soil) | 11.2 | 10.6 | - |
| Total nitrogen (g N kg−1 soil) | 1.07 | 0.95 | - |
| Available nutrients | |||
| Phosphorus (mg P kg−1 soil) | 96.5 | 117.2 | - |
| Potassium (mg K kg−1 soil) | 162.1 | 146.8 | - |
| Magnesium (mg kg−1 soil) | 63.0 | 42.7 | - |
| Cucumber | |||
| Texture (%) | |||
| Sand (2–0.05 mm) | 45.1 | 46.3 | 44.7 |
| Silt (0.05–0.002 mm) | 48.3 | 46.9 | 49.6 |
| Clay (<0.002 mm) | 6.6 | 6.8 | 5.7 |
| pHKCl | 7.08 | 6.61 | 6.85 |
| Organic carbon (g C kg−1 soil) | 12.9 | 11.7 | 13.5 |
| Total nitrogen (g N kg−1 soil) | 1.13 | 1.20 | 1.42 |
| Available nutrients | |||
| Phosphorus (mg P kg−1 soil) | 114.8 | 98.0 | 120.4 |
| Potassium (mg K kg−1 soil) | 188.9 | 177.3 | 203.5 |
| Magnesium (mg kg−1 soil) | 55.6 | 60.4 | 66.2 |
| Year | ||||||||
|---|---|---|---|---|---|---|---|---|
| Month | 2019 | 2020 | 2021 | Many-Year | 2019 | 2020 | 2021 | Many-Year |
| Temperature (°C) | Precipitation (mm) | |||||||
| January | - | 2.6 | −1.1 | −1.8 | - | 37.7 | 28.3 | 26.8 |
| February | - | 3.6 | −1.8 | −0.9 | - | 36.0 | 0.8 | 20.7 |
| March | - | 3.9 | 3.7 | 2.5 | - | 26.1 | 21.7 | 31.9 |
| April | - | 8.2 | 6.2 | 7.9 | - | 0.7 | 30.7 | 27.0 |
| May | - | 11.2 | 12.2 | 13.3 | - | 34.2 | 75.2 | 49.3 |
| June | - | 17.9 | 20.1 | 16.1 | - | 142.0 | 30.1 | 52.8 |
| July | - | 18.3 | 20.9 | 18.6 | - | 67.2 | 61.7 | 69.8 |
| August | 19.7 | 19.9 | 17.4 | 17.9 | 37.7 | 114.4 | 38.1 | 62.6 |
| September | 13.5 | 15.1 | - | 13.1 | 98.5 | 66.7 | - | 46.0 |
| October | 9.8 | 10.5 | - | 8.2 | 35.9 | 72.9 | - | 31.5 |
| November | 5.5 | 6.0 | - | 2.9 | 69.6 | 12.4 | - | 32.4 |
| December | 2.7 | 1.8 | - | −0.6 | 21.1 | 33.8 | - | 34.0 |
| Feature | AgNPs + Urea | Urea | Water | AgNPs + Urea | Urea | Water |
|---|---|---|---|---|---|---|
| Optimal Conditions | Stress Conditions | |||||
| Barley | ||||||
| Germination energy, % | 84.3 a (±5.2) | 85.0 a (±4.9) | 86.0 a (±4.4) | 77.1 a (±1.9) | 75.3 a,b (±1.4) | 72.2 b (±2.3) |
| Germination capacity, % | 93.2 a (±3.6) | 92.5 a (±3.5) | 93.1 a (±4.2) | 90.2 a (±2.8) | 86.9 a (±2.6) | 90.4 a (±2.5) |
| Abnormal sprouts, pcs/100 seeds | 2.4 a (±0.45) | 2.1 a (±0.27) | 2.3 a (±0.35) | 2.1 a (±0.24) | 2.2 a (±0.26) | 2.2 a (±0.18) |
| Sprout length, mm | 87.4 a (±4.9) | 88.0 a (±4.3) | 87.2 a (±5.7) | 62.5 a (±1.5) | 60.4 a,b (±2.3) | 56.6 b (±2.3) |
| Pathogen infestation, % | 5.3 a (±0.37) | 5.7 a (±0.42) | 5.5 a (±0.22) | 5.1 b (±0.22) | 9.0 a (±0.24) | 8.8 a (±0.18) |
| Peas | ||||||
| Germination energy, % | 76.7 a (±3.7) | 78.0 a (±3.4) | 77.3 a (±4.0) | 66.2 a (±4.3) | 64.6 a (±4.2) | 64.2 a (±4.2) |
