The Structure of Bitumen: Conceptual Models and Experimental Evidences
Abstract
:1. Introduction
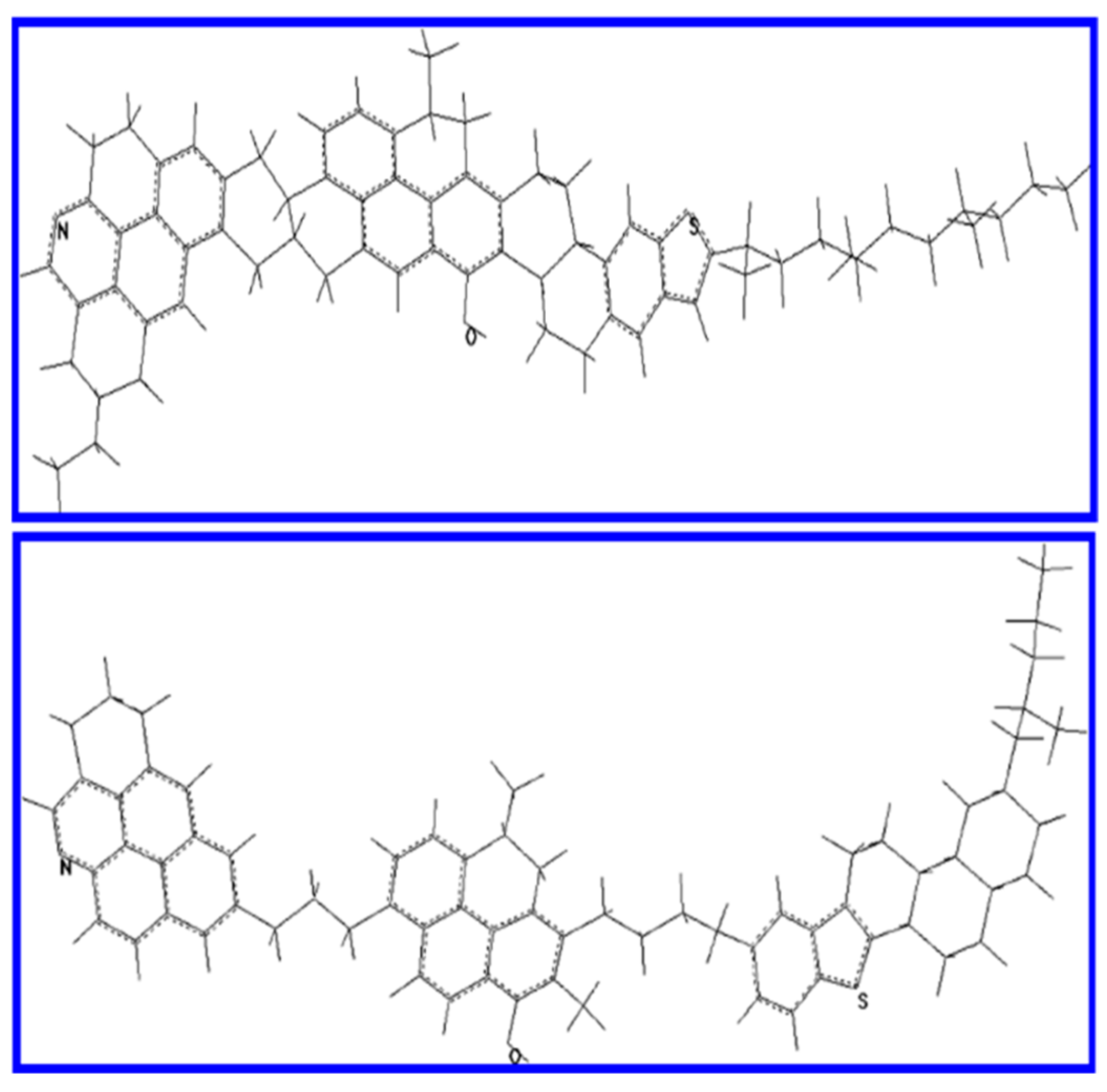
2. Asphaltenes and the Colloidal Nature of Bitumen Microstructure
2.1. Aggregation of Asphaltenes in Bitumen and Model Solvents
2.2. Reversible Aging and Thermal Behavior of Bitumen
2.3. Critical Micelle, Critical Nanoaggregation and Critical Cluster Concentrations (CMC, CNAC, CCC)
3. Experimental Investigations
3.1. Atomic Force Microscopy (AFM)
3.2. Scattering Techniques and Fractal Nature of Bitumen Microsctructure
4. Structure of Bitumen Aggregates by Computer and Molecular Dynamics Simulations
5. Novel Challenges in Bitumen Research
6. Conclusions
- A huge number of research reports have demonstrated that ASP aggregates exist on the colloidal length scale. One of the strengths of the colloidal model is the fact that ASP molecules are able to self-associate when in solution, in order to generate what we can call extended aggregates;
- Among the various efforts made to summarize the wide range of observations into a molecular picture, the colloidal model remains the most widely accepted and successful. This model assumes that polar molecules in crude oil residua interact to form molecular associations. These molecular associations are believed to be dispersed in a bulk solvent, which consists of saturated components, aromatic compounds, and less polar heteroatom-containing compounds;
- The size of the supramolecular associations, which are held together by forces varying in strength, is a function of the nature of the polar compounds, temperature, rate of shear, and the effectiveness with which the associations are solvated;
- Various properties of bitumen, such as its adhesion property or rheological behavior, are highly affected by the binder’s chemical composition and the macroscopic organization of ASP. However, not a lot is known about the relationship between the macroscopic organization of ASP and the rheological properties, resins and polymers that are added and incorporated into the bitumen matrix. The aging of bitumen is another important topic, and this is thought to be associated with the evolution of the ASP content and the macroscopic organization with time and exposure to oxidation.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
List of Abbreviations
| AFM | Atomic Force Microscopy |
| ASP | Asphaltene(s) |
| CAC | Critical Aggregate Concentration |
| CCC | Critical Cluster Concentration |
| CMC | Critical Micelle Concentration |
| CNAC | Critical Nanoaggregation Concentration |
| DPD | Dissipative Particle Dynamics |
| DPF | Dispersed Polar Fluid |
| FTIR | Fourier Transform Infrared spectroscopy |
| MW | Molecular Weight |
| NMR | Nuclear Magnetic Resonance |
| GPC | Gel Phase Chromatography |
| PAC | Particle Agglomeration Control |
| PMB | Polymer Modified Bitumen |
| Rg | Radius of gyration |
| RAP | Reclaimed Asphalt Pavement |
| SANS | Small-Angle Neutron Scattering |
| SAXS | Small Angle X-ray Scattering |
| SARA | Saturate, Aromatic, Resin and Asphaltene |
| Tg | Glass Transition Temperature |
| VPO | Vapor Pressure Osmometry |
| WAXS | Wide-Angle X-ray Scattering |
| XRD | X-ray Diffraction |
References
- Speight, J.G. The Chemistry and Technology of Petroleum, 5th ed.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2014. [Google Scholar]
- Speight, J.G. Asphalt Materials Science and Technology; Butterworth-Heinemann: Boston, MA, USA, 2016; Chapters 1 and 4. [Google Scholar]
- ASTM D4124; Standard Test Method for Separation of Asphalt into Four Fractions Annual Book of Standards. ASTM International: West Conshohocken, PA, USA, 2014.
- Moir, M.E. Asphaltenes, What Art Thou? In The Boduszynski Continuum: Contributions to the Understanding of the Molecular Composition of Petroleum; Ovalles, C., Moir, M., Eds.; American Chemical Society (ACS): Washington, DC, USA, 2018; Volume 1282, Chapter 1; pp. 3–24. [Google Scholar] [CrossRef] [Green Version]
- Sedghi, M.; Goual, L. Role of resins on asphaltene stability. Energy Fuels 2010, 24, 2275–2280. [Google Scholar] [CrossRef]
- Fakher, S.; Ahdaya, M.; Elturki, M.; Imqam, A. Critical review of asphaltene properties and factors impacting its stability in crude oil. J. Pet. Explor. Prod. Technol. 2020, 10, 1183–1200. [Google Scholar] [CrossRef] [Green Version]
- ASTM International. ASTM D6560-17; Standard Test Method for Determination of Asphaltenes (Heptane Insolubles) in Crude Petroleum and Petroleum Products. ASTM International: West Conshohocken, PA, USA, 2017. Available online: www.astm.org (accessed on 22 April 2020).