| Germination capacity, % | 88.9 a (±4.8) | 88.7 a (±3.7) | 90.3 a (±4.0) | 82.5 a (±6.1) | 80.8 a (±4.1) | 82.6 a (±5.0) |
| Abnormal sprouts, pcs/100 seeds | 3.9 a (±0.29) | 4.2 a (±0.36) | 3.9 a (±0.32) | 3.6 a (±0.38) | 3.8 a (±0.43) | 3.8 a (±0.29) |
| Sprout length, mm | 53.1 a (±3.5) | 54.0 a (±6.1) | 52.8 a (±3.4) | 45.6 a (±2.8) | 44.5 a (±3.1) | 44.4 a (±2.9) |
| Pathogen infestation, % | 7.8 b (±0.53) | 9.7 a (±0.67) | 10.0 a (±0.78) | 7.5 b (±0.49) | 12.8 a (±1.33) | 12.6 a (±0.90) |
| Rape | ||||||
| Germination energy, % | 90.2 a (±4.2) | 87.1 a (±4.4) | 87.0 a (±5.4) | 86.3 a (±2.3) | 81.9 b (±1.1) | 82.2 b (±1.4) |
| Germination capacity, % | 95.0 a (±3.7) | 96.1 a (±2.8) | 94.9 a (±6.1) | 91.7 a (±3.2) | 87.2 a,b (±2.1) | 86.4 b (±2.0) |
| Abnormal sprouts, pcs/100 seeds | 2.7 a (±0.52) | 2.6 a (±0.36) | 3.1 a (±0.32) | 3.3 a (±0.45) | 2.9 a (±0.23) | 3.3 a (±0.50) |
| Sprout length, mm | 57.8 a (±1.2) | 56.3 a,b (±1.6) | 54.8 b (±1.5) | 47.9 a (±1.6) | 44.5 b (±1.5) | 44.0 b (±1.7) |
| Pathogen infestation, % | 12.7 a (±0.78) | 13.4 a (±1.16) | 13.0 a (±0.74) | 12.4 b (±0.63) | 18.5 a (±0.94) | 18.1 a (±0.98) |
| Feature | AgNPs + Urea | Urea | Water | AgNPs + Urea | Urea | Water |
|---|---|---|---|---|---|---|
| Optimal Conditions | Stress Conditions | |||||
| Radish | ||||||
| Germination energy, % | 82.5 a (±5.4) | 84.0 a (±5.0) | 83.4 a (±4.8) | 74.8 a (±1.4) | 73.3 a,b (±1.4) | 71.1 b (±2.3) |
| Germination capacity, % | 88.5 a (±4.1) | 86.7 a (±3.7) | 87.7 a (±4.6) | 80.8 a (±2.5) | 78.2 a (±2.0) | 75.0 b (±2.5) |
| Abnormal sprouts, pcs/100 seeds | 3.7 a (±0.41) | 3.9 a (±0.29) | 3.6 a (±0.29) | 3.5 a (±0.41) | 3.6 a (±0.24) | 3.4 a (±0.22) |
| Sprout length, mm | 46.7 a (±4.8) | 44.7 a (±2.9) | 45.0 a (±2.2) | 41.9 a (±3.1) | 38.9 a (±1.8) | 39.6 a (±2.4) |
| Pathogen infestation, % | 10.3 a (±1.48) | 11.1 a (±0.82) | 11.4 a (±1.00) | 12.4 b (±1.25) | 18.6 a (±1.39) | 19.5 a (±1.90) |
| Cucumber | ||||||
| Germination energy, % | 76.5 a (±1.6) | 74.2 a,b (±1.4) | 72.9 b (±1.8) | 70.5 a (±1.4) | 67.3 b (±1.4) | 66.2 b (±1.8) |
| Germination capacity, % | 85.3 a (±2.4) | 84.7 a (±3.4) | 83.0 a (±3.4) | 81.7 a (±3.2) | 75.3 b (±3.6) | 76.4 b (±2.7) |
| Abnormal sprouts, pcs/100 seeds | 4.1 a (±0.24) | 4.3 a (±0.35) | 4.0 a (±0.24) | 4.3 b (±0.14) | 4.7 a (±0.18) | 4.7 a (±0.24) |
| Sprout length, mm | 42.7 a (±3.3) | 40.9 a (±2.2) | 42.8 a (±3.2) | 38.2 a (±2.0) | 34.6 b (±1.9) | 33.8 b (±1.4) |
| Pathogen infestation, % | 8.7 b (±0.67) | 17.2 a (±1.88) | 16.6 a (±1.43) | 7.0 b (±0.92) | 15.5 a (±2.22) | 16.2 a (±1.23) |
| Lettuce | ||||||