- Speight, J.G. Handbook of Petroleum Product Analysis, 2nd ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2015. [Google Scholar]
- Kokal, S.L.; Sayegh, S.G. Asphaltenes: The cholesterol of petroleum. In Proceedings of the Middle East Oil Show, Society of Petroleum Engineers (SPE), Manama, Bahrain, 11 March 1995; Volume 1, pp. 169–181. [Google Scholar] [CrossRef]
- Kor, P.; Kharrat, R.; Ayoubi, A. Comparison and evaluation of several models in prediction of asphaltene deposition profile along an oil well: A case study. J. Pet. Explor. Prod. Technol. 2017, 7, 497–510. [Google Scholar] [CrossRef] [Green Version]
- Alimohammadi, S.; Zendehboudi, S.; James, L. A comprehensive review of asphaltene deposition in petroleum reservoirs: Theory, challenges, and tips. Fuel 2019, 252, 753–791. [Google Scholar] [CrossRef]
- Pauli, A.T.; Branthaver, J.F. Relationship between asphaltenes, Heithaus compatibility parameters, and asphalt viscosity. Pet. Sci. Technol. 1998, 16, 1125–1147. [Google Scholar] [CrossRef]
- Ashoori, S.; Sharifi, M.; Masoumi, M.; Mohammad Salehi, M. The relationship between SARA fractions and crude oil stability. Egypt. J. Pet. 2017, 26, 209–213. [Google Scholar] [CrossRef] [Green Version]
- Madeira, N.C.L.; Rainha, K.P.; Mendonca, J.; Lacerda, V.; Chinelatto, L.S.; de Menezes, S.M.C.; Porto, C.F.; Pinto, F.E.; Filgueiras, P.R.; Romao, W.; et al. Study of the influence of resins on the asphaltene aggregates by 1H DOSY NMR. Energy Fuels 2020, 34, 5679–5688. [Google Scholar] [CrossRef]
- Giavarini, C.; Mastrofini, D.; Scarsella, M.; Barré, L.; Espinat, D. Macrostructure and rheological properties of chemically modified residues and bitumens. Energy Fuels 2000, 14, 495–502. [Google Scholar] [CrossRef]
- Petersen, J.C.; Glaser, R. Asphalt oxidation mechanisms and the role of oxidation products on age hardening revisited. Road Mater. Pavement Des. 2011, 12, 795–819. [Google Scholar] [CrossRef]
- Oliviero Rossi, C.; Ashimova, S.; Calandra, P.; De Santo, M.P.; Angelico, R. Mechanical resilience of modified bitumen at different cooling rates: A rheological and atomic force microscopy investigation. Appl. Sci. 2017, 5, 779. [Google Scholar] [CrossRef] [Green Version]
- Oliviero Rossi, C.; Caputo, P.; Loise, V.; Miriello, D.; Teltayev, B.; Angelico, R. Role of a food grade additive in the high temperature performance of modified bitumens. Colloid Surf. A 2017, 532, 618–624. [Google Scholar] [CrossRef]
- Oliviero Rossi, C.; Caputo, P.; Ashimova, S.; Fabozzi, A.; D’Errico, G.; Angelico, R. Effects of natural antioxidant agents on the bitumen aging process: An EPR and rheological investigation. Appl. Sci. 2018, 8, 1405. [Google Scholar] [CrossRef] [Green Version]
- Werner, A.; Behar, F.; de Hemptinne, J.C.; Behar, E. Viscosity and phase behaviour of petroleum fluids with high asphaltene contents. Fluid Phase Equilib. 1998, 147, 343–356. [Google Scholar] [CrossRef]
- Meyer, V.; Pilliez, J.; Habas, J.-P.; Montel, F.; Creux, P. Rheological evidence of the diffusional aggregation of asphaltenes in extra-heavy crude oils. Energy Fuels 2008, 22, 3154–3159. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Chaudhuri, P.; Kumar, B.; Panja, S.S. Review on aggregation of asphaltene vis-a-vis spectroscopic studies. Fuel 2016, 185, 541–554. [Google Scholar] [CrossRef]
- Zhang, M.; Hao, P.; Dong, S.; Li, Y.; Yuan, G. Asphalt binder micro-characterization and testing approaches: A review. Measurement 2020, 151, 107255–107269. [Google Scholar] [CrossRef]
- Nellensteyn, F.J. The constitution of asphalt. J. Inst. Pet. Technol. 1924, 10, 311–325. [Google Scholar]
- Pfeiffer, J.P.; Saal, R.N.J. Asphaltic bitumen as colloid system. J. Phys. Chem. 1940, 44, 139–149. [Google Scholar] [CrossRef]
- Pfeiffer, J.P. The Properties of Asphaltic Bitumen; Elsevier Publishing Company, Inc.: New York, NY, USA, 1950. [Google Scholar]
- Traxler, R.N.; Romberg, J.W. Asphalt, a colloidal material. Ind. Eng. Chem. 1952, 44, 155–158. [Google Scholar] [CrossRef]
- Kawanaka, S.; Leontaritis, K.J.; Park, S.J.; Mansoori, G.A. Thermodynamic and colloidal models of asphaltene flocculation. In Oil-Field Chemistry; Borchardt, J., Ed.; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1989; pp. 443–458. [Google Scholar] [CrossRef]
- Storm, D.A.; Barresi, R.J.; De Canio, S.J. Colloidal nature of vacuum residue. Fuel 1991, 70, 779–782. [Google Scholar] [CrossRef]
- Lian, H.; Lin, J.-R.; Yen, T.F. Peptization studies of asphaltene and solubility parameter spectra. Fuel 1994, 73, 423–428. [Google Scholar] [CrossRef]
- Yen, T.F.; Erdman, J.G.; Pollack, S.S. Investigation of the structure of petroleum asphaltenes by X-ray Diffraction. Anal. Chem. 1961, 33, 1587–1594. [Google Scholar] [CrossRef]
- Yen, T.F. Structure of petroleum asphaltene and its significance. Energy Sources 1974, 1, 447–463. [Google Scholar] [CrossRef]
- Dickie, J.P.; Yen, T.F. Macrostructures of the asphaltic fractions by various instrumental methods. Anal. Chem. 1967, 39, 1847–1852. [Google Scholar] [CrossRef]
- Mullins, O.C. The modified Yen model. Energy Fuels 2010, 24, 2179–2207. [Google Scholar] [CrossRef]
- Altegelt, K.H.; Harle, O.L. The effect of asphaltenes on asphalt viscosity. Ind. Eng. Chem. Prod. Res. Dev. 1975, 14, 240–246. [Google Scholar] [CrossRef]
- Koots, J.A.; Speight, J.G. The relation of petroleum resins to asphaltene constituents. Fuel 1975, 54, 179–184. [Google Scholar] [CrossRef]
- Petersen, J.C. Chemical composition of asphalt as related to asphalt durability: State of the art. Transport. Res. Rec. 1984, 999, 13–30. [Google Scholar]
- Christensen, D.W.; Anderson, D.A. Interpretation of dynamic mechanical test data for paving grade asphalt cements. J. Assoc. Asph. Paving Technol. 1992, 61, 67–116. [Google Scholar]
- Anderson, D.A.; Christensen, D.W.; Bahia, H. Physical properties of asphalt cement and the development of performance-related specifications. J. Assoc. Asph. Paving Technol. 1991, 60, 437–532. [Google Scholar]
- Redelius, P.G. Solubility parameters and bitumen. Fuel 2000, 79, 27–35. [Google Scholar] [CrossRef]
- Lesueur, D. The colloidal structure of bitumen: Consequences on the rheology and on the mechanisms of bitumen modification. Adv. Colloid Interface Sci. 2009, 145, 42–82. [Google Scholar] [CrossRef] [PubMed]
- Rogel, E. Asphaltene aggregation: A molecular thermodynamic approach. Langmuir 2002, 18, 1928–1937. [Google Scholar] [CrossRef]
- Acevedo, S.; Escobar, G.; Ranaudo, M.A.; Pinate, J.; Amorın, A.; Diaz, M.; Silva, P. Observations about the structure and dispersion of petroleum asphaltenes aggregates obtained from dialysis fractionation and characterization. Energy Fuels 1997, 11, 774–778. [Google Scholar] [CrossRef]