| Germination energy, % | 64.6 a (±3.1) | 65.0 a (±3.0) | 65.9 a (±4.9) | 59.8 a (±3.9) | 57.7 a (±3.7) | 58.6 a (±4.1) |
| Germination capacity, % | 82.4 a (±3.5) | 83.2 a (±4.0) | 80.1 a (±3.2) | 84.7 a (±2.7) | 84.6 a (±2.7) | 79.7 b (±2.5) |
| Abnormal sprouts, pcs/100 seeds | 5.1 a (±0.32) | 5.2 a (±0.38) | 4.8 a (±0.24) | 5.0 b (±0.26) | 5.8 a (±0.24) | 6.1 a (±0.36) |
| Sprout length, mm | 48.8 a (±1.4) | 47.1 a (±1.8) | 45.2 b (±1.5) | 41.9 a (±2.2) | 40.7 a,b (±2.4) | 37.0 b (±1.5) |
| Pathogen infestation, % | 11.0 b (±1.51) | 19.4 a (±1.60) | 20.1 a (±1.75) | 9.7 b (±1.25) | 15.1 a (±1.23) | 16.3 a (±1.32) |
| Treatment | Rape | Cucumber | ||||
|---|---|---|---|---|---|---|
| LAI | IPAR | Chlorophyll | LAI | IPAR | Chlorophyll | |
| 2019 | ||||||
| AgNPs + Urea | 2.37 a (±0.08) | 71.4 a (±1.5) | 632 a (±14.0) | 3.57 a (±0.12) | 81.2 a (±1.9) | 461 (±8.8) |
| Urea | 2.20 a,b (±0.10) | 68.1 b (±1.7) | 603 b (±12.6) | 3.33 b (±0.09) | 77.3 b (±1.6) | 446 a,b (±9.9) |
| Water | 2.11 b (±0.10) | 67.8 b (±1.3) | 605 b (±13.4) | 3.26 b (±0.07) | 77.3 b (±1.5) | 439 b (±9.1) |
| 2020 | ||||||
| AgNPs + Urea | 4.12 a (±0.07) | 85.6 a (±1.5) | 567 a (±11.2) | 4.63 a (±0.12) | 88.6 a (±1.7) | 516 a (±24.6) |
| Urea | 3.82 b (±0.14) | 80.9 b (±1.9) | 558 a,b (±13.4) | 4.67 a (±0.14) | 87.1 a (±1.9) | 509 a (±19.1) |
| Water | 3.88 b (±0.07) | 81.2 b (±1.5) | 542 b (±9.9) | 4.55 a (±0.15) | 87.4 a (±1.3) | 505 a (±14.7) |
| 2021 | ||||||
| AgNPs + Urea | 5.23 a (±0.11) | 93.5 a (±3.7) | 559 a (±7.6) | 4.22 a (±0.06) | 85.6 a (±1.3) | 527 a (±16.4) |
| Urea | 5.25 a (±0.10) | 93.5 a (±2.4) | 542 b (±8.7) | 4.10 b (±0.06) | 83.0 a,b (±1.5) | 528 a (±12.8) |
| Water | 5.16 a (±0.13) | 92.6 a (±2.4) | 542 b (±8.8) | 4.06 b (±0.04) | 82.4 b (±1.7) | 519 a (±17.6) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaskulski, D.; Jaskulska, I.; Majewska, J.; Radziemska, M.; Bilgin, A.; Brtnicky, M. Silver Nanoparticles (AgNPs) in Urea Solution in Laboratory Tests and Field Experiments with Crops and Vegetables. Materials 2022, 15, 870. https://doi.org/10.3390/ma15030870
Jaskulski D, Jaskulska I, Majewska J, Radziemska M, Bilgin A, Brtnicky M. Silver Nanoparticles (AgNPs) in Urea Solution in Laboratory Tests and Field Experiments with Crops and Vegetables. Materials. 2022; 15(3):870. https://doi.org/10.3390/ma15030870
Chicago/Turabian StyleJaskulski, Dariusz, Iwona Jaskulska, Joanna Majewska, Maja Radziemska, Ayla Bilgin, and Martin Brtnicky. 2022. "Silver Nanoparticles (AgNPs) in Urea Solution in Laboratory Tests and Field Experiments with Crops and Vegetables" Materials 15, no. 3: 870. https://doi.org/10.3390/ma15030870