- Acevedo, S.; Castro, A.; Negrin, J.G.; Fernandez, A.; Escobar, G.; Piscitelli, V.; Delolme, F.; Dessalces, G. Relations between asphaltene structures and their physical and chemical properties: The rosary-type structure. Energy Fuels 2007, 21, 2165–2175. [Google Scholar] [CrossRef]
- Acevedo, S.; Zuloaga, C.; Rodríguez, P. Aggregation-dissociation studies of asphaltene solutions in resins performed using the combined freeze fracture-transmission electron microscopy technique. Energy Fuels 2008, 22, 2332–2340. [Google Scholar] [CrossRef]
- Wiehe, I.A.; Kennedy, R.J. The oil compatibility model and crude oil incompatibility. Energy Fuels 2000, 14, 56–59. [Google Scholar] [CrossRef]
- Yen, T.F. The colloidal aspect of a macrostructure of petroleum asphalt. Fuel Sci. Technol. Int. 1992, 10, 723–733. [Google Scholar] [CrossRef]
- Mullins, O.C. Review of the molecular structure and aggregation of asphaltenes and petroleomics. SPE J. 2008, 13, 48–57. [Google Scholar] [CrossRef]
- Mullins, O.C.; Sabbah, H.; Eyssautier, J.; Pomerantz, A.E.; Barré, L.; Andrews, A.B.; Ruiz-Morales, Y.; Mostowfi, F.; Mc Farlane, R.; Goual, L.; et al. Advances in asphaltene science and the Yen-Mullins model. Energy Fuels 2012, 26, 3986–4003. [Google Scholar] [CrossRef]
- Mullins, O.C.; Seifert, D.J.; Zuo, J.Y.; Zeybek, M. Clusters of asphaltene nanoaggregates observed in oilfield reservoirs. Energy Fuels 2013, 27, 1752–1761. [Google Scholar] [CrossRef]
- Li, S.; Liu, C.; Que, G.; Liang, W.; Zhu, Y. A study of the interactions responsible for colloidal structures in petroleum residua. Fuel 1997, 76, 1459–1463. [Google Scholar] [CrossRef]
- Liu, D.; Kong, X.; Li, M.; Wang, Z. Study on the aggregation of residue-derived asphaltene molecules. Energy Fuels 2010, 24, 3624–3627. [Google Scholar] [CrossRef]
- Wang, J.X.; Buckley, J.S. A two-component solubility model of the onset of asphaltene flocculation in crude oils. Energy Fuels 2001, 15, 1004–1012. [Google Scholar] [CrossRef]
- Agrawala, M.; Yarranton, H.W. An asphaltene association model analogous to linear polymerization. Ind. Eng. Chem. Res. 2001, 40, 4664–4672. [Google Scholar] [CrossRef]
- Yarranton, H.W.; Ortiz, D.P.; Barrera, D.M.; Baydak, E.N.; Barré, L.; Frot, D.; Eyssautier, J.; Zeng, H.; Xu, Z.; Dechaine, G.; et al. On the size distribution of self-associated asphaltenes. Energy Fuels 2013, 27, 5083–5106. [Google Scholar] [CrossRef]
- Gray, M.R.; Tykwinski, R.R.; Stryker, J.M.; Tan, X. Supramolecular assembly model for aggregation of petroleum asphaltenes. Energy Fuels 2011, 25, 3125–3134. [Google Scholar] [CrossRef]
- Speight, J.G. Chemical and physical studies of petroleum asphaltenes. In Asphaltenes and Asphalts. I. Developments in Petroleum Science; Yen, T.F., Chilingarian, G.V., Eds.; Elsevier: Amsterdam, The Netherlands, 1994; Volume 40, Chapter 2. [Google Scholar]
- Speight, J.G. Asphaltenes and the Structure of Petroleum Petroleum Chemistry and Refining; Taylor & Francis Publishers: Philadelphia, PA, USA, 1998; Chapter 4; pp. 103–120. [Google Scholar]
- Lesueur, D.; Gérard, J.F.; Claudy, P.; Létoffé, J.M.; Planche, J.P.; Martin, D. A structure related model to describe asphalt linear viscoelasticity. J. Rheol. 1996, 40, 813–836. [Google Scholar] [CrossRef]
- León, O.; Rogel, E.; Espidel, J.; Torres, G. Asphaltenes: Structural characterization, self-association, and stability behavior. Energy Fuels 2000, 14, 6–10. [Google Scholar] [CrossRef]
- Evdokimov, I.N.; Eliseev, N.Y.; Akhmetov, B.R. Assembly of asphaltene molecular aggregates as studied by near-UV/visible spectroscopy II. Concentration dependencies of absorptivities. J. Petrol. Sci. Eng. 2003, 37, 145–152. [Google Scholar] [CrossRef]
- Evdokimov, I.N.; Eliseev, N.Y.; Akhmetov, B.R. Asphaltene dispersions in dilute oil solutions. Fuel 2006, 85, 1465–1472. [Google Scholar] [CrossRef]
- Gutierrez, L.B.; Ranaudo, M.A.; Mendez, B.; Acevedo, S. Fractionation of asphaltene by complex formation with p-nitrophenol. A method for structural studies and stability of asphaltene colloids. Energy Fuels 2001, 15, 624–628. [Google Scholar] [CrossRef]
- Castillo, J.; Fernandez, A.; Ranaudo, M.A.; Acevedo, S. New techniques and methods for the study of aggregation, adsorption, and solubility of asphaltenes. Impact of these properties on colloidal structure and flocculation. Pet. Sci. Technol. 2001, 19, 75–106. [Google Scholar] [CrossRef]
- Kleizienė, R.; Panasenkienė, M.; Vaitkus, A. Effect of aging on chemical composition and rheological properties of neat and modified bitumen. Materials 2019, 12, 4066. [Google Scholar] [CrossRef] [Green Version]
- Branco, V.A.M.; Mansoori, G.A.; De Almeida Xavier, L.C.; Park, S.J.; Manafi, H. Asphaltene flocculation and collapse from petroleum fluids. J. Petrol. Sci. Eng. 2001, 32, 217–230. [Google Scholar] [CrossRef]
- Dutta Majumdar, R.; Gerken, M.; Mikula, R.; Hazendonk, P. Validation of the Yen-Mullins model of Athabasca oil-sands asphaltenes using solution-state 1H NMR relaxation and 2D HSQC spectroscopy. Energy Fuels 2013, 27, 6528–6537. [Google Scholar] [CrossRef]
- Rassamdana, H.; Dabir, B.; Nematy, M.; Farhani, M.; Sahimi, M. Asphalt flocculation and deposition: I. The onset of precipitation. AIChE J. 1996, 42, 10–22. [Google Scholar] [CrossRef]
- Ekulu, G.; Nicolas, C.; Achard, C.; Rogalski, M. Characterization of aggregation processes in crude oils using differential scanning calorimetry. Energy Fuels 2005, 19, 1297–1302. [Google Scholar] [CrossRef]
- Savvidis, T.G.; Fenistein, D.; Barré, L.; Béhar, E. Aggregated structure of flocculated asphaltenes. AIChE J. 2001, 47, 206–211. [Google Scholar] [CrossRef]
- Strausz, O.P.; Peng, P.; Murgich, J. About the colloidal nature of asphaltenes and the MW of covalent monomeric units. Energy Fuels 2002, 16, 809–822. [Google Scholar] [CrossRef]
- Moschopedis, S.E.; Speight, J.G. Investigation of hydrogen bonding by oxygen functions in Athabasca bitumen. Fuel 1976, 55, 187–192. [Google Scholar] [CrossRef]
- Sharma, A.; Groenzin, H.; Tomita, A.; Mullins, O.C. Probing order in asphaltenes and aromatic ring systems by HRTEM. Energy Fuels 2002, 16, 490–496. [Google Scholar] [CrossRef]
- Creek, J.L. Freedom of action in the state of asphaltenes: Escape from conventional wisdom. Energy Fuels 2005, 19, 1212–1224. [Google Scholar] [CrossRef]
- Struik, L.C.E. Physical Aging in Amorphous Polymers and Other Materials; Elsevier Scientific Publishing Company: Amsterdam, The Netherlands, 1978. [Google Scholar]
- Bahia, H.; Anderson, D.A. Glass transition behavior and physical hardening of asphalt binders. J. Assoc. Asph. Paving Technol. 1993, 62, 93–129. [Google Scholar]
- Anderson, D.A.; Marasteanu, M.O. Physical hardening of asphalt binders relative to their glass transition temperatures. Transp. Res. Rec. 1999, 1661, 27–34. [Google Scholar] [CrossRef]
- Masson, J.-F.; Polomark, G.M.; Collins, P. Time-dependent microstructure of bitumen and its fractions by modulated differential scanning calorimetry. Energy Fuels 2002, 16, 470–476. [Google Scholar] [CrossRef] [Green Version]
- Hesp, S.; Iliuta, S.; Shirokoff, J.W. Reversible aging in asphalt binders. Energy Fuels 2007, 21, 1112–1121. [Google Scholar] [CrossRef] [Green Version]
- Schmets, A.; Kringos, N.; Pauli, T.; Redelius, P.; Scarpas, T. On the existence of wax-induced phase separation in bitumen. Int. J. Pavement Eng. 2010, 11, 555–563. [Google Scholar] [CrossRef]
- Tabatabaee, H.A.; Velasquez, R.; Bahia, H.U. Predicting low temperature physical hardening in asphalt binders. Constr. Build. Mater. 2012, 34, 162–169. [Google Scholar] [CrossRef]
- Fischer, H.R.; Dillingh, B.; Ingenhut, B. Fast solidification kinetics of parts of bituminous binders. Mater. Struct. 2016, 49, 3335–3340. [Google Scholar] [CrossRef] [Green Version]
- Laukkanen, O.-V.; Winter, H.H.; Soenen, H.; Seppälä, J. An empirical constitutive model for complex glass-forming liquids using bitumen as a model material. Rheol. Acta 2018, 57, 57–70. [Google Scholar] [CrossRef] [Green Version]
- Ding, H.; Qiu, Y.; Rahman, A. Low-temperature reversible aging properties of selected asphalt binders based on thermal analysis. J. Mater. Civ. Eng. 2019, 31, 04018402–04018408. [Google Scholar] [CrossRef]
- Ding, H.; Zhang, H.; Liu, H.; Qiu, Y. Thermoreversible aging in model asphalt binders. Constr. Build. Mater. 2021, 303, 124355–124363. [Google Scholar] [CrossRef]
- Berkowitz, M.; Filipovich, M.; Baldi, A.; Hesp, S.A.M.; Aguiar-Moya, J.P.; Lorıá-Salazar, L.G. Oxidative and thermoreversible aging effects on performance-based rheological properties of six Latin American asphalt binders. Energy Fuels 2019, 33, 2604–2613. [Google Scholar] [CrossRef]
- Sheu, E.Y.; De Tar, M.M.; Storm, D.A. Dielectric properties of asphaltene solutions. Fuel 1994, 73, 45–50. [Google Scholar] [CrossRef]
- Taylor, S.E. Use of surface tension measurements to evaluate aggregation of asphaltenes in organic solvents. Fuel 1992, 71, 1338–1339. [Google Scholar] [CrossRef]
- Merino-Garcia, D.; Andersen, S.I. Calorimetric evidence about the application of the concept of CMC to asphaltene self-association. J. Dispers. Sci. Technol. 2005, 26, 217–225. [Google Scholar] [CrossRef]
- Merino-Garcia, D.; Murgich, J.; Andersen, S.I. Asphaltene self-association: Modeling and effect of fractionation with a polar solvent. Pet. Sci. Technol. 2004, 22, 735–758. [Google Scholar] [CrossRef]
- Juyal, P.; Merino-Garcia, D.; Andersen, S.I. Effect on molecular interactions of chemical alteration of petroleum asphaltenes. I. Energy Fuels 2005, 19, 1272–1281. [Google Scholar] [CrossRef]
- Victorov, A.I.; Firoozabadi, A. Thermodynamic micellization model of asphaltene precipitation from petroleum fluids. AIChE J. 1996, 42, 1753–1764. [Google Scholar] [CrossRef]
- Priyanto, S.; Mansoori, G.A.; Suwono, A. Measurement of property relationships of nano-structure micelles and coacervates of asphaltene in a pure solvent. Chem. Eng. Sci. 2001, 56, 6933–6939. [Google Scholar] [CrossRef] [Green Version]
- Mansoori, G.A. Modeling of asphaltene and other heavy organic depositions. J. Pet. Sci. Eng. 1997, 17, 101–111. [Google Scholar] [CrossRef]
- Yudin, I.K.; Nikolaenko, G.L.; Gorodetskii, E.E.; Markhashov, E.L.; Agayan, V.A.; Anisimov, M.A.; Sengers, J.V. Crossover kinetics of asphaltene aggregation in hydrocarbon solutions. Phys. A Stat. Theor. Phys. 1998, 251, 235–244. [Google Scholar] [CrossRef]
- Yudin, I.K.; Nikolaenko, G.L.; Gorodetskii, E.E.; Markhashov, E.L.; Frot, D.; Briolant, Y.; Agayan, V.A.; Anisimov, M.A. Universal behavior of asphaltene aggregation in hydrocarbon solutions. Pet. Sci. Technol. 1998, 16, 395–414. [Google Scholar] [CrossRef]
- Andersen, S.I.; Birdi, K.S. Aggregation of asphaltenes as determined by calorimetry. J. Colloid Interface Sci. 1991, 142, 497–502. [Google Scholar] [CrossRef]
- Andersen, S.I.; Christensen, S.D. The critical micelle concentration of asphaltenes as measured by calorimetry. Energy Fuels 2000, 14, 38–42. [Google Scholar] [CrossRef]
- Sheu, E.Y.; De Tar, M.M.; Storm, D.A. Surface activity and dynamics of asphaltenes. In Asphaltene Particles in Fossil Fuel Exploration, Recovery, Refining and Production Processes; Sharma, M.K., Yen, T.F., Eds.; Plenum Press: New York, NY, USA, 1994. [Google Scholar]
- Sheu, E.Y. Physics of asphaltene micelles and microemulsions-theory and experiment. J. Phys. Condens. Matter. 1996, 8, A125–A141. [Google Scholar] [CrossRef]
- da Silva Ramos, A.C.; Haraguchi, L.; Notrispe, F.R.; Loh, W.; Mohamed, R.S. Interfacial and colloidal behavior of asphaltenes obtained from Brazilian crude oils. J. Pet. Sci. Eng. 2001, 32, 201–216. [Google Scholar] [CrossRef]
- Bouhadda, Y.; Bendedouch, D.; Sheu, E.; Krallafa, A. Some preliminary results on a physico-chemical characterization of a Hassi Messaoud petroleum asphaltene. Energy Fuels 2000, 14, 845–853. [Google Scholar] [CrossRef]
- Rogel, E.; León, O.; Torres, G.; Espidel, J. Aggregation of asphaltenes in organic solvents using surface tension measurements. Fuel 2000, 79, 1389–1394. [Google Scholar] [CrossRef]
- Larbi, A.; Daaou, M.; Faraoun, A. Investigation of structural parameters and self-aggregation of Algerian asphaltenes in organic solvents. Pet. Sci. 2015, 12, 509–517. [Google Scholar] [CrossRef] [Green Version]
- Yarranton, H.W.; Alboudwarej, H.; Jakher, R. Investigation of asphaltene association with vapor pressure osmometry and interfacial tension measurements. Ind. Eng. Chem. Res. 2000, 39, 2916–2924. [Google Scholar] [CrossRef]
- Buenrostro-Gonzalez, E.; Andersen, S.I.; Garcia-Martinez, J.A.; Lira-Galeana, C. Solubility/molecular structure relationships of asphaltenes in polar and nonpolar media. Energy Fuels 2002, 16, 732–741. [Google Scholar] [CrossRef]
- Zhang, Y.; Takanohashi, T.; Sato, S.; Saito, I.; Tanaka, R. Observation of stepwise association of petroleum-derived asphaltene and maltene components by surface tension measurements. J. Jpn. Petrol. Inst. 2004, 47, 32–36. [Google Scholar] [CrossRef] [Green Version]
- Friberg, S.E. Micellization. In Asphaltenes, Heavy Oils and Petroleomics; Mullins, O.C., Sheu, E.Y., Hammami, A., Marshall, A.G., Eds.; Springer: New York, NY, USA, 2007; Chapter 7. [Google Scholar]
- Andreatta, G.; Bostrom, N.; Mullins, O.C. High-Q ultrasonic determination of the critical nanoaggregate concentration of asphaltenes and the critical micelle concentration of standard surfactants. Langmuir 2005, 21, 2728–2736. [Google Scholar] [CrossRef]
- Andreatta, G.; Goncalves, C.C.; Buffin, G.; Bostrom, N.; Quintella, C.M.; Arteaga-Larios, F.; Peréz, E.; Mullins, O.C. Nanoaggregates and structure-function relations in asphaltenes. Energy Fuels 2005, 19, 1282–1289. [Google Scholar] [CrossRef]
- Mostowfi, F.; Indo, K.; Mullins, O.C.; Mcfarlane, R. Asphaltene nanoaggregates studied by centrifugation. Energy Fuels 2009, 23, 1194–1200. [Google Scholar] [CrossRef]
- Zeng, H.; Song, Y.-Q.; Johnson, D.L.; Mullins, O.C. Critical nanoaggregate concentration of asphaltenes by direct-current (DC) electrical conductivity. Energy Fuels 2009, 23, 1201–1208. [Google Scholar] [CrossRef]
- Goual, L.; Sedghi, M.; Zeng, H.; Mostowfi, F.; McFarlane, R.; Mullins, O.C. On the formation and properties of asphaltene nanoaggregates and clusters by DC-conductivity and centrifugation. Fuel 2011, 90, 2480–2490. [Google Scholar] [CrossRef]
- Sheu, E.Y.; Long, Y.; Hamza, H. Asphaltene self-association and precipitation in solvents, AC conductivity measurements. In Asphaltenes, Heavy Oils and Petroleomics; Mullins, O.C., Sheu, E.Y., Hammami, A., Marshall, A.G., Eds.; Springer: New York, NY, USA, 2007; Chapter 10. [Google Scholar]
- Castillo, J.; Gutierrez, H.; Ranaudo, M.; Villarroel, O. Measurement of the refractive index of crude oil and asphaltene solutions: Onset flocculation determination. Energy Fuels 2010, 24, 492–495. [Google Scholar] [CrossRef]
- Goual, L.; Sedghi, M.; Mostowfi, F.; McFarlane, R.; Pomerantz, A.E.; Saraji, S.; Mullins, O.C. Cluster of asphaltene nanoaggregates by DC conductivity and centrifugation. Energy Fuels 2014, 28, 5002–5013. [Google Scholar] [CrossRef]
- Zielinski, L.; Saha, I.; Freed, D.E.; Hürlimann, M.D.; Liu, Y. Probing asphaltene aggregation in native crude oils with low-field NMR. Langmuir 2010, 26, 5014–5021. [Google Scholar] [CrossRef]
- Goncalves, S.; Castillo, J.; Fernández, A.; Hung, J. Absorbance and fluorescence spectroscopy on the aggregation behavior of asphaltene-toluene solutions. Fuel 2004, 83, 1823–1828. [Google Scholar] [CrossRef]
- Zhao, B.; Shaw, J.M. Composition and size distribution of coherent nanostructures in Athabasca bitumen and Maya crude oil. Energy Fuels 2007, 21, 2795–2804. [Google Scholar] [CrossRef]
- Marques, J.; Merdrignac, I.; Baudot, A.; Barré, L.; Guillaume, D.; Espinat, D.; Brunet, S. Asphaltenes size polydispersity reduction by nano- and ultrafiltration separation methods e comparison with the flocculation method. Oil Gas Sci. Technol. Rev. IFP 2008, 63, 139–149. [Google Scholar] [CrossRef]
- Lisitza, N.V.; Freed, D.E.; Sen, P.N.; Song, Y.Q. Study of asphaltene nanoaggregation by nuclear magnetic resonance (NMR). Energy Fuels 2009, 23, 1189–1193. [Google Scholar] [CrossRef]
- Eyssautier, J.; Levitz, P.; Espinat, D.; Jestin, J.; Gummel, J.; Grillo, I.; Barré, L. Insight into asphaltene nanoaggregate structure inferred by small angle neutron and X-ray scattering. J. Phys. Chem. B 2011, 115, 6827–6837. [Google Scholar] [CrossRef]
- Acevedo, S.; García, L.A.; Rodríguez, P. Changes of diameter distribution with temperature measured for asphaltenes and their fractions A1 and A2. Impact of these measurements in colloidal and solubility issues of asphaltenes. Energy Fuels 2012, 26, 1814–1819. [Google Scholar] [CrossRef]
- Mullins, O.C.; Betancourt, S.S.; Cribbs, M.E.; Dubost, F.X.; Creek, J.L.; Andrews, A.B.; Venkataramanan, L. The colloidal structure of crude oil and the structure of oil reservoirs. Energy Fuels 2007, 21, 2785–2794. [Google Scholar] [CrossRef]
- Seidl, P.R.; Chrisman, E.C.A.N.; Carvalho, C.C.V.; Leal, K.Z.; de Menezes, S.M.C. NMR analysis of asphaltenes separated from vacuum residues by selected solvents. J. Dispers. Sci. Technol. 2004, 25, 349–353. [Google Scholar] [CrossRef]
- Carbognani, L.; Rogel, E. Solid petroleum asphaltenes seem surrounded by alkyl layers. Pet. Sci. Technol. 2003, 21, 537–556. [Google Scholar] [CrossRef]
- Murgich, J. Intermolecular forces in aggregates of asphaltenes and resins. Pet. Sci. Technol. 2002, 20, 983–997. [Google Scholar] [CrossRef]
- Murgich, J.; Abanero, J.A.; Strausz, O.P. Molecular recognition in aggregates formed by asphaltene and resin molecules from the athabasca oil sand. Energy Fuels 1999, 13, 278–286. [Google Scholar] [CrossRef]
- Rogel, E. Thermodynamic modeling of asphaltene aggregation. Langmuir 2004, 20, 1003–1012. [Google Scholar] [CrossRef]
- Eyssautier, J.; Espinat, D.; Gummel, J.; Levitz, P.; Becerra, M.; Shaw, J.; Barré, L. Mesoscale organization in a physically separated vacuum residue: Comparison to asphaltenes in a simple solvent. Energy Fuels 2012, 26, 2680–2687. [Google Scholar] [CrossRef]
- Bazyleva, A.; Hasan, M.A.; Fulem, M.; Becerra, M.; Shaw, J.M. Bitumen and heavy oil rheological properties: Reconciliation with viscosity measurements. J. Chem. Eng. Data 2010, 55, 1389–1397. [Google Scholar] [CrossRef]
- Loeber, L.; Sutton, O.; Morel, J.; Valleton, J.-M.; Muller, J. New direct observations of asphalts and asphalt binders by scanning electron microscopy and atomic force microscopy. J. Microsc. 1996, 182, 32–39. [Google Scholar] [CrossRef]
- Allen, R.G.; Little, D.N.; Bhasin, A. Structural characterization of micromechanical properties in asphalt using atomic force microscopy. J. Mater. Civil Eng. 2012, 24, 1317–1327. [Google Scholar] [CrossRef]
- Jahangir, R.; Little, D.; Bhasin, A. Evolution of asphalt binder microstructure due to tensile loading determined using AFM and image analysis techniques. Int. J. Pavement Eng. 2015, 16, 337–349. [Google Scholar] [CrossRef]
- Masson, J.-F.; Leblond, V.; Margeson, J.; Bundalo-Perc, S. Low temperature bitumen stiffness and viscous paraffinic nano and micro domains by cryogenic AFM and PDM. J. Microsc. 2007, 227, 191–202. [Google Scholar] [CrossRef]
- Pauli, T.; Grimes, W.; Beiswenger, J.; Schmets, A. Surface structuring of wax in complex media. J. Mater. Civ. Eng. 2015, 27, C4014001. [Google Scholar] [CrossRef]
- Sheu, E.Y. Small angle scattering and asphaltenes. J. Phys. Condens. Matter 2006, 18, S2485–S2498. [Google Scholar] [CrossRef] [Green Version]
- Barré, L.; Jestin, J.; Morisset, A.; Palermo, T.; Simon, S. Relation between nanoscale structure of asphaltene aggregates and their macroscopic solution properties. Oil Gas Sci. Technol. 2009, 64, 617–628. [Google Scholar] [CrossRef]
- Redelius, P.G. The structure of asphaltenes in bitumen. Road Mater. Pavement Des. 2006, 7 (Suppl. S1), 143–162. [Google Scholar] [CrossRef]
- Edgar, G. Measure, Topology, and Fractal Geometry; Springer Science+Business Media, LLC: Berlin, Germany, 2008. [Google Scholar]
- Liu, Y.C.; Sheu, E.Y.; Chen, S.H.; Storm, D.A. Fractal structure of asphaltenes in toluene. Fuel 1995, 74, 1352–1356. [Google Scholar] [CrossRef]
- Barré, L.; Espinat, D.; Rosenberg, E.; Scarsella, M. Colloidal structure of heavy crudes and asphaltene solutions. Oil Gas Sci. Technol. Rev. IFP 1997, 52, 161–175. [Google Scholar] [CrossRef] [Green Version]
- Anisimov, M.A.; Yudin, I.K.; Nikitin, V.; Nikolaenko, G.; Chernoutsan, A.; Toulhoat, H.; Frot, D.; Briolant, Y. Asphaltene aggregation in hydrocarbon solutions studied by photon correlation spectroscopy. J. Phys. Chem. 1995, 99, 9576–9580. [Google Scholar] [CrossRef]
- Acevedo, S.; Escobar, G.; Ranaudo, M.A.; Gutierrez, L.B. Discotic shape of asphaltenes obtained from g.p.c. data. Fuel 1994, 73, 1807–1809. [Google Scholar] [CrossRef]
- Fenistein, D.; Barré, L.; Brosseta, D.; Espinat, D.; Livet, A.; Roux, J.-N.; Scarsella, M. Viscosimetric and neutron scattering study of asphaltene aggregates in mixed toluene/heptane solvents. Langmuir 1998, 14, 1013–1020. [Google Scholar] [CrossRef]
- Roux, J.-N.; Broseta, D.; Demé, B. SANS Study of asphaltene aggregation: Concentration and solvent quality effects. Langmuir 2001, 17, 5085–5092. [Google Scholar] [CrossRef]
- Gmachowski, L.; Paczuski, M. Fractal dimension of asphaltene aggregates determined by turbidity. Colloid Surf. A 2011, 384, 461–465. [Google Scholar] [CrossRef]
- Eyssautier, J.; Frot, D.; Barré, L. Structure and dynamic properties of colloidal asphaltene aggregates. Langmuir 2012, 28, 11997–12004. [Google Scholar] [CrossRef]
- Porte, G.; Zhou, H.; Lazzeri, V. Reversible description of asphaltene colloidal association and precipitation. Langmuir 2003, 19, 40–47. [Google Scholar] [CrossRef]
- Gawrys, K.L.; Spiecker, P.M.; Kilpatrick, P.K. The role of asphaltene solubility and chemical composition on asphaltene aggregation. Pet. Sci. Technol. 2003, 21, 461–489. [Google Scholar] [CrossRef]
- Alvarez-Ramírez, F.; Ruiz-Morales, Y. Island versus archipelago architecture for asphaltenes: Polycyclic Aromatic Hydrocarbon Dimer theoretical studies. Energy Fuels 2013, 27, 1791–1808. [Google Scholar] [CrossRef]
- Schuler, B.; Zhang, Y.; Liu, F.; Pomerantz, A.E.; Ballard Andrews, A.; Gross, L.; Pauchard, V.; Banerjee, S.; Mullins, O.C. Overview of asphaltene nanostructures and thermodynamic applications. Energy Fuels 2020, 34, 15082–15105. [Google Scholar] [CrossRef]
- Schuler, B.; Meyer, G.; Pena, D.; Mullins, O.C.; Gross, L. Unraveling the molecular structures of asphaltenes by atomic force microscopy. J. Am. Chem. Soc. 2015, 137, 9870–9876. [Google Scholar] [CrossRef]
- Schuler, B.; Fatayer, S.; Meyer, G.; Rogel, E.; Moir, M.; Zhang, Y.; Harper, M.R.; Pomerantz, A.E.; Bake, K.D.; Witt, M.; et al. Heavy oil based mixtures of different origins and treatments studied by atomic force microscopy. Energy Fuels 2017, 31, 6856–6861. [Google Scholar] [CrossRef]
- Gawrys, K.L.; Blankenship, G.A.; Kilpatrick, P.K. Solvent entrainment in and flocculation of asphaltenic aggregates probed by small-angle neutron scattering. Langmuir 2006, 22, 4487–4497. [Google Scholar] [CrossRef]
- Thiyagarajan, P.; Hunt, J.E.; Winans, R.E.; Anderson, K.B.; Miller, J.T. Temperature-dependent structural changes of asphaltenes in 1-Methylnaphthalene. Energy Fuels 1995, 9, 829–833. [Google Scholar] [CrossRef]
- Fenistein, D.; Barré, L. Experimental measurement of the mass distribution of petroleum asphaltene aggregates using ultracentrifugation and small-angle X-ray scattering. Fuel 2001, 80, 283–287. [Google Scholar] [CrossRef]
- Gawrys, K.L.; Kilpatrick, P.K. Asphaltenic aggregates are polydisperse oblate cylinders. J. Colloid Interf. Sci. 2005, 288, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Barré, L.; Simon, S.; Palermo, T. Solution properties of asphaltenes. Langmuir 2008, 24, 3709–3717. [Google Scholar] [CrossRef] [PubMed]
- Sheu, E.Y. Petroleum asphaltene-properties, characterization, and issues. Energy Fuels 2002, 16, 74–82. [Google Scholar] [CrossRef]
- Hoepfner, M.P.; Fogler, H.S. Multiscale scattering investigations of asphaltene cluster breakup, nanoaggregate dissociation, and molecular ordering. Langmuir 2013, 29, 15423–15432. [Google Scholar] [CrossRef] [PubMed]
- Haji-Akbari, N.; Masirisuk, P.; Hoepfner, M.P.; Fogler, H.S. A unified model for aggregation of asphaltenes. Energy Fuels 2013, 27, 2497–2505. [Google Scholar] [CrossRef]
- Mason, T.G.; Liu, M.Y. Asphaltene nanoparticle aggregation in mixtures of incompatible crude oils. Phys. Rev. E 2003, 67, 050401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mason, T.G.; Liu, M.Y. Time-resolved small angle neutron scattering measurements of asphaltene nanoparticle aggregation kinetics in incompatible crude oil mixtures. J. Chem. Phys. 2003, 119, 565–571. [Google Scholar] [CrossRef] [Green Version]
- Headen, T.F.; Boek, E.S.; Stellbrink, J.; Scheven, U.M. Small angle neutron scattering (SANS and V-SANS) study of asphaltene aggregates in crude oil. Langmuir 2009, 25, 422–428. [Google Scholar] [CrossRef]
- Eyssautier, J.; Hénaut, I.; Levitz, P.; Espinat, D.; Barré, L. Organization of asphaltenes in a vacuum residue: A small-angle X-ray scattering (SAXS)—viscosity approach at high temperatures. Energy Fuels 2012, 26, 2696–2704. [Google Scholar] [CrossRef]
- Hoepfner, M.P.; Vilas Bôas Fávero, C.; Haji-Akbari, N.; Fogler, H.S. The fractal aggregation of asphaltenes. Langmuir 2013, 29, 8799–8808. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, R.; Sato, E.; Hunt, J.; Winans, R.; Sato, S.; Takanohashi, T. Characterization of asphaltene aggregates using X-ray diffraction and small-angle X-ray scattering. Energy Fuels 2004, 18, 1118–1125. [Google Scholar] [CrossRef]
- Tuzikov, F.V.; Larichev, Y.V.; Borisova, L.S.; Kozhevnikov, I.V.; Mart’yanov, O.N. Small-angle scattering study of colloidal particles in heavy crude oils. Petrol. Chem. 2011, 51, 281–285. [Google Scholar] [CrossRef]
- Hoepfner, M.P.; Yang, Y. Ultra-small-angle X-ray scattering as a probe of petroleum heterogeneities from the nano- to the macroscale. In Chemistry Solutions to Challenges in the Petroleum Industry; Rahimi, P., Ovalles, C., Zhang, Y., Adams, J.J., Eds.; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2019; Chapter 4; pp. 67–87. [Google Scholar] [CrossRef]
- Dwiggins, C.W., Jr. A small angle X-ray scattering study of the colloidal nature of petroleum. J. Phys. Chem. 1965, 69, 3500–3506. [Google Scholar] [CrossRef]
- Pollack, S.S.; Yen, T.F. Structural studies of asphaltics by x-ray small angle scattering. Anal. Chem. 1970, 42, 623–629. [Google Scholar] [CrossRef]
- Dwiggins, C.W., Jr. Study of the colloidal nature of petroleum with an automated Bonse-Hart X-ray small-angle scattering unit. J. Appl. Cryst. 1978, 11, 615–619. [Google Scholar] [CrossRef]
- Herzog, P.; Tchoubar, D.; Espinat, D. Macrostructure of asphaltene dispersions by small-angle X-ray scattering. Fuel 1988, 67, 245–250. [Google Scholar] [CrossRef]
- Storm, D.A.; Sheu, E.Y.; De Tar, M.M. Macrostructure of asphaltenes in vacuum residue by small-angle X-ray scattering. Fuel 1993, 72, 977–981. [Google Scholar] [CrossRef]
- Xu, Y.; Koga, Y.; Strausz, O.P. Characterization of Athabasca asphaltenes by small-angle X-ray scattering. Fuel 1995, 74, 960–964. [Google Scholar] [CrossRef]
- Espinat, D.; Fenistein, D.; Barré, L.; Frot, D.; Briolant, Y. Effects of temperature and pressure on asphaltenes agglomeration in toluene. A light, X-ray, and neutron scattering investigation. Energy Fuels 2004, 18, 1243–1249. [Google Scholar] [CrossRef]
- Sirota, E.B. Swelling of asphaltenes. Petrol. Sci. Technol. 1998, 16, 415–431. [Google Scholar] [CrossRef]
- Sirota, E.B. Physical structure of asphaltenes. Energy Fuels 2005, 19, 1290–1296. [Google Scholar] [CrossRef]
- Sirota, E.B.; Lin, M.Y. Physical behavior of asphaltenes. Energy Fuels 2007, 21, 2809–2815. [Google Scholar] [CrossRef]
- Siddiqui, M.N.; Ali, M.F.; Shirokoff, J. Use of X-ray diffraction in assessing the aging pattern of asphalt fractions. Fuel 2002, 81, 51–58. [Google Scholar] [CrossRef]
- Shirokoff, J.; Siddiqui, M.N.; Ali, M.F. Characterization of the structure of Saudi crude asphaltenes by X-ray diffraction. Energy Fuels 1997, 11, 561–565. [Google Scholar] [CrossRef] [Green Version]
- Gebresellasie, K.; Lewis, J.C.; Shirokoff, J. X-ray spectral line shape analysis of asphalt binders. Energy Fuels 2013, 27, 2018–2024. [Google Scholar] [CrossRef]
- Kananpanah, S.; Bayat, M.; Mousavian, M.A.; Solaimany Nazar, A.R. Impacts of physico-chemical properties of asphaltene aggregates on optimization of the thermal de-asphaltene process. J. Petrol. Sci. Eng. 2016, 147, 718–725. [Google Scholar] [CrossRef]
- Díaz-Sánchez, H.; Rojas-Trigos, J.B.; Leyva, C.; Trejo-Zárraga, F. An approach for determination of asphaltene crystallite by X-ray diffraction analysis: A case of study. Petrol. Sci. Technol. 2017, 35, 1415–1420. [Google Scholar] [CrossRef]
- Rassamdana, H.; Sahimi, M. Asphalt flocculation and deposition: II. Formation and growth of fractal aggregates. AIChE J. 1996, 42, 3318–3332. [Google Scholar] [CrossRef]
- Ravey, J.C.; Ducouret, G.; Espinat, D. Asphaltene macrostructure by small angle neutron scattering. Fuel 1988, 67, 1560–1567. [Google Scholar] [CrossRef]
- Sheu, E.Y.; Liang, K.S.; Sinha, S.K.; Overfield, R.E. Polydispersity analysis of asphaltene solutions in toluene. J. Colloid Interf. Sci. 1992, 153, 399–410. [Google Scholar] [CrossRef]
- Tanaka, R.; Hunt, J.E.; Winans, R.E.; Thiyagarajan, P.; Sato, S.; Takanohashi, T. Aggregates structure analysis of petroleum asphaltenes with small-angle neutron scattering. Energy Fuels 2003, 17, 127–134. [Google Scholar] [CrossRef]
- Headen, T.F.; Boek, E.S.; Jackson, G.; Totton, T.S.; Müller, E.A. Simulation of asphaltene aggregation through molecular dynamics: Insights and limitations. Energy Fuels 2017, 31, 1108–1125. [Google Scholar] [CrossRef]
- Sjöblom, J.; Simon, S.; Xu, Z. Model molecules mimicking asphaltenes. Adv. Colloid Interface Sci. 2015, 218, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farooq, U.; Lædre, S.; Gawel, K. Review of asphaltenes in an electric field. Energy Fuels 2021, 35, 7285–7304. [Google Scholar] [CrossRef]
- Kusaka, I.; Wang, Z.-G.; Seinfeld, J.H. Ion-induced nucleation. II. Polarizable multipolar molecules. J. Chem. Phys. 1995, 103, 8993–9009. [Google Scholar] [CrossRef] [Green Version]
- Rogel, E. Studies on asphaltene aggregation via computational chemistry. Colloid Surf. A 1995, 104, 85–93. [Google Scholar] [CrossRef]
- Kowalewski, I.; Vandenbroucke, M.; Huc, A.Y. Preliminary results on molecular modeling of asphaltenes using structure elucidation programs in conjunction with molecular simulation programs. Energy Fuels 1996, 10, 97–107. [Google Scholar] [CrossRef]
- Murgich, J.; Rodríguez, J.; Aray, Y. Molecular recognition and molecular mechanics of micelles of some model asphaltenes and resins. Energy Fuels 1996, 10, 68–76. [Google Scholar] [CrossRef]
- Pacheco-Sánchez, J.H.; Zaragoza, I.P.; Martínez-Magadán, J.M. Asphaltene aggregation under vacuum at different temperatures by molecular dynamics. Energy Fuels 2003, 17, 1346–1355. [Google Scholar] [CrossRef]
- Jian, C.; Tang, T.; Bhattacharjee, S. Probing the effect of side-chain length on the aggregation of a model asphaltene using molecular dynamics simulations. Energy Fuels 2013, 27, 2057–2067. [Google Scholar] [CrossRef]
- Zhang, L.; Greenfield, M.L. Molecular orientation in model asphalts using molecular simulation. Energy Fuels 2007, 21, 1102–1111. [Google Scholar] [CrossRef]
- Carauta, A.N.M.; Seidl, P.R.; Chrisman, E.C.A.N.; Correia, J.C.G.; Menechini, P.D.O.; Silva, D.M.; Leal, K.Z.; de Menezes, S.M.C.; de Souza, W.F.; Teixeira, M.A.G. Modeling solvent effects on asphaltene dimers. Energy Fuels 2005, 19, 1245–1251. [Google Scholar] [CrossRef]
- Alvarez-Ramirez, F.; Ramirez-Jaramillo, E.; Ruiz-Morales, Y. Calculation of the interaction potential curve between asphaltene-asphaltene, asphaltene-resin, and resin-resin systems using density functional theory. Energy Fuels 2006, 20, 195–204. [Google Scholar] [CrossRef]
- Ortega-Rodríguez, A.; Cruz, S.A.; Gil-Villegas, A.; Guevara-Rodríguez, F.; Lira-Galeana, C. Molecular view of the asphaltene aggregation behavior in asphaltene-resin mixtures. Energy Fuels 2003, 17, 1100–1108. [Google Scholar] [CrossRef]
- Sedghi, M.; Goual, L.; Welch, W.; Kubelka, J. Effect of asphaltene structure on association and aggregation using molecular dynamics. J. Phys. Chem. B 2013, 117, 5765–5776. [Google Scholar] [CrossRef] [PubMed]
- da Costa, M.L.; Stoyanov, S.R.; Gusarov, S.; Seidl, P.R.; Carneiro, J.W.; Kovalenko, A. Computational study of the effect of dispersion interactions on the thermochemistry of aggregation of fused polycyclic aromatic hydrocarbons as model asphaltene compounds in solution. J. Phys. Chem. A 2014, 118, 896–908. [Google Scholar] [CrossRef]
- da Costa, M.L.; Stoyanov, S.R.; Gusarov, S.; Tan, X.; Gray, M.R.; Stryker, J.M.; Tykwinski, R.; Carneiro, J.W.; Seidl, P.R.; Kovalenko, A. Density functional theory investigation of the contributions of π–π stacking and hydrogen-bonding interactions to the aggregation of model asphaltene compounds. Energy Fuels 2012, 26, 2727–2735. [Google Scholar] [CrossRef]
- Diallo, M.S.; Cagin, T.; Faulon, J.L.; Goddard III, W.A. Thermodynamic properties of asphaltenes: A predictive approach based on computer assisted structure elucidation and atomistic simulations. In Asphaltenes and Asphalts; Yen, T.F., Chilingarian, G.V., Eds.; Elsevier: Amsterdam, The Netherlands, 2000; Volume 2, Chapter 5. [Google Scholar]
- Rogel, E.; Carbognani, L. Density estimation of asphaltenes using molecular dynamics simulations. Energy Fuels 2003, 17, 378–386. [Google Scholar] [CrossRef]
- Aray, Y.; Hernández-Bravo, R.; Parra, J.G.; Rodríguez, J.; Coll, D.S. Exploring the structure-solubility relationship of asphaltene models in toluene, heptane, and amphiphiles using a molecular dynamic atomistic methodology. J. Phys. Chem. A 2011, 115, 11495–11507. [Google Scholar] [CrossRef]
- Barcenas, M.; Duda, Y. Irreversible colloidal agglomeration in presence of associative inhibitors: Computer simulation study. Phys. Lett. A 2007, 365, 454–457. [Google Scholar] [CrossRef]
- Barcenas, M.; Duda, Y. Inhibition of irreversible cluster-cluster aggregation of colloids. Colloid Surf. A 2009, 334, 137–141. [Google Scholar] [CrossRef]
- Barcenas, M.; Orea, P. Molar-mass distributions of asphaltenes in the presence of inhibitors: Experimental and computer calculations. Energy Fuels 2011, 25, 2100–2108. [Google Scholar] [CrossRef]
- Noid, W.G.; Ayton, G.S.; Izvekov, S.; Voth, G.A. The multiscale coarse-graining method: A systematic approach to coarse-graining. In Coarse-Graining of Condensed Phase and Biomolecular Systems; Voth, G.A., Ed.; CRC Press: Boca Raton, FL, USA, 2009; Chapter 3. [Google Scholar]
- Español, P.; Warren, P.B. Perspective: Dissipative particle dynamics. J. Chem. Phys. 2017, 146, 150901–150916. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.-F.; Sun, L.-L.; Xu, J.-B.; Wu, H.; Wen, H. Aggregate structure in heavy crude oil: Using a dissipative particle dynamics based mesoscale platform. Energy Fuels 2010, 24, 4312–4326. [Google Scholar] [CrossRef]
- Wang, S.; Xu, J.; Wen, H. The aggregation and diffusion of asphaltenes studied by GPU-accelerated dissipative particle dynamics. Comput. Phys. Commun. 2014, 185, 3069–3078. [Google Scholar] [CrossRef]
- Dunn, N.J.H.; Gutama, B.; Noid, W.G. Simple simulation model for exploring the effects of solvent and structure on asphaltene aggregation. J. Phys. Chem. B 2019, 123, 6111–6122. [Google Scholar] [CrossRef]
- Loise, V.; Caputo, P.; Porto, M.; Calandra, P.; Angelico, R.; Oliviero Rossi, C. A review on Bitumen rejuvenation: Mechanisms, materials, methods and perspectives. Appl. Sci. 2019, 9, 4316. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Sun, H.; Jiang, H.; Xu, X.; Liang, M.; Hou, Y.; Yao, Z. Experimental assessment of reclaimed bitumen and RAP asphalt mixtures incorporating a developed rejuvenator. Constr. Build. Mater. 2019, 215, 660–669. [Google Scholar] [CrossRef]
- Porto, M.; Caputo, P.; Loise, V.; Eskandarsefat, S.; Teltayev, B.; Oliviero Rossi, C. Bitumen and bitumen modification: A review on latest advances. Appl. Sci. 2019, 9, 742. [Google Scholar] [CrossRef] [Green Version]
- Joohari, I.B.; Giustozzi, F. Waste tyres crumb rubber as a sustainability enhancer for polymer-modified and hybrid polymer-modified bitumen. Int. J. Pavement Eng. 2021, 1–15. [Google Scholar] [CrossRef]
- Oliviero Rossi, C.; Spadafora, A.; Teltayev, B.; Izmailova, G.; Amerbayev, Y.; Bortolotti, V. Polymer modified bitumen: Rheological properties and structural characterization. Colloids Surf. A Physicochem. Eng. Asp. 2015, 480, 390–397. [Google Scholar] [CrossRef]
- Pouranian, M.R.; Shishehbor, M. Sustainability assessment of green asphalt mixtures: A review. Environments 2019, 6, 73. [Google Scholar] [CrossRef] [Green Version]









| Modeling Approach | Main Features | Reference |
|---|---|---|
| colloidal | Bitumen is viewed as a colloidal dispersion of ASP micelles stabilized by resins | Nellensteyn [24] Pfeiffer et al. [25,26] Traxler et al. [27] Kawanaka et al. [28] Storm et al. [29] Lian et al. [30] |
| colloidal | ASPs are plate-like structures stacked together that form particles or crystallites | Yen et al. [31,32] Dickie et al. [33] Mullins [34] |
| solubility | ASPs are complexed by resins without forming micelles | Altegelt et al. [35] Koots et al. [36] Petersen [37] Christensen et al. [38] |
| solubility | Bitumen is a single-phase and homogenous fluid | Anderson et al. [39] Redelius [40] |
| colloidal | ASP micelles consist of an insoluble molecular core associated with surfactant-like resins in thermodynamic equilibrium | Lesueur [41] Rogel [42] |
| colloidal | Bitumen microstructure is formed by insoluble ASP aggregates stabilized by a fraction of more soluble ASP | Acevedo et al. [43,44,45] |
| colloidal | ASPs are stabilized by a series of nested shells with decreasing polarity | Wiehe et al. [46] |
| colloidal | Bitumen microstructure is constituted by ASP particles, micelles, and clusters of micelles held by molecular interactions | Yen et al. [47] |
| colloidal | Hierarchical microstructure of bitumen made by ASP molecules, nanoaggregates of ASP and cluster of nanoaggregates | Mullins et al. [48,49,50] |
| colloidal | Mechanism of micellization for ASP aggregates | Li et al. [51] Liu et al. [52] |
| solubility | Solution of ASP in oil is described in terms of thermodynamic liquid–liquid equilibrium | Wang et al. [53] |
| solubility | ASP association is interpreted according to a stepwise polymerization scheme | Agrawala et al. [54] Yarranton et al. [55] |
| colloidal | ASP nanoaggregates dispersed in solution generate structures with pores and host–guest complexes | Gray et al. [56] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Porto, M.; Angelico, R.; Caputo, P.; Abe, A.A.; Teltayev, B.; Rossi, C.O. The Structure of Bitumen: Conceptual Models and Experimental Evidences. Materials 2022, 15, 905. https://doi.org/10.3390/ma15030905
Porto M, Angelico R, Caputo P, Abe AA, Teltayev B, Rossi CO. The Structure of Bitumen: Conceptual Models and Experimental Evidences. Materials. 2022; 15(3):905. https://doi.org/10.3390/ma15030905
Chicago/Turabian StylePorto, Michele, Ruggero Angelico, Paolino Caputo, Abraham A. Abe, Bagdat Teltayev, and Cesare Oliviero Rossi. 2022. "The Structure of Bitumen: Conceptual Models and Experimental Evidences" Materials 15, no. 3: 905. https://doi.org/10.3390/ma15030905








